NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Nabilone is an orally available cannabinoid agonist that is used to treat chemotherapy induced nausea and vomiting and to stimulate appetite, particularly in patients with wasting disease or cachexia. Nabilone is associated with a minimal rate of serum enzyme elevations during therapy and has not been linked to cases of clinically apparent liver injury with jaundice.
Background
Nabilone (Nab’ i lone) is a synthetic cannabinoid which is similar to the principal psychoactive constituent of the marijuana plant (Cannabis sativa). Nabilone is a partial agonist of the cannabinoid receptors which are found in the central nervous system (CB1 receptor), but also peripherally (largely CB2 receptors). Activation of CB receptors results in effects on appetite, mood, cognition, memory and perception. Nabilone therapy has been shown to decrease nausea and vomiting in patients undergoing cancer chemotherapy. Nabilone was approved for use in the United States in 1985 and current indications are prevention of cancer chemotherapy associated nausea and vomiting. Nabilone is available as 1 mg capsules under the brand name Cesamet. The typical adult oral dose is 1 to 2 mg twice daily, the initial dose being 1 to 3 hours before the chemotherapeutic agent is given. Nabilone is usually reserved for patients who fail to response to or are intolerant of conventional antiemetics, such as the serotonin [5-HT3] receptor antagonists. Off-label uses of nabilone include as an appetite stimulant and for chronic pain relief, but its efficacy is not well established for either of these indications. Common side effects include fatigue, sedation, somnolence, dizziness, euphoria, abnormal thinking, paranoid reactions, impairment of driving and operation of heavy equipment, conjunctivitis, diarrhea, nausea, vomiting, abdominal pain, orthostatic hypotension and tachycardia. Rare side effects include hallucinations and seizures. Nabilone is classified as a Schedule II drug, indicating that it has clear potential for physical and psychological dependency and abuse.
Hepatotoxicity
Serum aminotransferase elevations during nabilone therapy are not common, generally mild and similar to the rate in controls who are receiving cancer chemotherapy. There have been no convincing cases of clinically apparent liver injury attributable to nabilone published in the literature and, thus, significant liver injury from nabilone must be exceeding rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Nabilone is metabolized by the liver and undergoes extensive first-pass metabolism to both active and inactive metabolites. Despite its hepatic metabolism by CYP microsomal enzymes, it has not been implicated in causing drug-drug interactions. The lack of reported cases of liver injury and low rate of drug-drug interactions due to nabilone may be due to the low doses and limited duration of typical therapy.
Drug Class: Gastrointestinal Agents, Antiemetic Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nabilone – Cesamet®
DRUG CLASS
Gastrointestinal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Nabilone | 51022-71-0 | C24-H36-O3 |
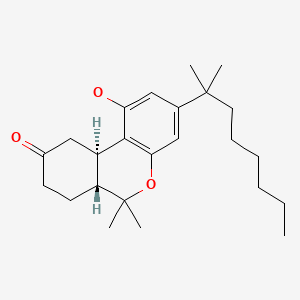
|
ANNOTATED BIBLIOGRAPHY
References updated: 02 October 2021
Abbreviations used: AIDS, acquired immune deficiency syndrome; CB, cannabinoids.
- Zimmerman HJ. Antiemetic and prokinetic compounds. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 721.(Expert review of hepatotoxicity published in 1999 does not discuss nabilone).
- Sharkey KA, MacNaughton WK. Antinauseants and antiemetics. Gastrointestinal motility bowel motility and water flux, emesis, and biliary and pancreatic disease. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 934-8.(Textbook of pharmacology and therapeutics).
- Marijuana. In, PDR for Herbal Medicines. 4th ed. Montvale, New Jersey: Thomson Healthcare Inc. 2007: pp. 562-7.(Compilation of short monographs on herbal medications and dietary supplements).
- Fabre LF, McLendon D. The efficacy and safety of nabilone(a synthetic cannabinoid) in the treatment of anxiety. J Clin Pharmacol. 1981;21:377S–382S. [PubMed: 6117575](Among 25 patients with anxiety disorders participating in dose finding studies of nabilone for up to 28 days, the most common side effects were dry mouth and eyes, drowsiness, headaches and insomnia; "nabilone did not alter any value in the clinical chemistry battery").
- Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, Lefkowitz L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10:89–97. [PubMed: 7730690](Among 139 patients with AIDS-related anorexia and weight loss treated with dronabinol or placebo for up to 4 weeks, side effects included euphoria, dizziness, drowsiness and difficulty thinking and "no treatment-related toxicity was found on. laboratory tests").
- Beal JE, Olson R, Lefkowitz L, Laubenstein L, Bellman P, Yangco B, Morales JO, et al. Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia. J Pain Symptom Manage. 1997;14:7–14. [PubMed: 9223837](Among 94 patients with late stage AIDS treated with dronabinol [2.5-5 mg daily] for up to 12 months, there were "no significant changes in hematology or blood chemistry parameters").
- Wissel J, Haydn T, Mueller J, Brenneis C, Berger T, Poewe W, Schelosky LD. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain: a double-blind placebo-controlled cross-over trial. J Neurol. 2006;253:1337–41. [PubMed: 16988792](Among 13 patients with upper motor neuron disease and spasticity treated with nabilone [1 mg daily] for up to 9 weeks, pain and spasticity decreased and "no severe side effects were reported").
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, no cases were attributed to cannabinoid agonists or antiemetics).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to antiemetic agents).
- Borgelt LM, Franson KL, Nussbaum AM, Wang GS. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33:195–209. [PubMed: 23386598](Review of history and status of cannabis used for medical purposes including severe pain, muscle spasms, anorexia, nausea and vomiting, and glaucoma; adverse events are common, but usually mild and reversible, most frequently dry mouth, dizziness, drowsiness, and changes in cognition and mood).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to antiemetics).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, the most common implicated agents being nimesulide [n=53: 30%], cyproterone [n=18], nitrofurantoin [n=17], antituberculosis drugs [n=13], and flutamide [n=12: 7%]; no cannabinoid agonist or antiemetic was listed).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to nabilone or other antiemetics).
- Polito S, MacDonald T, Romanick M, Jupp J, Wiernikowski J, Vennettilli A, Khanna M, et al. Safety and efficacy of nabilone for acute chemotherapy-induced vomiting prophylaxis in pediatric patients: A multicenter, retrospective review. Pediatr Blood Cancer. 2018;65:e27374. [PubMed: 30051617](Among 110 pediatric patients treated with nabilone for prevention of chemotherapy induced nausea and vomiting [usually in combination with a serotonin [5-HT3] receptor antagonist], adverse events arose in 34%, most commonly sedation [20%], dizziness [10%] and euphoria [4%], leading to discontinuation in 9%, but without serious adverse events; no mention of ALT elevations or hepatotoxicity).
- Herrmann N, Ruthirakuhan M, Gallagher D, Verhoeff NPLG, Kiss A, Black SE, Lanctôt KL. Randomized Placebo-Controlled Trial of Nabilone for Agitation in Alzheimer's Disease. Am J Geriatr Psychiatry. 2019;27:1161–1173. [PubMed: 31182351](Among 38 elderly patients with Alzheimer disease treated with nabilone [titrated] or placebo for 6 weeks in a crossover trial, nabilone therapy was associated with a slight decrease in agitation but increase in sedation; no mention of ALT elevations or hepatoxicity).
- Cannabis and cannabinoids. Med Lett Drugs Ther. 2019;61(1585):179–182. [PubMed: 31770357](Concise review of the beneficial and adverse effects of cannabis and cannabinoids, 3 of which are FDA approved products, two synthetic agents that are used for prevention of chemotherapy induced nausea and vomiting [nabilone and dronabinol] and one a purified natural product used to treat rare forms of severe epilepsy [cannabidiol]; all of the cannabinoids can cause dizziness, somnolence, euphoria, abnormal thinking, difficulty concentrating, hypotension, hypertension, syncope and tachycardia as well as addiction and dependence with long term use; no mention of ALT elevations or hepatoxicity).
- Sholler DJ, Huestis MA, Amendolara B, Vandrey R, Cooper ZD. Therapeutic potential and safety considerations for the clinical use of synthetic cannabinoids. Pharmacol Biochem Behav. 2020;199:173059. [PMC free article: PMC7725960] [PubMed: 33086126](Extensive review of the endocannabinoid system and potential therapies for synthetic cannabinoids [CB] and CB receptor antagonists; while there are CB receptors in the liver [predominantly CB2], there is no evidence that the CBs in clinical practice cause liver injury).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Dronabinol.[LiverTox: Clinical and Researc...]Review Dronabinol.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Nabilone for Non-chemotherapy Associated Nausea and Weight Loss due to Medical Conditions: A Review of the Clinical Effectiveness and Guidelines[ 2014]Review Nabilone for Non-chemotherapy Associated Nausea and Weight Loss due to Medical Conditions: A Review of the Clinical Effectiveness and Guidelines. 2014 Sep 12
- Review Oral nabilone capsules in the treatment of chemotherapy-induced nausea and vomiting and pain.[Expert Opin Investig Drugs. 2008]Review Oral nabilone capsules in the treatment of chemotherapy-induced nausea and vomiting and pain.Davis MP. Expert Opin Investig Drugs. 2008 Jan; 17(1):85-95.
- Review Nabilone for the Treatment of Nausea and Vomiting or Anorexia: A Review of Clinical Effectiveness and Guidelines[ 2019]Review Nabilone for the Treatment of Nausea and Vomiting or Anorexia: A Review of Clinical Effectiveness and GuidelinesHo C, MacDougall D. 2019 Feb 27
- A cross-over comparison of nabilone and prochlorperazine for emesis induced by cancer chemotherapy.[Am J Clin Oncol. 1985]A cross-over comparison of nabilone and prochlorperazine for emesis induced by cancer chemotherapy.Niiranen A, Mattson K. Am J Clin Oncol. 1985 Aug; 8(4):336-40.
- Nabilone - LiverToxNabilone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...