NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Nabumetone is a long acting nonsteroidal antiinflammatory drug (NSAID) that is available by prescription only and is used in therapy of chronic arthritis. Nabumetone has been linked to rare instances of clinically apparent, idiosyncratic drug induced liver disease.
Background
Nabumetone (nab ue' me tone) is a naphthyl alkanone and orally available antiinflammatory agent. Like other NSAIDs, nabumetone is a potent cyclo-oxygenase (Cox-1 and -2) inhibitor, which blocks the formation of prostaglandins that are important mediators in pain and inflammatory pathways. Nabumetone is a pro-drug and exerts anti-cyclo-oxygenase activity only after absorption and activation in the liver. Like other NSAIDs, it has analgesic, antipyretic and antiinflammatory activities. Nabumetone was approved in the United States in 1991 and its current indications are for treatment of chronic arthritis due to osteoarthritis or rheumatoid arthritis. Nabumetone is available by prescription only, and currently more than 4 million prescriptions are filled yearly. Generic formulations are available of 500 and 750 mg; specific commercially available names include Relafen. The usual dose in adults is 1000 mg once daily, increasing to as much as 2000 mg daily based upon response and tolerance. As with other NSAIDs, nabumetone is generally well tolerated, but side effects can included headache, dizziness, somnolence, dyspepsia, nausea, abdominal discomfort, heartburn, peripheral edema and hypersensitivity reactions. Rare but serious adverse events from NSAIDs include gastrointestinal ulceration and bleeding, increased risk for cardiovascular disease, renal dysfunction and hypersensitivity reactions including anaphylaxis, exfoliative dermatitis and Stevens Johnson syndrome.
Hepatotoxicity
Prospective studies show that 1% to 5% of patients taking nabumetone experience at least transient serum aminotransferase elevations. These may resolve even with drug continuation. Marked aminotransferase elevations (>3 times ULN) occur in 0.5% of patients, a rate similar to that in placebo treated controls. Clinically apparent liver injury with jaundice from nabumetone is rare and in large clinical trials, no instances of acute liver injury with jaundice were reported. Since its approval and release, nabumetone has been reported to cause rare instances of serious hepatic adverse events (~1.3 per million prescriptions), but there have been no cases of clinically apparent liver injury due to nabumetone described in the published literature. Furthermore, nabumetone is not mentioned as a cause in large case series on drug induced liver injury or acute liver failure. Thus, the latency, clinical features and outcome of nabumetone induced liver injury have not been described and clinically apparent hepatotoxicity due to nabumetone must be very rare.
Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which nabumetone causes hepatotoxicity is not known. Nabumetone undergoes rapid biotransformation in the liver to methoxy-2-naphthylacetic acid, the principal active metabolite.
Outcome and Management
The minor aminotransferase elevations that occur in a small proportion of patients during chronic therapy with nabumetone are usually subclinical and not progressive. The clinically apparent liver injury from nabumetone has not been characterized, but most NSAID related hepatotoxicity is self-limited and mild-to-moderate in severity. The cross reactivity in liver injury between various NSAIDs has not been well defined, but can occur. For these reasons, switching to another NSAID after clinically apparent liver injury from nabumetone should be done with caution.
Drug Class: Nonsteroidal Antiinflammatory Drugs
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Nabumetone – Generic, Relafen®
DRUG CLASS
Nonsteroidal Antiinflammatory Drugs
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Nabumetone | 42924-53-8 | C15-H16-O2 |
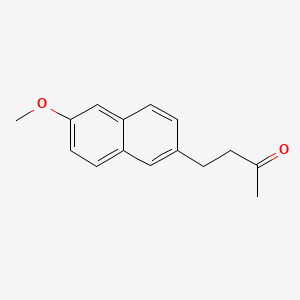
|
ANNOTATED BIBLIOGRAPHY
References updated: 20 March 2020
Abbreviations: NSAIDs, nonsteroidal antiinflammatory drugs.
- Zimmerman HJ. Drugs used to treat rheumatic and musculospastic disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 517-53.(Expert review of hepatotoxicity published in 1999; mentions an unpublished case of cholestatic hepatitis in a patient taking nabumetone).
- Lewis JH, Stine JG. Nonsteroidal anti-inflammatory drugs and leukotriene receptor antagonists: pathology and clinical presentation of hepatotoxicity. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013. pp. 370-402.(Review of hepatotoxicity of NSAIDs mentions that nabumetone is associated with mild elevations in ALT in <1% of patients and that there have been no reports of severe liver injury).
- Grossner T, Smyth EM, Fitzgerald GA. Pharmacotherapy of inflammation, fever, pain and gout. In, Brunton LL, Hilal-Dandan R, Knollman BC. Goodman & Gilman’s The pharmacological basis of therapeutics, 13th ed. New York: McGraw-Hill, 2018. pp. 685-709.(Textbook of pharmacology and therapeutics).
- Jackson RE, Mitchell FN, Brindley DA. Safety evaluation of nabumetone in United States clinical trials. Am J Med. 1987;83:115–20. [PubMed: 3318422](Summary of safety evaluation of nabumetone in 1924 US patients with rheumatoid arthritis or osteoarthritis found similar pattern of adverse events to other NSAIDs; 18 patients [1%] had ALT >2 times ULN, but 6 had some degree of abnormality before therapy; no patient developed hepatitis or jaundice).
- Jenner PN. A 12-month postmarketing surveillance study of nabumetone. A preliminary report. Drugs. 1990;40 Suppl 5:80–6. [PubMed: 2081502](Analysis of postmarketing surveillance of nabumetone using follow up forms sent to physicians; 7 gastrointestinal bleeds, one fatal; but no mention of cases of clinically apparent liver injury).
- Zimmerman HJ. Update of hepatotoxicity due to classes of drugs in common clinical use: non-steroidal drugs, anti-inflammatory drugs, antibiotics, antihypertensives, and cardiac and psychotropic agents. Semin Liver Dis. 1990;10:322–38. [PubMed: 2281340](Extensive review article on liver injury due to NSAIDs; nabumetone is not discussed).
- Bernhard GC. Worldwide safety experience with nabumetone. J Rheumatol Suppl. 1992;36:48–57. [PubMed: 1474535](Summary of adverse effects in premarketing studies of nabumetone; 11 of 1677 patients had ALT elevations >3 times ULN, but all resolved with stopping; in postmarketing surveillance on 44,953 patients treated for 3 weeks to 8 years, no case of hepatitis or liver serious adverse event reported).
- Eversmeyer W, Poland M, DeLapp RE, Jensen CP. Safety experience with nabumetone versus diclofenac, naproxen, ibuprofen, and piroxicam in osteoarthritis and rheumatoid arthritis. Am J Med. 1993;95:10S–18S. [PubMed: 8356997](Major focus of analysis was on gastrointestinal side effects in this study of patients given nabumetone [n=3,315], diclofenac [296], naproxen [279], piroxicam [286] or ibuprofen [235] for 12 weeks; ALT elevations >2 to 3 times ULN occurred in 0.2 to 0.5% of patients on nabumetone, 1.1 to 4% on diclofenac, and 0.4% on naproxen, ibuprofen or piroxicam; no mention of hepatitis or jaundice).
- Friedel HA, Langtry HD, Buckley MM. Nabumetone. A reappraisal of its pharmacology and therapeutic use in rheumatic diseases. Drugs. 1993;45:131–56. [PubMed: 7680981](Thorough review of structure, pharmacology, efficacy and safety of nabumetone; among 1677 patients, 0.4% showed marked elevations in both ALT and AST, but no mention of clinically apparent liver injury or jaundice).
- Bellamy N, Bensen WG, Beaulieu A, Siminovitch KA, Kraag GR, Lussier A, Ahmad S, et al. A multicenter study of nabumetone and diclofenac sustained release in patients with osteoarthritis. J Rheumatol. 1995;22:915–20. [PubMed: 8587082](Among 382 patients enrolled in controlled trials of 6 months of nabumetone vs diclofenac, there was similar efficacy, but better tolerance with nabumetone; ALT >2 times ULN occurred in <1% [n=2] of nabumetone vs 3% of diclofenac recipients; no mention of hepatitis or jaundice).
- Schnitzer TJ, Ballard IM, Constantine G, McDonald P. Double-blind, placebo-controlled comparison of the safety and efficacy of orally administered etodolac and nabumetone in patients with active osteoarthritis of the knee. Clin Ther. 1995;17:602–12. [PubMed: 8565024](Mild ALT elevations occurred in 3 of 89 nabumetone, none of 90 etodolac, and 2 of 90 placebo treated patients; no mention of outcome or symptoms).
- Weaver A, Rubin B, Caldwell J, McMahon FG, Lee D, Makarowski W, Offenberg H, et al. Comparison of the efficacy and safety of oxaprozin and nabumetone in the treatment of patients with osteoarthritis of the knee. Clin Ther. 1995;17:735–45. [PubMed: 8565037](In an analysis of a six week placebo controlled trial, ALT elevations occurred in none of 109 nabumetone, but in 9 of 109 oxaprozin treated patients).
- Morgan GJ Jr, Kaine J, DeLapp R, Palmer R. Treatment of elderly patients with nabumetone or diclofenac: gastrointestinal safety profile. J Clin Gastroenterol. 2001;32:310–4. [PubMed: 11276273](335 elderly patients with osteoarthritis were enrolled in randomized clinical trials of nabumetone [1 or 2 g/day] vs diclofenac for 12 weeks; ALT >2 times ULN occurred in 0% [0/155] of nabumetone vs 4% of diclofenac recipients).
- Rubenstein JH, Laine L. Systematic review: the hepatotoxicity of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2004;20:373–80. [PubMed: 15298630](NSAIDs are the most commonly used drugs in the US and account for a large proportion of cases of hepatic injury, but the frequency is quite rare. Among 7 population based studies, hospitalization occurred in 22.4/100,000 patient-years [rate ratio 1.5] and ~1 death/100,000 patient-years due to liver injury; not increased with age or associated with gender; in case controlled studies, higher odds ratio with sulindac, indomethacin, piroxicam and diclofenac, no mention of nabumetone).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](Survey of all cases of drug induced liver injury with fatal outcome from Swedish Adverse Drug Reporting system from 1966-2002; among 103 cases, 3 attributed to naproxen, but none to nabumetone).
- Rostom A, Goldkind L, Laine L. Nonsteroidal anti-inflammatory drugs and hepatic toxicity: a systematic review of randomized controlled trials in arthritis patients. Clin Gastroenterol Hepatol. 2005;3:489–98. [PubMed: 15880319](Review of randomized clinical trials of NSAIDS for frequency of adverse events; ALT >3 times ULN in 0.43% of ibuprofen, 0.43% naproxen, 0.42% celecoxib, 1.8% rofecoxib, 3.55% diclofenac and 0.29% of placebo recipients, rare liver related serious adverse events or deaths with any; no mention on nabumetone).
- Arellano FM, Yood MU, Wentworth CE, Oliveria SA, Rivero E, Verma A, Rothman KJ. Use of cyclo-oxygenase 2 inhibitors (COX-2) and prescription non-steroidal anti-inflammatory drugs (NSAIDS) in UK and USA populations. Implications for COX-2 cardiovascular profile. Pharmacoepidemiol Drug Saf. 2006;15:861–72. [PubMed: 17086563](Survey of NSAID use in UK and US indicates ibuprofen, naproxen and diclofenac are the most commonly used NSAIDs; nabumetone not in top 10 agents used).
- Lapeyre-Mestre M, de Castro AM, Bareille MP, Garcia del Pozo J, Requejo AA, Arias LM, Montastruc J-L, et al. Non-steroidal anti-inflammatory drug-related hepatic damage in France and Spain: analysis from national spontaneous reporting systems. Fundam Clin Pharmacol. 2006;20:391–5. [PubMed: 16867024](Analysis of reports of liver injury from NSAIDs from France and Spain from 1982-2001; among more than 29,000 liver adverse event reports, only 3 were for nabumetone and no details given).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, NSAIDs were implicated as a sole agent in 8 cases [4 diclofenac, 2 celecoxib, 1 meloxicam, and 1 oxaprozin] and as one of several agents in 3 cases [1 diclofenac, 1 celecoxib, 1 ibuprofen]; nabumetone not listed).
- Bannwarth B. Safety of nonselective NSAID nabumetone: focus on gastrointestinal toxicity. Drug Saf. 2008;31:485–503. [PubMed: 18484783](Review of chemistry, pharmacology and gastrointestinal tolerance of nabumetone; during first 7 years on the market, the frequency of hepatic adverse event reports was 3.4 per million prescriptions and serious hepatic events 1.3 per million, which was markedly lower than for diclofenac [13.6 and 4.3 per million] and similar to naproxen [1.8 and 2.9 per million] and piroxicam [0.2 and 1.2 per million]).
- Bessone F. Non-steroidal anti-inflammatory drugs: What is the actual risk of liver damage? World J Gastroenterol. 2010;16:5651–61. [PMC free article: PMC2997980] [PubMed: 21128314](Review of estimated frequency of drug induced liver injury due to NSAIDs from large published epidemiological studies; nabumetone not discussed).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 7 to NSAIDs with 4 attributed to bromfenac, 2 to diclofenac and 1 to etodolac, but none to nabumetone).
- Gulmez SE, Larrey D, Pageaux GP, Lignot S, Lassalle R, Jové J, Gatta A, et al. Transplantation for acute liver failure in patients exposed to NSAIDs or paracetamol (acetaminophen): the multinational case-population SALT study. Drug Saf. 2013;36:135–44. [PMC free article: PMC3568201] [PubMed: 23325533](Among 600 patients undergoing liver transplantation for acute liver failure at 52 European liver transplant centers between 2005 and 2007, 301 were considered idiopathic and had received a medication within 30 days of onset; including acetaminophen in 192 and NSAIDs in 44, including diclofenac [the most commonly used NSAID] in 7; nabumetone was not specifically mentioned).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 6 attributed to diclofenac [ranking 2nd], but none for nabumetone or other NSAIDs).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, the most common class of implicated agents being NSAIDs [n=62, 32%], and specific agents were nimesulide [n=53], piroxicam [5], diclofenac [2], gold salts [1], and naproxen [1]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 28 were attributed to NSAIDs [Schmeltzer 2016]).
- Schmeltzer PA, Kosinski AS, Kleiner DE, Hoofnagle JH, Stolz A, Fontana RJ, Russo MW., Drug-Induced Liver Injury Network (DILIN). Liver injury from nonsteroidal anti-inflammatory drugs in the United States. Liver Int. 2016;36:603–9. [PMC free article: PMC5035108] [PubMed: 26601797](Among 1221 cases of drug induced liver injury enrolled in a prospective, US database between 2004 and 2014, 30 cases [2.5%] were attributed to NSAIDs, most commonly diclofenac [n=16], but also celecoxib [3], meloxicam [3], etodolac [2], ibuprofen [2], oxaprozin [2], valdecoxib [1] and sulindac [1], but none for nabumetone).
- Donati M, Conforti A, Lenti MC, Capuano A, Bortolami O, Motola D, Moretti U, et al. DILI-IT Study Group. Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case-control study in Italy. Br J Clin Pharmacol. 2016;82:238–48. [PMC free article: PMC4917796] [PubMed: 26991794](Among 179 cases of acute liver injury and 1770 controls admitted to 9 Italian hospitals between 2010 and 2014, NSAIDs used more frequently in cases compared to controls included nimesulide [17% vs 10%: odds ratio 1.88] and ibuprofen [14% vs 10%: odds ratio 1.59] and risk was higher in those taking higher doses).
- Zoubek ME, González-Jimenez A, Medina-Cáliz I, Robles-Díaz M, Hernandez N, Romero-Gómez M, Bessone F, et al. High Prevalence of ibuprofen drug-induced Liver injury in Spanish and Latin-American registries. Clin Gastroenterol Hepatol. 2018;16:292–4. [PubMed: 28782674](Analysis of a Spanish and Latin-American registries identified 73 cases of NSAID induced liver injury, the most common agents being nimesulide [38%], diclofenac [34%] and ibuprofen [17%]; nabumetone and other NSAIDs not mentioned).
- Meunier L, Larrey D. Recent advances in hepatotoxicity of non-steroidal anti-inflammatory drugs. Ann Hepatol. 2018;17:187–91. [PubMed: 29469052](Review of the hepatotoxicity of NSAIDS mentions the most commonly implicated are diclofenac, nimesulide, sulindac, ibuprofen, piroxicam, naproxen and aspirin).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Oxaprozin.[LiverTox: Clinical and Researc...]Review Oxaprozin.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Meloxicam.[LiverTox: Clinical and Researc...]Review Meloxicam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Nabumetone: new preparation. Just another NSAID.[Prescrire Int. 2000]Nabumetone: new preparation. Just another NSAID.. Prescrire Int. 2000 Apr; 9(46):43-6.
- Review Etodolac.[LiverTox: Clinical and Researc...]Review Etodolac.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Nabumetone: a "nonacidic" nonsteroidal antiinflammatory drug.[Ann Pharmacother. 1993]Review Nabumetone: a "nonacidic" nonsteroidal antiinflammatory drug.Dahl SL. Ann Pharmacother. 1993 Apr; 27(4):456-63.
- Nabumetone - LiverToxNabumetone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...