NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Mitotane is a unique antineoplastic agent used solely in the therapy of metastatic, unresectable adrenocortical carcinoma. Mitotane has been associated with a high rate of serum enzyme elevation during therapy, but has had limited clinical use and has not been linked to instances of clinically apparent acute liver injury.
Background
Mitotane (mye' toe tane) is an isomer of DDT (dichloro-diphenyl-trichloroethane) which was found to cause adrenal atrophy in dogs and was later developed as an antineoplastic agent for advanced or metastatic adrenocortical carcinoma. Mitotane is directly cytotoxic to adrenal tissue and appears to act by inhibition of sterol-O-acyl-transferase (SOAT) activity which blocks the esterification of cholesterol. Cholesterol is the precursor of adrenocortical hormones and it accumulates in adrenocortical cells. The prevention of esterification causes an excess of free cholesterol and other fatty acids which are is toxic to the hormone producing adrenal cells. Treatment with mitotane regularly causes a decrease in corticosteroid synthesis and can result in adrenal insufficiency and Addisonian crisis. Mitotane was approved for use in the chemotherapy of advanced adrenal carcinoma in the United States in 1970. It is available as tablets of 500 mg under the brand name Lysodren. The typical initial dose is 2 to 6 grams daily in 3 to 4 divided doses, which can be increased to achieve a blood concentration of 14 to 20 mg/L. Side effects of mitotane therapy are common and can include anorexia, nausea, vomiting, diarrhea, depression, dizziness, decreased memory and concentration, rash, gynecomastia, arthralgia and leukopenia. Potentially severe adverse events include adrenal insufficiency and crisis.
Hepatotoxicity
Serum aminotransferase elevations occur in up to half of patients on conventional doses of mitotane therapy, but elevations above 5 times the upper limit of normal are uncommon (<1%). There have been no individual published case reports of clinically apparent liver injury attributed to mitotane, but its general use has been limited, as adrenocortical carcinoma is rare. Furthermore, the nonspecific side effects of mitotane therapy are often dose limiting and may be mediated, at least in part, by hepatic dysfunction or injury perhaps due to the same lipotoxicity that accounts for adrenocortical injury.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
While hepatotoxicity from mitotane may be rare, it is likely due to direct hepatotoxicity. Mitotane is extensively metabolized in the liver via the microsomal enzyme system (predominantly 2C9), and production of a toxic or immunogenic intermediate may trigger liver injury.
Outcome and Management
The severity of the liver injury linked to mitotane therapy has been generally mild, transient and without symptoms or jaundice. Mitotane has not been linked to cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome. There is no information on cross sensitivity to hepatic injury between mitotane and other antineoplastic agents.
Drug Class: Antineoplastic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Mitotane – Lysodren®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
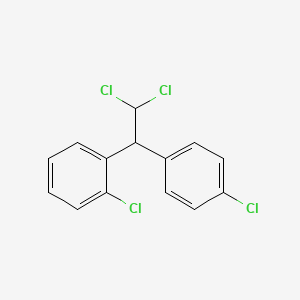
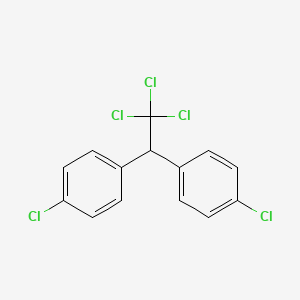
CHEMICAL FORMULAS AND STRUCTURES
ANNOTATED BIBLIOGRAPHY
References updated: 19 February 2020
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity of cancer chemotherapeutic agents published in 1999; mentions that mitotane can cause elevations in serum aminotransferase levels).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam, Elsevier, 2013, p. 541-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; mitotane is not discussed).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Cytotoxic agents. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1167-203.(Textbook of pharmacology and therapeutics).
- Montgomery DA, Welbourn RB. Adrenocortical carcinoma treated with O,P' -DD. Br Med J. 1965;1(5446):1356–8. [PMC free article: PMC2165741] [PubMed: 14280300](3 year old girl presented with Cushing syndrome and was found to have a large adrenocortical carcinoma, which was resected but recurred with metastases to the liver and lung that responded transiently to mitotane therapy, but ultimately led to her death; mentions that blood counts and liver tests did not change during therapy).
- Hutter AM Jr, Kayhoe DE. Adrenal cortical carcinoma. Results of treatment with o,p'DDD in 138 patients. Am J Med. 1966;41:581–92. [PubMed: 5923599](Among 138 patients with adrenocortical carcinoma treated with mitotane, toxicities were largely dose related and included gastrointestinal [anorexia, nausea, vomiting, diarrhea] and CNS effects [lethargy, somnolence, neuromotor disturbances], while "the absence of bone marrow and liver toxicity deserves emphasis").
- Hoffman DL, Mattox VR. Treatment of adrenocortical carcinoma with o,p'-DDD. Med Clin North Am. 1972;56:999–1012. [PubMed: 5034421](Among 19 patients with adrenocortical carcinoma treated with mitotane [o,p-DDD] between 1959 and 1971, most showed evidence of suppression of corticosteroid production and objective tumor regression occurred in 4, but mitotane was poorly tolerated and was suspected to be causing liver injury in one patient, but a liver biopsy was said to be normal).
- Hajjar RA, Hickey RC, Samaan NA. Adrenal cortical carcinoma. A study of 32 patients. Cancer. 1975;35:549–54. [PubMed: 234292](Review of 32 cases of adrenocortical carcinoma seen at MD Anderson Hospital between 1950 and 1972, 15 of whom received mitotane, most of whom had little response to treatment although side effects were frequent; no mention of ALT elevations and hepatotoxicity).
- Hogan TF, Citrin DL, Johnson BM, Nakamura S, Davis TE, Borden EC. o,p'-DDD (mitotane) therapy of adrenal cortical carcinoma: observations on drug dosage, toxicity, and steroid replacement. Cancer. 1978;42:2177–81. [PubMed: 719602](Among 4 patients with adrenocortical carcinoma treated with mitotane, all had severe toxicity including adrenal insufficiency).
- Gutierrez ML, Crooke ST. Mitotane (o,p'-DDD). Cancer Treat Rev. 1980;7:49–55. [PubMed: 7397707](Review of the development of mitotane, an isomer of DDT (o,p-DDD) which was found to cause severe adrenal cortical atrophy in dogs, and was later developed as a therapy of human adrenocortical carcinoma where clinical responses were found in 35-54% of patients and toxicities included gastrointestinal [79%] and CNS toxicity [49%], and more rarely skin rash, eye, genitourinary and cardiovascular injury; no mention of ALT elevations or hepatotoxicity).
- Luton JP, Cerdas S, Billaud L, Thomas G, Guilhaume B, Bertagna X, Laudat MH, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med. 1990;322:1195–201. [PubMed: 2325710](Review of the clinical features and courses of 105 patients with adrenocortical carcinoma seen between 1963 and 1987 of whom 59 received mitotane which controlled hormone levels in 75% of patients, although survival did not seem to be improved; side effects were common and largely gastrointestinal [anorexia, nausea] or neurologic [ataxia, somnolence] and "most patients had hepatic disturbances" including increases in ALT, AST, GT and Alk P, but no mention of jaundice or clinically apparent liver injury).
- Haak HR, Hermans J, van de Velde CJ, Lentjes EG, Goslings BM, Fleuren GJ, Krans HM. Optimal treatment of adrenocortical carcinoma with mitotane: results in a consecutive series of 96 patients. Br J Cancer. 1994;69:947–51. [PMC free article: PMC1968906] [PubMed: 8180029](Among 96 patients with adrenocortical carcinoma with mitotane between 1959 and 1992, patient responses and improved survival was found in those who achieved high levels of mitotane [≥14 g/L] and side effects were mainly anorexia, nausea, diarrhea and [with high doses] CNS toxicity; no mention of ALT elevations or hepatotoxicity).
- Webb CB, Twedt DC. Acute hepatopathy associated with mitotane administration in a dog. J Am Anim Hosp Assoc. 2006;42:298–301. [PubMed: 16822769](8 year old mixed breed bitch developed jaundice one month after starting and 2 weeks after stopping mitotane for suspected hyperadrenocorticism [bilirubin 9.1 mg/dL, ALT 1091 U/L, Alk P 2371 U/L, GGT 37 U/L], liver biopsy showing parenchymal collapse and vacuolated hepatocytes, recovering after treatment with ursodiol, vitamin E and S-adenosylmethionine).
- Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372–80. [PubMed: 17554118](Among 177 patients with adrenocortical carcinoma undergoing surgical resection, the 47 who received adjuvant mitotane had a prolonged overall and recurrence free survival; side effects included ALT or AST elevations in 49%, but all were transient or symptomatic and none were above 5 times ULN).
- Wängberg B, Khorram-Manesh A, Jansson S, Nilsson B, Nilsson O, Jakobsson CE, Lindstedt S, et al. The long-term survival in adrenocortical carcinoma with active surgical management and use of monitored mitotane. Endocr Relat Cancer. 2010;17:265–72. [PubMed: 20026647](Among 43 patients with adrenocortical carcinoma treated with surgery and monitored mitotane, survival was improved in those with drug levels above 14 mg/L, but only 24 patients [56%] could tolerate and achieve these levels; no mention of ALT elevations or hepatotoxicity).
- Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, et al. FIRM-ACT Study Group. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–97. [PubMed: 22551107](Among 304 patients with adrenocortical carcinoma treated with mitotane and either etoposide with doxorubicin and cisplatin [mit-EDC] or streptozocin [mit-S], objective responses were more frequent with mit-EDC, but overall survival was not different, and rates and patterns of side effects were similar between the two groups).
- Kerkhofs TM, Baudin E, Terzolo M, Allolio B, Chadarevian R, Mueller HH, Skogseid B, et al. Comparison of two mitotane starting dose regimens in patients with advanced adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:4759–67. [PubMed: 24057287](Among 40 patients with adrenocortical carcinoma treated with 2 doses of mitotane, adverse event rates were similar and included elevations in GGT [peak 123 to 311 U/L] and, to a lesser extent, increases in ALT and AST, although no patient developed clinically apparent liver injury).
- Lerario AM, Worden FP, Ramm CA, Hesseltine EA, Stadler WM, Else T, Shah MH, et al. The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: a multi-institutional NCI-sponsored trial. Horm Cancer. 2014;5:232–9. [PMC free article: PMC4298824] [PubMed: 24849545](Among 20 patients treated with mitotane, a clinical response occurred in 40%, but side effects were frequent and progression free survival only 8 weeks; one patient had persistent ALT elevations above 5 times ULN and one died of multiorgan failure).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, ten were attributed to antineoplastic agents, but none to mitotane).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 45 were attributed to antineoplastic agents, but none to mitotane).
- Molitch ME. Current approaches to the pharmacological management of Cushing's disease. Mol Cell Endocrinol. 2015;408:185–9. [PubMed: 25450859](Review of the medical therapy of Cushing disease mentions that mitotane is an adrenolytic agent used to treat adrenal carcinoma but is rarely used for benign disease and that “abnormal liver function tests” are common adverse events).
- Sbiera S, Leich E, Liebisch G, Sbiera I, Schirbel A, Wiemer L, Matysik S, et al. Mitotane inhibits sterol-O-acyl transferase 1 triggering lipid-mediated endoplasmic reticulum stress and apoptosis in adrenocortical carcinoma cells. Endocrinology. 2015;156:3895–908. [PubMed: 26305886](Treatment of an adrenocortical cell line with mitotane showed a marked alteration of lipid-related genes and ER stress that appeared to be due to inhibition of cholesterol esterification by sterol-O-acyl transferase and accumulation of toxic levels of free cholesterol, oxysterols and free fatty acids).
- Lalli E. Mitotane revisited: a new target for an old drug. Endocrinology. 2015;156:3873–5. [PubMed: 26474360](Commentary of the findings of Sbiera [2015] and the proposed mechanism of action of mitotane in causing apoptosis of adrenocortical cells).
- Maiter D, Bex M, Vroonen L. T'Sjoen G, Gil T, Banh C, Chadarevian R. Efficacy and safety of mitotane in the treatment of adrenocortical carcinoma: A retrospective study in 34 Belgian patients. Ann Endocrinol (Paris). 2016;77(5):578–85. [PubMed: 27063476](Among 34 patients with adrenocortical carcinoma treated with mitotane, adverse events were common but "never serious"; no mention of ALT elevations or hepatotoxicity).
- Gupta N, Rivera M, Novotny P, Rodriguez V, Bancos I, Lteif A. Adrenocortical carcinoma in children: a clinicopathological analysis of 41 patients at the Mayo Clinic from 1950 to 2017. Horm Res Paediatr. 2018;90:8–18. [PubMed: 29804118](Among 41 children with adrenocortical carcinoma followed at the Mayo Clinic between 1950 and 2017, 20 received chemotherapy with mitotane, 8 of whom received it as monotherapy after surgical resection; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Phase II evaluation of cisplatin and etoposide followed by mitotane at disease progression in patients with locally advanced or metastatic adrenocortical carcinoma: a Southwest Oncology Group Study.[Cancer. 2000]Phase II evaluation of cisplatin and etoposide followed by mitotane at disease progression in patients with locally advanced or metastatic adrenocortical carcinoma: a Southwest Oncology Group Study.Williamson SK, Lew D, Miller GJ, Balcerzak SP, Baker LH, Crawford ED. Cancer. 2000 Mar 1; 88(5):1159-65.
- Complete Responses to Mitotane in Metastatic Adrenocortical Carcinoma-A New Look at an Old Drug.[Oncologist. 2017]Complete Responses to Mitotane in Metastatic Adrenocortical Carcinoma-A New Look at an Old Drug.Reidy-Lagunes DL, Lung B, Untch BR, Raj N, Hrabovsky A, Kelly C, Gerst S, Katz S, Kampel L, Chou J, et al. Oncologist. 2017 Sep; 22(9):1102-1106. Epub 2017 May 30.
- Japanese single-institution analysis of mitotane for patients with adrenocortical carcinoma.[Endocr J. 2021]Japanese single-institution analysis of mitotane for patients with adrenocortical carcinoma.Ohmoto A, Shigematsu Y, Fukuda N, Wang X, Urasaki T, Hayashi N, Sato Y, Nakano K, Yunokawa M, Ono M, et al. Endocr J. 2021 Dec 28; 68(12):1383-1390. Epub 2021 Jun 26.
- Review Management of endocrine manifestations and the use of mitotane as a chemotherapeutic agent for adrenocortical carcinoma.[J Clin Oncol. 2009]Review Management of endocrine manifestations and the use of mitotane as a chemotherapeutic agent for adrenocortical carcinoma.Veytsman I, Nieman L, Fojo T. J Clin Oncol. 2009 Sep 20; 27(27):4619-29. Epub 2009 Aug 10.
- Review Adjunctive treatment of adrenocortical carcinoma.[Curr Opin Endocrinol Diabetes ...]Review Adjunctive treatment of adrenocortical carcinoma.Terzolo M, Berruti A. Curr Opin Endocrinol Diabetes Obes. 2008 Jun; 15(3):221-6.
- Mitotane - LiverToxMitotane - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...