NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Mesalamine, also known as mesalazine and 5-aminosalicylate, is an orally available, antiinflammatory agent used for the treatment of ulcerative colitis to both induce and maintain remissions in disease. Mesalamine therapy has been associated with a low rate of serum enzyme elevations during therapy and with rare instances of clinically apparent acute liver injury.
Background
Mesalamine (me sal’ a meen) is used to treat disease flares in ulcerative colitis and to maintain disease remission. Mesalamine has antiinflammatory, antioxidant and antimicrobial activity and is locally active in the large intestine in reducing inflammation and injury. Mesalamine appears to act by inhibition of lipooxygenase activity, thereby inhibiting production of leukotrienes and leading to reduction in interleukin 1 (IL-1) and tumor necrosis factor (TNF) alpha. Mesalamine was approved for use in the United States in 1985 and remains a first line agent in the therapy of ulcerative colitis, both for induction and maintenance of clinical remission. It is poorly effective in Crohn disease, its major activity being in the large, rather than the small intestine. Mesalamine by itself is largely absorbed in the small intestine and little reaches the colon. For this reason, it is formulated to avoid absorption in the upper intestine and to be released in active form in the colon. The delayed absorption is accomplished either by enteric coating or by formulation as a pro-drug that is activated in the large intestine by local conditions or bacterial enzymes. Formulations of mesalamine include extended or delayed release formulations (Pentasa, Asacol, Lialda, Apriso) and mesalamine pro-drugs including sulfasalazine, olsalazine and balsalazide. Typical doses range considerable and vary by preparation, but are generally in the range of 1.5 to 4.8 g of mesalamine daily. Mesalamine is also available as an enema (Rowasa and others) and rectal suppositories (Canasa), which are useful for ulcerative proctitis and in ameliorating symptoms in mildly active, distal ulcerative colitis.
Balsalazide (bal sal’ a zide) is a pro-drug of mesalamine that consists of 5-aminosalicylate with an azo bond to a phenyl-hydroxybenzoid acid moiety which is cleaved off in the large intestine by bacterial enzymes, releasing free mesalamine. Balsalazide was approved as treatment of mildly to moderately active ulcerative colitis in adults in the United States in 2000 and is available generically and under the brand name Colazal in capsules of 750 mg; the usual dosage being 2.25 to 6.75 g daily in three divided doses.
Olsalazine (ol sal’ a zeen) is a prodrug of mesalamine that consists of two molecules of 5-aminocsalicylate (5’5’-azodisalicylate) joined at the amino-terminus with an azo bond that is cleaved by bacterial action in the colon, releasing two molecules of mesalamine. Olsalazine was approved in the United States for maintenace of remission of ulcerative colitis in patients intolerant of sulfasalazine in 2007 and is available under the brand name Dipentum in capsules of 250 mg, the usual dose being 500 mg twice daily.
Sulfasalazine (sul" fa sal' a zeen) is a prodrug of mesalamine that consists of 5-aminosalicylate joined with an azo bond to sulfapyridine. Sulfasalazine has been used extensively in the treatment of ulcerative colitis, but adverse reactions to the sulfonamide component of the agent can be problematic and has led to a decline in its use in favor of mesalamine by itself. Because the adverse effects and hepatotoxicity of sulfasalazine is largely due to the sulfonamide component, it is discussed separately in LiverTox.
Mesalamine is generally well tolerated and adverse event rates are similar to what is reported with placebo therapy of ulcerative colitis. Side effects may include diarrhea, headache, dizziness and nausea. Hypersensitivity reactions and pancreatitis can occur, but are rare.
Hepatotoxicity
In large registration trials of various forms of mesalamine, serum enzyme elevations were no more common with the products than with placebo therapy and were less common than with sulfasalazine. In these large studies, there were no reported instances of clinically apparent liver injury. Since approval and wide scale usage, however, there have been isolated reports of acute and chronic liver injury with jaundice attributed to mesalamine therapy. Clinically apparent liver injury is estimated to occur at an incidence of 3.2 cases per million prescriptions. Several patterns of injury have been reported, including asymptomatic and mild elevations in serum ALT levels, mild hepatitis accompanying a hypersensitivity reaction within a few days or weeks of starting (sometimes following a similar reaction to sulfasalazine), drug fever with accompanying serum enzyme elevations or mild hepatitis, and more typically, idiosyncratic cholestatic or hepatocellular liver injury (Cases 1 and 2), which typically arises after 1 to 6 months of treatment and is not accompanied by signs of hypersensitivity (rash, fever, eosinophilia). Most cases of liver injury with jaundice have been mild-to-moderate in severity and resolve rapidly upon stopping. In cases with hypersensitivity reactions, corticosteroid therapy is often used and appears to speed recovery. There have been no convincing reports of fatal acute liver injury, chronic hepatitis or vanishing bile duct syndrome attributable to mesalamine.
While fewer cases of hepatotoxicity have been reported with balsalazide and olsalazide than mesalamine, reports of enzyme elevations, jaundice, hepatitis and hepatic failure and death have been reported to the sponsor for these prodrugs. For this reason, the likelihood score for hepatotoxicity of all three agents are estimated to be the same. The hepatotoxicity of sulfasalazine is much more frequent than with mesalamine and is discussed separately.
Likelihood score (Mesalamine, Balsalazide and Olsalazine): C (probable cause of clinically apparent liver injury).
Likelihood score (Sulfasalazine): A (well documented cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of mesalamine hepatotoxicity is not well known, but may be immunologic, particularly those cases associated with immunoallergic features or drug fever. Mesalamine is formulated to avoid absorption in the upper intestine and only 10% to 20% of the dose is systematically absorbed. Mesalamine is metabolized by the liver to an acetylated molecule that is excreted in the urine. Interestingly, some cases of hypersensitivity reactions have been reported after administration of mesalamine rectally or by edema.
Outcome and Management
Most cases of liver injury attributed to mesalamine have been mild-to-moderate in severity and rapidly reversed when the drug is stopped. There have been published instances of severe hypersensitivity reactions to mesalamine, but no typical instances of acute liver failure or vanishing bile duct syndrome. Rechallenge leads to recurrence and should be avoided. There is occasional cross sensitivity to reactions to sulfasalazine; some degree of cross sensitivity should be expected between the delayed and extended formulations and the various other pro-drugs of mesalamine (balsalazide and olsalazine).
While sulfasalazine contains mesalamine, its hepatotoxicity generally resembles that of the sulfonamides and it is dicussed and its references are given separately in LiverTox.
Drug Class: Gastrointestinal Agents, Inflammatory Bowel Disease Agents; See also Sulfasalazine
CASE REPORTS
Case 1. Acute cholestatic hepatitis attributed to mesalamine therapy.
[Modified from: Stoschus B, Meybehm M, Spengler U, Scheurlen C, Sauerbruch T. Cholestasis associated with mesalazine therapy in a patient with Crohn's disease. J Hepatol 1997; 26: 425-8. PubMed Citation]
A 30 year old man with Crohn disease of the ileum was treated with oral mesalazine (the term used for mesalamine in Europe) and developed nausea, fatigue, weight loss, right upper quadrant pain and jaundice four months later. He had no history of liver disease and was known to have normal routine liver tests before starting mesalamine (Table). He denied alcohol use and was evidently not taking other medications. On examination, he was jaundiced and had right upper quadrant tenderness, but no evidence of chronic liver disease and no fever or rash. Laboratory testing showed serum bilirubin of 6.0 mg/dL, ALT 393 U/L, GGT 166 U/L and alkaline phosphatase 201 U/L. The total white blood cell count was normal as was the absolute eosinophil count (294/μL). Tests for hepatitis A, B and C and for cytomegalovirus, Epstein-Barr and herpes simplex viruses were negative as were routine serum autoantibodies. An ultrasound of the abdomen showed no evidence of biliary obstruction. A liver biopsy showed canalicular and cytoplasmic hepatocellular cholestasis, minimal lymphocytic infiltrates and spotty hepatocyte necrosis with no fibrosis or fat, suggestive of drug induced liver injury. Mesalamine was stopped and his symptoms resolved quickly. Six weeks after presentation all liver tests had returned to normal.
Key Points
| Medication: | Mesalamine, 4 g daily |
| Pattern: | Hepatocellular (R=16) |
| Severity: | 3+ (jaundiced and hospitalized) |
| Latency: | 4 months |
| Recovery: | Complete within 6 weeks |
| Other medications: | None mentioned |
Laboratory Values
Comment
A young man with Crohn disease developed jaundice and right upper quadrant pain 4 months after starting mesalamine. The prominence of pain, fatigue and nausea suggests that the injury was hepatocellular, which was also supported by the R ratio (~16) obtained at the time of hospital admission. On the other hand, the liver biopsy showed a prominence of cholestasis and only mild inflammation and necrosis, which led the authors to refer to the injury as cholestatic. Regardless, the course was self-limited and reasonably convincing as an example of mesalamine induced liver injury.
Case 2. Acute hepatitis with jaundice attributed to mesalamine therapy.
[Modified from: Barroso N, Leo E, Guil A, Larrauri J, Tirado C, Zafra C, Gavilán F, Reina FR. [Non-immunoallergic hepatotoxicity due to mesalazine]. Gastroenterol Hepatol 1999; 22: 176-9. Spanish. PubMed Citation]
A 57 year old woman with inflammatory bowel disease was treated with oral mesalamine (5-aminosalcyclate) and developed nausea and epigastric pain followed by pruritus, dark urine and jaundice six months later. She had no history of liver disease or drug allergies. She denied alcohol use and was not taking other medications, over-the-counter drugs or herbal preparations. On examination, she was jaundiced but had no fever, rash or arthritis. Laboratory testing showed a total bilirubin of 15.1 mg/dL (direct 13.4), ALT 916 U/L, AST 1049 U/L, GGT 203 U/L, and alkaline phosphatase 462 U/L (Table). Complete blood cell counts and INR were normal. Tests for hepatitis A, B and C and for cytomegalovirus, Ebstein-Barr and herpes simplex viruses were negative as were serum ANA and SMA. An ultrasound of the abdomen was normal and showed no evidence of biliary obstruction. A liver biopsy showed an acute hepatitis without fibrosis or steatosis. Mesalamine was stopped, but she continued to worsen for several weeks with cholestatic features, serum bilirubin rising to 25.7 mg/dL and alkaline phosphate to 769 U/L. However, she did not develop signs of liver failure and INR values were only mildly elevated. Thereafter she began to improve, and all tests were normal three months later.
Key Points
| Medication: | Mesalamine, 1.5 g daily |
| Pattern: | Hepatocellular (R=13.8 initially, falling to 1.7) |
| Severity: | 3+ (jaundiced and hospitalized) |
| Latency: | 6 months |
| Recovery: | Complete within 12 weeks |
| Other medications: | None |
Laboratory Values
| Days After Starting | Days After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 6 months | 0 | 916 | 462 | 15.1 | Admission, 2 weeks after onset of symptoms. R=13.8 |
| 7 months | 28 days | 467 | 670 | 23.8 | R=4.2 |
| 7.5 months | 38 days | 186 | 769 | 10.5 | R=1.7 |
| 8 months | 51 days | 104 | 458 | 3.1 | |
| 10 months | 110 days | 28 | 234 | 0.5 | Asymptomatic |
| Normal Values | <40 | <280 | <1.2 | ||
Comment
A woman with inflammatory bowel disease developed jaundice and itching 6 to 7 months after starting mesalamine. She was taking no other medications and other causes of acute liver injury were adequately excluded. While the initial laboratory tests showed a hepatocellular pattern of injury, the disease became distinctly cholestatic over the next several weeks. Recovery was delayed, but ultimately complete. Unlike cases associated with sulfasalazine, most cases of clinically apparent liver injury attributed to 5-ASA have had few features of hypersensitivity.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Mesalamine – Generic, Pentasa®
Balsalazide – Generic, Colazal®
Olsalazine – Dipentum®
Sulfasalazine – Generic, Azulfidine®
DRUG CLASS
Gastrointestinal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Mesalamine | 89-57-6 | C7-H7-N-O3 |
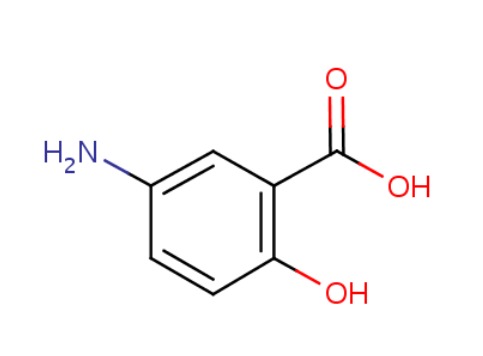 |
| Balsalazide | 80573-04-2 | C17-H15-N3-O6 |
 |
| Olsalazine | 6054-98-4 | C14-H10-N2-O6.2Na |
 |
| Sulfasalazine | 599-79-1 | C18-H14-N4-O5-S |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 25 September 2017
- Zimmerman HJ. Drugs used in inflammatory bowel disease. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 723-4.(Expert review of hepatotoxicity published in 1999 mentions that some patients with a hypersensitivity response and liver injury due to sulfasalazine redevelop similar injury when treated with 5-aminosalicylate or a derivative and that other instances of drug induced liver injury has been linked to mesalamine).
- Wallace JL, Sharkey KA. Pharmacotherapy of inflammatory bowel disease. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1350-62.(Textbook of pharmacology and therapeutics).
- Fich A, Schwartz J, Braverman D, Zifroni A, Rachmilewitz D. Sulfasalazine hepatotoxicity. Am J Gastroenterol 1984; 79: 401-2. [PubMed: 6144268](32 year old man developed fever and rash 1 month after starting sulfasalazine for ulcerative colitis [bilirubin 0.4 mg/dL, AST 420 U/L, Alk P 70 U/L], recovering within 3 weeks of stopping, but redeveloped fever and conjunctivitis within hours of receiving a mesalamine enema [but liver tests were normal]).
- Burke DA, Manning AP, Williamson JM, Axon AT. Adverse reactions to sulphasalazine and 5-amino salicylic acid in the same patient. Aliment Pharmacol Ther 1987 Jun; 1(3): 201-8. [PubMed: 2908750](A 26 year old man developed fever and rash 18 days after starting sulfasalazine for ulcerative proctitis [bilirubin 0.8 mg/dL, AST 2287 U/L, Alk P 2 times ULN], 21% eosinophils], resolving within 2 weeks, but with recurrence of fever within a day of starting 5-ASA enemas [liver tests remained normal]).
- Riley SA, Mani V, Goodman MJ, Herd ME, Dutt S, Turnberg LA. Comparison of delayed-release 5-aminosalicylic acid (mesalazine) and sulfasalazine as maintenance treatment for patients with ulcerative colitis. Gastroenterology 1988; 94: 1383-9. [PubMed: 2896139](Among 100 patients with ulcerative colitis treated with sulfasalazine or mesalamine for 48 weeks found similar efficacy but fewer side effects with mesalamine, and .biochemical variables showed no consistent changes during treatment with either. drug).
- Pineda JR, Leal JC, de la Morena E, Abreu L. [Hepatotoxicity: a new side effect of 5-aminosalicylic acid]. Med Clin (Barc) 1989; 93: 516. [PubMed: 2622244](37 year old woman with ulcerative colitis on sulfasalazine for 4 years developed ALT elevations within 2 weeks of switching to mesalamine [ALT 22 rising to 74 U/L], which resolved with switching back and recurred upon restarting mesalamine [ALT 139 U/L, symptoms, bilirubin and Alk P not mentioned]).
- Gyssens IC, de Bock RF, Peetermans ME. [Sulfasalazine allergy: fever, skin rash, hepatitis and T-lymphocytes]. Ned Tijdschr Geneeskd 1989; 133: 1608-10. Dutch. [PubMed: 2571950](29 year old woman developed fever, rash and lymphadenopathy within 3 weeks of starting sulfasalazine for suspected Crohn disease [bilirubin not given, ALT 340 U/L, Alk P 419 U/L, 19% atypical lymphocytes], which resolved quickly on stopping, but with recurrence of fever and rash with subsequent treatment with TMP/SMZ [ALT normal]).
- Rachmilewitz D. Coated mesalazine(5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 1989; 298 (6666): 82-6. [PMC free article: PMC1835436] [PubMed: 2563951](Among 220 patients with ulcerative colitis treated with a resin coated mesalamine vs sulfasalazine for 8 weeks, efficacy was similar, but side effects were more common with sulfasalazine, particularly hypersensitivity reactions, but events necessitating withdrawal of mesalamine included "increased liver function values and cholestasis").
- Brimblecombe R. Mesalazine: a global safety evaluation. Scand J Gastroenterol Suppl 1990; 172: 66. [PubMed: 1972297](Summary of safety data from 4 controlled trials of mesalamine in 932 patients with ulcerative colitis or Crohn disease, found common side effects of mesalamine no more frequent than with comparator arms or placebo; in postmarketing studies, the most frequently reported side effects were rash, nausea, dyspepsia, diarrhea, headache and "elevated liver function tests"; no mention of clinically apparent liver injury).
- Hautekeete ML, Bourgeois N, Potvin P, Duville L, Reynaert H, Devis G, Adler M, et al. Hypersensitivity with hepatotoxicity to mesalazine after hypersensitivity to sulfasalazine. Gastroenterology 1992; 103: 1925-7. [PubMed: 1360436](21 year old woman with Crohn disease developed rash 3 weeks after starting sulfasalazine, which resolved rapidly on stopping, but recurred within 1 day of starting mesalamine which was followed by rash, conjunctivitis, worsening liver tests and coma [bilirubin 2.5 rising to 11.8 mg/dL, ALT 102 to 1379 U/L, Alk P 560 U/L, 10% atypical lymphocytes] and complicated course, but ultimate full recovery).
- Gendre JP, Mary JY, Florent C, Modigliani R, Colombel JF, Soulé JC, Galmiche JP, Lerebours E, Descos L, Viteau JM, et al. Oral mesalamine (Pentasa) as maintenance treatment in Crohn's disease: a multicenter placebo-controlled study. The Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID). Gastroenterology 1993; 104: 435-9. [PubMed: 8425685](Among 161 patients with Crohn disease in remission treated with mesalamine or placebo for up to 2 years, side effects were reported to be similar in the two groups and .no adverse biological effects were found.; no mention of hepatotoxicity or ALT levels).
- Miner P, Hanauer S, Robinson M, Schwartz J, Arora S. Safety and efficacy of controlled-release mesalamine for maintenance of remission in ulcerative colitis. Pentasa UC Maintenance Study Group. Dig Dis Sci 1995; 40: 296-304. [PubMed: 7851193](Among 205 patients with ulcerative colitis in remission treated with mesalamine [Pentasa: 4 g daily] or placebo for 12 months, mentions that one of 103 patients on mesalamine developed "hepatitis" [AST 674 U/L, bilirubin and Alk P not given], which resolved within 1 month of stopping).
- Marteau P, Nelet F, Le Lu M, Devaux C. Adverse events in patients treated with 5-aminosalicyclic acid: 1993-1994 pharmacovigilance report for Pentasa in France. Aliment Pharmacol Ther 1996; 10: 949-56. [PubMed: 8971293](Between 1993-1994, there were 130 adverse events due to mesalamine reported to a French Pharmacovigilance network, including 7 cases of liver test abnormalities; no specific information provided).
- An oral preparation of mesalamine as long-term maintenance therapy for ulcerative colitis. A randomized, placebo-controlled trial. The Mesalamine Study Group. Ann Intern Med 1996; 124: 204-11. [PubMed: 8533995](Among 264 patients with ulcerative colitis treated with mesalamine [0.8 or 1.6 g daily] or placebo for 6 months, "no significant changes were seen in hepatic laboratory profiles" and there were no hepatic severe adverse events).
- Stoschus B, Meybehm M, Spengler U, Scheurlen C, Sauerbruch T. Cholestasis associated with mesalazine therapy in a patient with Crohn's disease. J Hepatol 1997; 26: 425-8. [PubMed: 9059966](30 year old man developed jaundice without fever, rash or eosinophilia 4 months after starting mesalazine [4 g daily] for Crohn disease [bilirubin 6.0 mg/dL, ALT 393 U/L, Alk P 201 U/L], resolving within 6 weeks of stopping: Case 1).
- Green JR, Gibson JA, Kerr GD, Swarbrick ET, Lobo AJ, Holdsworth CD, Crowe JP, et al. Maintenance of remission of ulcerative colitis: a comparison between balsalazide 3 g daily and mesalazine 1.2 g daily over 12 months. ABACUS Investigator group. Aliment Pharmacol Ther 1998; 12: 1207-16. [PubMed: 9882028](Among 95 patients with ulcerative colitis treated with balsalazide [3 g daily] or mesalamine [1.2 g daily] for up to 12 months, mean serum ALT levels decreased slightly on balsalazide [2 U/L] but increased on mesalamine [2 U/L], but no hepatic adverse events were mentioned).
- Thomsen OO, Cortot A, Jewell D, Wright JP, Winter T, Veloso FT, Vatn M, Persson T, Pettersson E. A comparison of budesonide and mesalamine for active Crohn's disease. International Budesonide-Mesalamine Study Group. N Engl J Med 1998; 339: 370-4. [PubMed: 9691103](Among 182 patients with Crohn disease treated with budesonide [9 g once daily] or mesalamine [2 g twice daily] for 16 weeks, there were .no clinically significant changes in . biochemical variables in either group. and no hepatic serious adverse events reported).
- Hanauer SB. Dose-ranging study of mesalamine (PENTASA) enemas in the treatment of acute ulcerative proctosigmoiditis: results of a multicentered placebo-controlled trial. The U.S. PENTASA Enema Study Group. Inflamm Bowel Dis 1998; 4: 79-83. [PubMed: 9589293](Among 287 patients with ulcerative proctitis treated with mesalamine [1, 2 or 4 g] or placebo enemas daily for 8 weeks, adverse events were similar with mesalamine as with placebo treatments).
- Green JR, Lobo AJ, Holdsworth CD, Leicester RJ, Gibson JA, Kerr GD, Hodgson HJ, et al. Balsalazide is more effective and better tolerated than mesalamine in the treatment of acute ulcerative colitis. The Abacus Investigator Group. Gastroenterology 1998; 114: 15-22. [PubMed: 9428213](Among 101 patients with ulcerative colitis treated with balsalazide or mesalamine for 12 weeks, there were no serious hepatic adverse events and mean ALT levels decreased slightly during balsalazide [-5 U/L] but not mesalamine therapy [+1 U/L]).
- Braun M, Fraser GM, Kunin M, Salamon F, Tur-Kaspa R. Mesalamine-induced granulomatous hepatitis. Am J Gastroenterol 1999; 94: 1973-4. [PubMed: 10406274](42 year old man with ulcerative colitis developed fever two weeks after starting mesalamine which continued for 6 weeks [bilirubin not provided, ALT 147 U/L, Alk P 800 U/L], which resolved only when mesalamine was stopped and recurred within 1 day of rechallenge).
- Barroso N, Leo E, Guil A, Larrauri J, Tirado C, Zafra C, Gavilán F, Reina FR. [Non-immunoallergic hepatotoxicity due to mesalazine]. Gastroenterol Hepatol 1999; 22: 176-9. Spanish. [PubMed: 10349787](57 year old woman developed jaundice without fever or rash 6 months after starting mesalamine [bilirubin 15.1 mg/dL, ALT 916 U/L, Alk P 462 U/L], worsening for 2 weeks and then resolving over the following 3 months).
- Deltenre P, Berson A, Marcellin P, Degott C, Biour M, Pessayre D. Mesalazine (5-aminosalicylic acid) induced chronic hepatitis. Gut 1999; 44: 886-8. [PMC free article: PMC1727547] [PubMed: 10323894](65 year old man developed serum enzyme elevations 8 months after starting mesalamine [ALT 13 times ULN], which remained elevated for 8 more months despite stopping simvastatin [ALT 12 times ULN, GGT 2 times ULN, bilirubin normal, ANA 1:500], but then resolved rapidly once mesalamine was stopped; a liver biopsy showed chronic hepatitis, interface hepatitis and portal fibrosis).
- Oral balsalazide (Colazal) for ulcerative colitis. Med Lett Drugs Ther 2001; 43 (1109): 62-3. [PubMed: 11468602](Concise review of pharmacology, efficacy and safety of balsalazide shortly after its approval in the United States mentions that hepatitis and allergic reactions have been reported to occur with mesalamine, but not specifically with balsalazide).
- Kruis W, Schreiber S, Theuer D, Brandes JW, Schütz E, Howaldt S, Krakamp B, et al. Low dose balsalazide (1.5 g twice daily) and mesalazine (0.5 g three times daily) maintained remission of ulcerative colitis but high dose balsalazide (3.0 g twice daily) was superior in preventing relapses. Gut 2001; 49: 783-9. [PMC free article: PMC1728533] [PubMed: 11709512](Among 133 patients with ulcerative colitis treated with balsalazide [3.0 vs 6.0 g daily] or mesalazine [1.5 g daily] for 26 weeks, no hepatic severe adverse events were observed; no mention of serum enzyme test results).
- Ransford RA, Langman MJ. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut 2002; 51: 536-9. [PMC free article: PMC1773410] [PubMed: 12235076](Serious adverse events reported to the Committee on Safety of Medications [UK] between 1991 and 1999 included 2400 due to sulfasalazine [SSZ] and 1100 mesalamine [MSA]; blood dyscrasias and skin reactions being more common with SSZ, interstitial nephritis and pancreatitis with MSA and hepatitis representing 6% of SSZ and 3.2% of MSA reports; no details given).
- Hartleb M, Biernat L, Kochel A. Drug-induced liver damage--a three-year study of patients from one gastroenterological department. Med Sci Monit 2002; 8: CR292-6. [PubMed: 11951073](During a 3 year period, 14 patients with drug induced liver injury were seen at a referral hospital; causes included Augmentin [3], statins [3], tuberculosis drugs [2], and one case due to mesalamine: 57 year old woman found to have abnormal liver tests 6 weeks after starting mesalamine [bilirubin 0.7 mg/dL, ALT 1.9 times ULN, Alk P 2.9 times ULN], resolving after stopping).
- Levine DS, Riff DS, Pruitt R, Wruble L, Koval G, Sales D, Bell JK, Johnson LK. A randomized, double blind, dose-response comparison of balsalazide (6.75 g), balsalazide (2.25 g), and mesalamine (2.4 g) in the treatment of active, mild-to-moderate ulcerative colitis. Am J Gastroenterol 2002; 97: 1398-407. [PubMed: 12094857](Among 154 patients with ulcerative colitis treated with balsalazide [6.75 or 2.25 g/daily] or [mesalamine 2.4 g daily], there were no severe hepatic adverse events and "no clinically significant changes in routine laboratory assessments").
- Mansfield JC, Giaffer MH, Cann PA, McKenna D, Thornton PC, Holdsworth CD. A double-blind comparison of balsalazide, 6.75 g, and sulfasalazine, 3 g, as sole therapy in the management of ulcerative colitis. Aliment Pharmacol Ther 2002; 16: 69-77. [PubMed: 11856080](Among 50 patients with ulcerative colitis receiving balsalazide or sulfasalazine has sole therapy for 8 weeks, there were no significant hepatic adverse events and "no significant changes in any of the...biochemical tests preformed").
- Green JR, Mansfield JC, Gibson JA, Kerr GD, Thornton PC. A double-blind comparison of balsalazide, 6.75 g daily, and sulfasalazine, 3 g daily, in patients with newly diagnosed or relapsed active ulcerative colitis. Aliment Pharmacol Ther 2002; 16: 61-8. [PubMed: 11856079](Among 57 patients with ulcerative colitis treated with either balsalazide or sulfasalazine for 12 weeks, there were no hepatic severe adverse events and "biochemistry assessment revealed no significant changes within or between the groups").
- Loftus EV Jr, Kane SV, Bjorkman D. Systematic review: short-term adverse effects of 5-aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther 2004; 19: 179-89. [PubMed: 14723609](Systematic review of 46 controlled trials of mesalazine, balsalazide and olsalazine for 2-12 weeks in ulcerative colitis found rates of adverse events similar to rates among placebo recipients, but lower than those with sulfasalazine; diarrhea in 2-10%, nausea in 3-8%, headache in 4-5%, abdominal pain in 4-6%, and rash in 2-4%; liver abnormalities reported in 2% of patients on mesalamine).
- Hanauer SB, Strömberg U. Oral Pentasa in the treatment of active Crohn's disease: A meta-analysis of double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol 2004; 2: 379-88. [PubMed: 15118975](An analysis of 3 controlled trials of oral mesalamine vs placebo for 16 weeks in a total of 615 patients with Crohn disease, does not discuss adverse events).
- Hanauer SB, Sandborn WJ, Kornbluth A, Katz S, Safdi M, Woogen S, Regalli G, et al. Delayed-release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: the ASCEND II trial. Am J Gastroenterol 2005; 100: 2478-85. [PubMed: 16279903](Among 386 patients with ulcerative colitis treated with 2.4 vs 4.8 g of mesalamine daily for 6 weeks, no hepatic severe adverse events occurred and "there were no clinically significant changes in laboratory values in either treatment group").
- Once-daily mesalamine (Lialda) for ulcerative colitis. Med Lett Drugs Ther 2007; 49 (1257): 25-6. [PubMed: 17375030](Concise review of the efficacy and safety of an extended release mesalamine [Lialda] shortly after its approval in the US mentions adverse effects are similar to those of other mesalamine products including headache, flatulence, dyspepsia, abdomen pain and diarrhea; pancreatitis, but not hepatotoxicity, is also mentioned).
- Hanauer SB, Sandborn WJ, Dallaire C, Archambault A, Yacyshyn B, Yeh C, Smith-Hall N. Delayed-release oral mesalamine 4.8 g/day (800 mg tablets) compared to 2.4 g/day (400 mg tablets) for the treatment of mildly to moderately active ulcerative colitis: The ASCEND I trial. Can J Gastroenterol 2007; 21: 827-34. [PMC free article: PMC2658575] [PubMed: 18080055](Among 301 patients with ulcerative colitis treated with mesalamine [2.4 or 4.8 g daily], there were no hepatic adverse events and "no clinically significant changes in laboratory values from baseline").
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to mesalamine or other 5-ASA containing products).
- Wiggins JB, Rajapakse R. Balsalazide: a novel 5-aminosalicylate prodrug for the treatment of active ulcerative colitis. Expert Opin Drug Metab Toxicol 2009; 5: 1279-84. [PubMed: 19743890](Review of safety and efficacy of balsalazide states that "although potential side effects are numerous, in clinical practice 5-ASAs are well tolerated"; no mention or discussion of hepatotoxicity).
- Encapsulated mesalamine granules(Apriso) for ulcerative colitis. Med Lett Drugs Ther 2009; 51 (1312): 38-9. [PubMed: 19448588](Concise review of pharmacology, efficacy and safety of encapsulated mesalamine granules [Apriso] shortly after its approval in the United States mentions that hepatotoxicity has been reported with other mesalamine-containing products).
- Scherl EJ, Pruitt R, Gordon GL, Lamet M, Shaw A, Huang S, Mareya S, Forbes WP. Safety and efficacy of a new 3.3 g b.i.d. tablet formulation in patients with mild-to-moderately-active ulcerative colitis: a multicenter, randomized, double-blind, placebo-controlled study. Am J Gastroenterol 2009; 104: 1452-9. [PubMed: 19491859](Among 249 patients with ulcerative colitis treated with balsalazide [3.3 g twice daily] or placebo, "there were no clinically significant or notable mean changes from baseline to week 8" in chemistry results).
- Sandborn WJ, Regula J, Feagan BG, Belousova E, Jojic N, Lukas M, Yacyshyn B, et al. Delayed-release oral mesalamine 4.8 g/day (800-mg tablet) is effective for patients with moderately active ulcerative colitis. Gastroenterology 2009; 137: 1934-43. [PubMed: 19766640](Among 772 patients with ulcerative colitis treated with mesalamine [2.4 or 4.8 g daily] for 6 weeks, side effects were similar in the two groups with 10 severe adverse events, one of which was "drug hypersensitivity" but no details given).
- Quiros JA, Heyman MB, Pohl JF, Attard TM, Pieniaszek HJ, Bortey E, Walker K, et al. Safety, efficacy, and pharmacokinetics of balsalazide in pediatric patients with mild-to-moderate active ulcerative colitis: results of a randomized, double-blind study. J Pediatr Gastroenterol Nutr 2009; 49: 571-9. [PMC free article: PMC3258511] [PubMed: 19633577](Among 68 children with ulcerative colitis treated with balsalazide [2.25 or 6.75 g daily] for 8 weeks, there were no hepatic adverse events and a "median decrease" in serum ALT levels).
- Sandborn WJ, Korzenik J, Lashner B, Leighton JA, Mahadevan U, Marion JF, Safdi M, et al. Once-daily dosing of delayed-release oral mesalamine (400-mg tablet) is as effective as twice-daily dosing for maintenance of remission of ulcerative colitis. Gastroenterology 2010; 138: 1286-96, 1296. [PubMed: 20064514](Among 1023 patients with ulcerative colitis treated with mesalamine [1.6-2.4 g with once or twice daily dosing], one patient developed "cholestatic jaundice" and one cholangitis, but no details were provided).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](World wide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children; mesalamine and other 5-ASA based medications were not listed among the top 41 causes).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to mesalamine, olsalazine or balsalazide).
- Khokhar OS, Lewis JH. Hepatotoxicity of agents used in the management of inflammatory bowel disease. Dig Dis 2010; 28: 508-18. [PubMed: 20926880](Extensive review of hepatotoxicity of agents used for IBD mentions that mesalamine only rarely causes acute liver injury, at an estimated rate of 3.2 cases per million prescriptions with predominantly cholestatic liver injury, with or without immunoallergic features).
- Love BL, Miller AD. Extended-release mesalamine granules for ulcerative colitis. Ann Pharmacother 2012; 46: 1529-36. [PubMed: 23115226](Summary of 2 controlled trials of an extended release mesalamine [Apriso] in 562 adults with ulcerative colitis in remission mentions that adverse event rates were "often similar to that of placebo or active controls" and ALT elevations occurred in less than 3% of patients).
- Masuda H, Takahashi Y, Nishida Y, Asai S. Comparison of the effect of mesalazine and sulfasalazine on laboratory parameters: a retrospective observational study. Eur J Clin Pharmacol 2012; 68: 1549-55. [PubMed: 22546896](Retrospective analysis of laboratory test results from 303 patients treated with mesalamine and 67 with sulfasalazine found no change in mean ALT levels with either drug).
- Drugs for inflammatory bowel disease. Treat Guidel Med Lett 2012; 10 (115): 19-28. [PubMed: 22361569](Concise review mentions that aminosalicylates are first line treatment for ulcerative colitis, are available in multiple formulations, induce remissions in 35-50% and maintain remissions in 55-75% of patients, can cause mild-to-moderate degrees of nausea and vomiting, diarrhea, headache, abdominal pain, and have been reported to cause rare instances of allergic reactions and hepatotoxicity).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 114: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to mesalamine or 5-ASA containing formulations).
- Girelli F, Bernardi S, Gardelli L, Bassi B, Parente G, Dubini A, Serra L, et al. A New Case of DRESS syndrome induced by sulfasalazine and triggered by amoxicillin. Case Rep Rheumatol 2013; 2013: 409152. [PMC free article: PMC3723001] [PubMed: 23936716](53 year old woman with seronegative arthritis developed fever, sore throat, facial edema, lymphadenopathy and rash 6 weeks after starting sulfasalazine [bilirubin 2.7 mg/dL, ALT 350 U/L, Alk P 2959 U/L], with prompt clinical response to methylprednisolone therapy).
- Raskin JB, Kamm MA, Jamal MM, Márquez J, Melzer E, Schoen RE, Szalóki T, et al. Mesalamine did not prevent recurrent diverticulitis in phase 3 controlled trials. Gastroenterology 2014; 147: 793-802. [PubMed: 25038431](Among 1182 adults with history of diverticulitis, recurrence rates were similar among those treated with mesalmamine [1.2, 2.4 or 4.8 g] or placebo daily; no mention of ALT elevations, but one patient died of hepatic failure and cirrhosis).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to mesalamine, balsalazide or olsalazine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was attributed to mesalamine but none to balsalazide or olsalazine).
- Bain JA. Mesalamine-induced fever: an important reminder to prescribers. J Gastrointestin Liver Dis 2015; 24: 259. [PubMed: 26114191](64 year old man developed recurrent fevers 2 months after starting mesalamine [no liver tests reported]).
- Stelzer T, Kohler S, Marques Maggio E, Heuss LT. An unusual cause of febrile hepatitis. BMJ Case Rep 2015; 2015. pii: bcr2014205857. [PMC free article: PMC4488648] [PubMed: 26113581](51 year old man with ulcerative colitis developed fever 2 months after starting mesalamine [bilirubin normal, ALT 319 U/L, Alk P 385 U/L, ANA 1:320], biopsy showing granulomas and resolving rapidly with stopping mesalamine and a short course of prednisone).
- Domínguez Jiménez JL, Pelado García EM, Copado Herrera R. [Mesalazine-induced acute hepatitis]. Gastroenterol Hepatol 2015; 38: 302-3. [PubMed: 25458543](53 year old man with ulcerative proctitis developed asymptomatic ALT elevations 2 months after starting mesalamine [bilirubin 0.9 mg/dL, ALT 1070 U/L, Alk P 85 U/L, no eosinophilia], with rapid improvement on stopping).
- Oliveira AM, Carvalho R, Martins A, Reis J. Acute hepatitis in the DRESS syndrome. GE Port J Gastroenterol 2016; 23: 304-308. [PMC free article: PMC5580171] [PubMed: 28868484](22 year old woman with migratory arthritis developed rash and fever 3 weeks after starting sulfasalazine [peak bilirubin 2.1 mg/dL, ALT 2102 U/L, Alk P 573 U/L, eosinophilia], resolving with prednisolone therapy and stopping sulfasalazine).
- Masood U, Sharma A, Nijjar S, Krenzer B. Unusual case of an alcoholic with liver injury from sulfasalazine use. J Basic Clin Pharm 2016; 8: 38-39. [PMC free article: PMC5201062] [PubMed: 28104973](57 year old man with alcoholism and rheumatoid arthritis developed jaundice 2 months after starting sulfasalazine [bilirubin 27.2 mg/dL, ALT 287 U/L, AST 363 U/L, Alk P 97 U/L, no eosinophilia], with ultrasound evidence of cirrhosis and ascites and ultimate recovery after stopping sulfasalazine).
- Rocci E, Park K, Hutchens K, Winterfield L. First report of mesalamine (5-aminosalicylic acid) as the causative agent in a case of acute generalized exanthamous pustulosis. Dermatol Online J 2017; 23. pii: 13030. [PubMed: 28329471](73 year old man with ulcerative colitis developed pustular rash, leukocytosis, "renal failure" and ALT elevations 1 week after starting mesalamine, with rapid resolution on stopping [no specific results provided]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- "Hepatotoxicity in inflammatory bowel disease: mesalazine, the forgotten drug".[Eur J Gastroenterol Hepatol. 2...]"Hepatotoxicity in inflammatory bowel disease: mesalazine, the forgotten drug".Garrido I, Santos AL, Lopes J, Lopes S, Macedo G. Eur J Gastroenterol Hepatol. 2021 Dec 1; 33(1S Suppl 1):e1067-e1070.
- Review Review article: high-dose aminosalicylates to induce and maintain remissions in ulcerative colitis.[Aliment Pharmacol Ther. 2006]Review Review article: high-dose aminosalicylates to induce and maintain remissions in ulcerative colitis.Hanauer SB. Aliment Pharmacol Ther. 2006 Oct; 24 Suppl 3:37-40.
- Review Balsalazide disodium for the treatment of ulcerative colitis.[Expert Rev Gastroenterol Hepat...]Review Balsalazide disodium for the treatment of ulcerative colitis.Patil SA, Moss AC. Expert Rev Gastroenterol Hepatol. 2008 Apr; 2(2):177-84.
- Review Review article: aminosalicylates in inflammatory bowel disease.[Aliment Pharmacol Ther. 2004]Review Review article: aminosalicylates in inflammatory bowel disease.Hanauer SB. Aliment Pharmacol Ther. 2004 Oct; 20 Suppl 4:60-5.
- Oral versus combination mesalazine therapy in active ulcerative colitis: a double-blind, double-dummy, randomized multicentre study.[Aliment Pharmacol Ther. 2001]Oral versus combination mesalazine therapy in active ulcerative colitis: a double-blind, double-dummy, randomized multicentre study.Vecchi M, Meucci G, Gionchetti P, Beltrami M, Di Maurizio P, Beretta L, Ganio E, Usai P, Campieri M, Fornaciari G, et al. Aliment Pharmacol Ther. 2001 Feb; 15(2):251-6.
- Mesalamine - LiverToxMesalamine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...