NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Mitomycin is a cytotoxic antibiotic which is used as anticancer therapy of advanced cancers of the stomach and pancreas. Mitomycin in combination with other anticancer agents frequently causes mild-to-moderate serum enzyme elevations during therapy and is capable of causing sinusoidal obstruction syndrome, but mitomycin by itself has not been specifically linked to instances of clinically apparent liver injury with jaundice.
Background
Mitomycin (mye” toe mye’ sin), which is also called mitomycin-C, is a cytotoxic antibiotic first isolated from Streptococcus caespitosus and later shown to have potent antitumor effects in vitro and in vivo. Mitomycin has clinical activity against several forms of malignancy, and combination therapies that include mitomycin have proven effective in several forms of solid tumors. Mitomycin was approved for use in the United States in 2002 and current formal indications include advanced or disseminated stomach and pancreatic cancer in combination with other antineoplastic. Because of its toxicities, however, mitomycin is now rarely used. Mitomycin is available as a solution or lyophilized powder for injection in vials of varying concentrations generically and under the commercial name Mutamycin. The typical dose of mitomycin varies by indication and is adjusted for body weight and renal function. Side effects of mitomycin include bone marrow suppression, nausea, vomiting, diarrhea, stomatitis, rash, fever and malaise. Uncommon but potentially severe adverse events include hemolytic uremic syndrome, hemolysis, neurological abnormalities, renal failure and interstitial pneumonitis.
Hepatotoxicity
Chemotherapy with mitomycin in combination with other agents is associated with serum enzyme elevations in a proportion of patients, depending upon the dose and other agents used. ALT elevations during mitomycin therapy are usually asymptomatic and transient and may resolve without dose modification. In many instances, it is difficult to attribute the liver test abnormalities to mitomycin, because of the exposure to other potentially hepatotoxic agents. High doses of mitomycin have been linked to cases of sinusoidal obstruction syndrome, typically presenting with right upper quadrant pain 10 to 30 days after the infusion, followed by weight gain, ascites and liver test abnormalities. Fatalities due to hepatic failure have occurred, but most patients recover within 1 to 3 months of onset. The frequency of sinusoidal obstruction syndrome limits the dosage of mitomycin that can be used in cancer chemotherapy and in myeloablation in preparation for bone marrow transplantation. There have been no convincing instances of acute, clinically apparent idiosyncratic liver injury with jaundice associated with mitomycin therapy.
Likelihood score: B[H] (very likely but now uncommon cause of sinusoid obstruction syndrome when given in high doses and in combination with other cytotoxic agents).
Mechanism of Injury
Mitomycin can be directly toxic to cells and the transient liver enzyme elevations during therapy, and induction of sinusoidal obstruction syndrome are probably due to direct toxicity to hepatocytes and sinusoidal endothelial cells.
Outcome and Management
The hepatic injury due to mitomycin varies in severity from mild, transient and asymptomatic liver enzyme elevations to acute liver failure due to sinusoidal obstruction syndrome. There is no satisfactory therapy for sinusoidal obstruction syndrome besides careful management of fluid balance and avoidance of further injury. Sinusoidal obstruction syndrome has become rare, largely due to avoidance of high dose chemotherapy with agents that have been linked with it such as busulfan, cyclosphosphamide and mitomycin. Intravenous defibrotide has been used to treat severe sinusoidal obstruction syndrome with some evidence of partial response.
Drug Class: Antineoplastic Agents
Other Drugs in the Subclass, Antibiotics, Cytotoxic: Bleomycin, Dactinomycin, Daunorubicin, Doxorubicin, Epirubicin, Idarubicin, Mitoxantrone, Plicamycin
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Mitomycin – Generic, Mutamycin®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Mitomycin | 50-07-7 | C15-H18-N4-O5 |
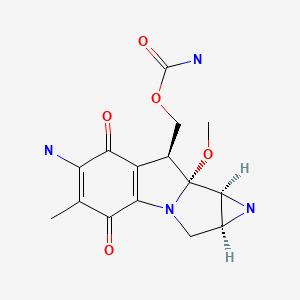
|
ANNOTATED BIBLIOGRAPHY
References updated: 19 February 2020
- Zimmerman HJ. Antibiotics. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 694-8.(Review of hepatotoxicity published in 1999 mentions that mitomycin produces steatosis in experimental animals and humans, but that this appears less important clinically than its potential for causing sinusoidal obstruction syndrome).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 541-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; mitomycin is not discussed).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Cytotoxic agents. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1167-203.(Textbook of pharmacology and therapeutics).
- Kenis Y, Stryckmans P. Chemotherapia (Basel). 1964;20:114–38. [Action of mitomycin C in 65 cases of malignant tumor. Comparison of repeated small doses and "massive" doses] French. [PubMed: 14164294](Experience in treating 65 patients with various malignancies with a wide range of doses of mitomycin; one patient developed toxic hepatitis, but no details were provided).
- Manheimer LH, Vital J. Mitomycin-C in the therapy of far-advanced malignant tumors. Cancer. 1966;19:207–12. [PubMed: 5905463](Among 46 patients with advanced cancer treated with mitomycin, there was "no appreciable effect upon the liver function").
- Matsunaga F, Shimoyama T, Mikawa K, Ishiwata J. Comparative study of methods of administering mitomycin C. Cancer. 1967;20:805–8. [PubMed: 4290387](A total of 135 patients with inoperable advanced cancers were treated with 3 different regimens of mitomycin; no discussion of ALT elevations or hepatotoxicity).
- Moertel CG, Reitemeier RJ, Hahn RG. Mitomycin C therapy in advanced gastrointestinal cancer. JAMA. 1968;204:1045–8. [PubMed: 5694750](Among 45 patients with advanced cancer treated with 5 day courses of mitomycin and 40 who were treated with long term courses, 3 patients developed severe hepatic decompensation in the context of advanced hepatic carcinomatosis and another developed combined hepatic and severe hematologic injury, both resolving together; few details given).
- Moore GE, Bross ID, Ausman R, Nadler S, Jones R Jr, Slack N, Rimm AA. Effects of mitomycin c (NSC-26980) in 346 patients with advanced cancer. Eastern Clinical Drug Evaluation Program. Cancer Chemother Rep. 1968;52:675–84. [PubMed: 5753587](Among 346 adults with malignancies given mitomycin C in a single dose regimen, toxicities included hematologic [84%], oral ulceration [2%], nausea and vomiting [22%], diarrhea [10%] and rash [5%]; no mention of hepatotoxicity, jaundice or ALT elevations).
- Robert J, Barbier P, Manaster J, Jacobs E. Hepatotoxicity of cytostatic drugs evaluated by liver function tests and appearance of jaundice. Digestion. 1968;1:229–32. [PubMed: 5696244](Among 417 courses of chemotherapy in 339 patients with various forms of cancer, elevations in bilirubin levels occurred in 9% and Alk P in 11%, but most could be attributed to other causes).
- Yamada E, Tanaka Y, Miyaishi S, Kuroyanagi Y. Kidoc, Kaneko M. One-shot infusion of non-surgically administered mitomycin C in the celiac artery for liver metastases. Clinical effects. Int Surg. 1970;54:391–404. [PubMed: 5489413](Among 53 patients with liver metastases treated with arterial infusion of mitomycin [30 mg], objective responses occurred in a proportion of those with gastric cancer, no mention of ALT elevations or hepatotoxicity).
- Whittington RM, Close HP. Clinical experience with mitomycin C (NSC-26980). Cancer Chemother Rep. 1970;54:195–8. [PubMed: 4334088](Among 281 patients with various malignancies treated with mitomycin in multiple 10 day courses, objective responses occurred in 7%; side effects were largely hematologic and gastrointestinal; no mention of ALT elevations or hepatotoxicity).
- Godfrey TE, Wilbur DW. Clinical experience with mitomycin C in large infrequent doses. Cancer. 1972;29:1647–52. [PubMed: 5064044](Among 106 patients with malignancies treated with intermittent infusions of mitomycin, "liver toxicity was not observed").
- Lazarus HM, Gottfried MR, Herzig RH, Phillips GL, Weiner RS, Sarna GP, Fay J, et al. Veno-occlusive disease of the liver after high-dose mitomycin C therapy and autologous bone marrow transplantation. Cancer. 1982;49:1789–95. [PubMed: 7042075](Among 29 patients with advanced malignancies undergoing autologous bone marrow transplantation after mitomycin ablation [60-90 mg over 3 days], 12 [41%] had rise of serum enzymes above 3 times ULN, 6 [21%] developed sinusoidal obstruction syndrome 15-70 days after the infusions, and 3 [10%] died of liver failure).
- Gottfried MR, Sudilovsky O. Hepatic veno-occlusive disease after high-dose mitomycin C and autologous bone marrow transplantation therapy. Hum Pathol. 1982;13:646–50. [PubMed: 6806170](Histological description of sinusoidal obstruction syndrome in 3 patients after mitomycin therapy and bone marrow transplantation described by Lazarus [1980]).
- Schlangen J, Wils J. Liver calcifications following hepatic artery infusion with 5-fluorouracil, adriamycin and mitomycin C (FAM). Rofo. 1984;140:607–8. [PubMed: 6429769](Among 27 patients with hepatic metastases from colorectal cancer treated with intraarterial infusions of fluorouracil, doxorubicin and mitomycin, one developed extensive liver calcifications shown on liver biopsy to be calcifications within necrotic tumor tissue; the patient was asymptomatic and liver test results were not provided).
- Shepard KV, Levin B, Karl RC, Faintuch J, DuBrow RA, Hagle M, Cooper RM, Beschorner J, Stablein D. Therapy for metastatic colorectal cancer with hepatic artery infusion chemotherapy using a subcutaneous implanted pump. J Clin Oncol. 1985;3:161–9. [PubMed: 3155793](Among 62 patients with colorectal cancer metastatic to the liver who underwent intraarterial infusions of FUDR and either mitomycin or dichloromethotrexate, 26 [49%] developed serum enzyme elevations, half of whom had jaundice).
- Karanes C, Ratanatharathorn V, Schilcher RB, Young JD, Emmer D, Hoschner JA, Leichman L, Baker LH. High-dose mitomycin-C with autologous bone marrow transplantation in patients with refractory malignancies. Influence of dose schedule on pharmacokinetics and nonhematopoietic toxicities. Am J Clin Oncol. 1986;9:444–8. [PubMed: 3096129](Among 12 patients with advanced malignancies undergoing bone marrow transplantation after high dose mitomycin monotherapy, one died of hepatic failure and sinusoidal obstruction syndrome occurred in at least half of patients, indicating that the frequency of severe hepatic toxicity is too great for mitomycin to be used alone for myeloablation).
- Sznol M, Ohnuma T, Holland JF. Hepatic toxicity of drugs used for hematologic neoplasia. Semin Liver Dis. 1987;7:237–56. [PubMed: 3317861](Overview of hepatotoxicity of antineoplastic agents, mentions that combination regimens including mitomycin have been linked to instances of sinusoidal obstruction syndrome).
- Shepard KV, Levin B, Faintuch J, Doria MI, DuBrow RA, Riddell RH. Hepatitis in patients receiving intraarterial chemotherapy for metastatic colorectal carcinoma. Am J Clin Oncol. 1987;10:36–40. [PubMed: 2950752](Among 51 patients with liver metastases from colorectal cancer treated with intraarterial infusions of FUDR and either mitomycin or dichloromethotrexate, 24 [47%] developed hepatitis, and two developed biliary strictures).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, several cases were attributed to antineoplastic agents [such as mercaptopurine, cyclophosphamide, docetaxel, temozolomide, bortezomib and imatinib], but none to mitomycin).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including 2 attributed to antineoplastic agents, 1 to melphalan and 1 to gemtuzumab).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, ten were attributed to antineoplastic agents, but none to plicamycin).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 cases were attributed to antineoplastic agents, but none to plicamycin).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Everolimus.[LiverTox: Clinical and Researc...]Review Everolimus.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Temsirolimus.[LiverTox: Clinical and Researc...]Review Temsirolimus.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Daratumumab.[LiverTox: Clinical and Researc...]Review Daratumumab.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Doxorubicin.[LiverTox: Clinical and Researc...]Review Doxorubicin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Pertuzumab.[LiverTox: Clinical and Researc...]Review Pertuzumab.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Mitomycin - LiverToxMitomycin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...