NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
OxyELITE Pro is the commercial name for a variety of multi-ingredient dietary supplements (MIDS) marketed for weight loss and body building. In 2013, one of the OxyELITE Pro products called “Super Thermogenic” was withdrawn from use in the United States after it was implicated in more than 50 cases of acute hepatitis including several instances of fatal, acute liver failure.
Background
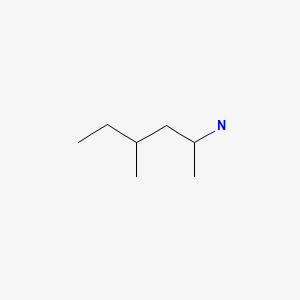
OxyELITE Pro is the proprietary name of a series of multi-ingredient nutritional supplements that are typically marketed as weight loss, body building, “fat burning” and performance enhancement aids. Initial ingredients in the products included caffeine and dimethylamylamine (DMAA) which in animal studies led to weight loss. Small phase 1 studies demonstrated that both blood pressure and pulse were increased with OxyELITE Pro, which was attributed to the effects of DMAA. In 2013, the FDA banned the use of DMAA in nutritional supplements and the composition of OxyELITE Pro was altered, with removal of DMAA and addition of aegeline, an herbal derivative that was believed to cause weight loss. Shortly thereafter reports of severe hepatitis were reported in persons taking “OxyELITE Pro Super Thermogenic”, some of which were severe enough to warrant emergency liver transplantation and some of which were fatal. In October 2013, under pressure from the FDA, the sponsor of OxyELITE Pro withdrew it from the market and requested retrieval of products already in distribution.
Aegeline is a component present in the fruit of the bael tree, Aegle marmelos that is native to India and is found throughout Southeast Asia. The bael tree is considered sacred in much of India and its fruit is used both as food and in Ayurvedic medicine. Extracts of bael leaves and fruit have had many uses in traditional medicine including treatment of digestive complaints and diarrhea. Its active ingredients include tannins, fatty oils, furocoumarins and furoquinolin alkaloids. The aegeline added to OxyELITE Pro was suspected to be a synthetic product, the purity of which was not clear. Other components in the OxyELITE product included caffeine (100 mg/capsule) and a propriety blend of Bauhinia purpurea leaf and pod extract, Bacopa monnieri leaf extract, dimethylamylamine (DMAA), Cirsium oligophyllum extract and Pausinystalia yohimbe bark extract (Yohimbe); none of these, however, have been implicated convincingly in causing liver injury.
Hepatotoxicity
OxyELITE Pro has been associated with at least 50 instances of clinically apparent acute liver injury. The onset of injury was usually within 2 to 20 weeks of starting regular use or of switching to the altered formulation of OxyELITE Pro products. The typical presenting symptoms were fatigue, nausea, and abdominal pain followed by dark urine and jaundice. The pattern of liver injury was hepatocellular and serum aminotransferase levels ranged to as high as several thousand U/L while alkaline phosphatase levels tended to be normal or minimally elevated (less than 3 times ULN). Liver biopsies showed an acute hepatitis-like picture and severe cases were associated with confluent, submassive or massive necrosis. Immunoallergic and features were not common, but autoantibodies were often detected. Indeed, instances of an autoimmune hepatitis-like pattern of injury occurred in a proportion of cases, some of which led to a chronic hepatitis and required long term immune suppressive therapy despite prompt withdrawal of the agent. The mortality rate overall was approximately 10% among cases with jaundice. In most non-fatal cases, symptoms resolved within 1 to 8 weeks and laboratory tests returned to normal by two to three months.
Likelihood score: A (well documented cause of clinically apparent liver injury).
Mechanism of Injury
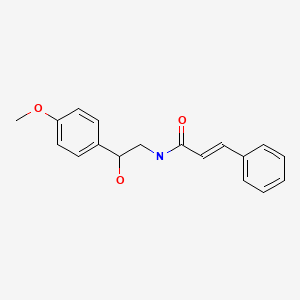
The cause of acute liver injury associated with OxyELITE Pro has been attributed to aegeline, which was added to the commercial product in early 2013. Aegeline is a derivative of the fruit and other components of the bael tree and is a commonly used herbal agent in Southeast Asia, employed in Ayurvedic medicine for digestive complains. Cases of acute liver injury have not been previously reported with aegeline use. The aegeline used in OxyELITE Pro implicated in liver injury, however, was a synthetic product produced in China and may have included contaminants, synthetic precursors, metabolic derivatives, or racemic forms of the chemical that are toxic or immunogenic.
Drug Class: Herbal and Dietary Supplements, ◦Multi-Ingredient Nutritional Supplements
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
OxyELITE Pro®
DRUG CLASS
Herbal and Dietary Supplements
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
ANNOTATED BIBLIOGRAPHY
References updated: 8 September 2021
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 731-4.(Expert review of hepatotoxicity published in 1999; OxyELITE Pro, aegeline and MDAA are not discussed).
- Seeff L, Stickel F, Navarro VJ. Hepatotoxicity of herbals and dietary supplements. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 631-58.(Review of hepatotoxicity of herbal and dietary supplements [HDS]; OxyELITE Pro, aegeline and MDAA are not mentioned).
- Bael. Aegle marmelos. In, PDR for Herbal Medicines. 4th ed. Montvale, New Jersey: Thomson Healthcare Inc. 2007: pp. 61-62.(Compilation of short monographs on herbal medications and dietary supplements).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, et al. Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 9% were attributed to herbal and dietary supplements, but none to OxyELITE Pro or MDAA).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including 12 [9%] attributed to herbal supplements, but none to OxyELITE Pro or MDAA).
- Bloomer RJ, Harvey IC, Farney TM, Bell ZW, Canale RE. Effects of 1,3-dimethylamylamine and caffeine alone or in combination on heart rate and blood pressure in healthy men and women. Phys Sportsmed. 2011;39:111–20. [PubMed: 22030947](10 healthy subjects received a single dose of DMAA with or without caffeine and were monitored for heart rate and blood pressure; blood pressure increased by 17-20% with DMAA, but not caffeine exposure).
- Farney TM, McCarthy CG, Canale RE, Allman RJ Jr, Bloomer RJ. Hemodynamic and hematologic profile of healthy adults ingesting dietary supplements containing 1,3-dimethylamylamine and caffeine. Nutr Metab Insights. 2011;5:1–12. [PMC free article: PMC3698476] [PubMed: 23882143](13 healthy volunteers took Jac3d or OxyELITE Pro [2 commercial products with DMAA and caffeine marked for weight loss] once daily for 2 weeks; heart rate and blood pressure increased with both agents 1-2 hours after administration, but returned to baseline within 24 hours and there were no significant changes in bilirubin, ALT, AST or Alk P).
- Forrester M. Exposures to 1,3-dimethylamylamine-containing products reported to Texas poison centers. Hum Exp Toxicol. 2013;32:18–23. [PubMed: 23060409](Between 2010 and 2011, 56 cases of poisoning due to DMAA were reported to 6 Texas poison centers, 18 of which were unintentional ingestions by toddlers, and 8 were suicidal; the usual adverse events being nausea, vomiting and tachycardia and no reports of associated liver injury).
- Centers for Disease Control and Prevention (CDC). Notes from the field: acute hepatitis and liver failure following the use of a dietary supplement intended for weight loss or muscle building--May-October 2013. MMWR Morb Mortal Wkly Rep. 2013;62(40):817–9. [PMC free article: PMC4585555] [PubMed: 24113901](29 cases of acute hepatitis were identified in Hawaii during a 5 month period in 2013 associated with taking a nutritional supplement, 24 being linked to OxyELITE Pro with onset after 7-130 days [average 60 days] and peak bilirubin 2.8-39.6 mg/dL [median 12.6], ALT 347-3091 U/L [median 1793 U/L], and Alk P 68-251 U/L [median 150 U/L], 1 patient dying and 2 undergoing liver transplantation [some cases also described in Roytman 2014]).
- Roytman MM, Pörzgen P, Lee CL, Huddleston L, Kuo TT, Bryant-Greenwood P, Wong LL, Tsai N. Outbreak of severe hepatitis linked to weight-loss supplement OxyELITE Pro. Am J Gastroenterol. 2014;109:1296–8. [PubMed: 25091255](Report of outbreak of acute liver injury in patients taking OxyELITE Pro at a single referral center in Hawaii, presenting between May and September 2013; 8 patients, ages 22 to 54 years, 1 man and 7 women, all Pacific-Islanders [bilirubin 1.4-41.6 mg/dL, ALT 633-2280 U/L, Alk P 146-190 U/L], 2 undergoing liver transplantation and 1 death without transplant).
- Foley S, Butlin E, Shields W, Lacey B. Experience with OxyELITE Pro and acute liver injury in active duty service members. Dig Dis Sci. 2014;59:3117–21. [PubMed: 24916713](7 cases of severe acute liver injury in US Military personnel taking OxyELITE Pro, ages 23 to 48 years, 3 women and 4 men, on supplement for 1 week to several years [bilirubin 1.2-32 mg/dL, ALT 176-3348 U/L, Alk P 136 U/L], 2 patients requiring liver transplantation).
- Klontz KC, DeBeck HJ, LeBlanc P, Mogen KM, Wolpert BJ, Sabo JL, Salter M, et al. The role of adverse event reporting in the FDA response to a multistate outbreak of liver disease associated with a dietary supplement. Public Health Rep. 2015;130:526–32. [PMC free article: PMC4529836] [PubMed: 26327730](Analysis of 55 spontaneous reports of liver injury with a history of OxyELITE Pro exposure to the FDA between 2012 and 2014; laboratory analysis identified no drugs, poisons, pharmaceuticals, toxic metals or known hepatotoxic substances in retrieved products).
- Rossi S, Navarro VJ. Herbs and liver injury: a clinical perspective. Clin Gastroenterol Hepatol. 2014;12:1069–76. [PubMed: 23924877](Review of HDS induced liver injury including regulatory problems, difficulties in diagnosis and assessing causality; mentions OxyELITE Pro products as being implicated in case series of liver injury from Hawaii).
- Seeff LB, Bonkovsky HL, Navarro VJ, Wang G. Herbal products and the liver: a review of adverse effects and mechanisms. Gastroenterology. 2015;148:517–532. [PubMed: 25500423](Extensive review of possible beneficial as well as harmful effects of herbal products on the liver mentions that multi-ingredient supplements have been implicated in many cases of liver injury including proprietary agents marketed under the names Herbalife, Hydroxycut and OxyELITE Pro).
- Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851–65. [PubMed: 25680499](Extensive review of liver injury due to HDS mentions that OxyELITE Pro products have been implicated in more than 30 cases of liver injury resulting in liver transplantation in 4 and death in one patient).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a prospective database between 2004 and 2012, HDS were implicated in 145 [16%], of which one was attributed to an OxyELITE Pro product [Navarro 2014]).
- Zheng EX, Navarro VJ. Liver injury from herbal, dietary, and weight loss supplements: a review. J Clin Transl Hepatol. 2015;3:93–8. [PMC free article: PMC4548352] [PubMed: 26357638](Review of literature on liver injury due to HDS products used for weight loss, focusing upon liver injury attributed to Herbalife, Hydroxycut and OxyELITE Pro products which was predominantly hepatocellular [acute hepatitis-like] and had a significant mortality rate).
- Johnston DI, Chang A, Viray M, Chatham-Stephens K, He H, Taylor E, Wong LL, et al. Hepatotoxicity associated with the dietary supplement OxyELITE Pro™ -- Hawaii, 2013. Drug Test Anal. 2016;8(3-4):319–27. [PMC free article: PMC4833726] [PubMed: 26538199](Clinical and epidemiologic analysis of an outbreak of acute liver injury in Hawaii attributed to use of OxyELITE Pro identified 36 cases, ages 16 to 66 years; 57% women; 23% Asian and 57% reporting 2 or more races; latency 7 days to 2 years, peak median ALT 1740 U/L, Alk P 141 U/L, bilirubin 9.4 mg/dL; 33% with autoimmune markers; 39% hospitalized; 2 patients required liver transplant and one died).
- Teschke R, Schwarzenboeck A, Frenzel C, Schulze J, Eickhoff A, Wolff A. The mystery of the Hawaii liver disease cluster in summer 2013: A pragmatic and clinical approach to solve the problem. Ann Hepatol. 2015;15:91–109. [PubMed: 26626645](Analysis of cases of liver injury attributed to OxyELITE Pro reported by the CDC, found “pervasive bias” and “poor quality of case management” and suggested that the liver injury was largely due to other causes).
- Teschke R, Eickhoff A. The Honolulu liver disease cluster at the Medical Center: its mysteries and challenges. Int J Mol Sci. 2016;17:E476. pii. [PMC free article: PMC4848932] [PubMed: 27043544](Review of the outbreak of acute liver injury in Honolulu [Roytman 2014] that was attributed to OxyELITE Pro arguing that other causes were not adequately excluded).
- Heidemann LA, Navarro VJ, Ahmad J, Hayashi PH, Stolz A, Kleiner DE, Fontana RJ. Severe acute hepatocellular injury attributed to OxyELITE Pro: a case series. Dig Dis Sci. 2016;61(9):2741–8. [PMC free article: PMC4982804] [PubMed: 27142670](Among 1596 patients enrolled in a prospective study of drug induced liver injury in the US between 2004 and 2015, 7 were attributed to exposure to OxyELITE Pro, 6 of which presented in 2013 with an acute hepatitis-like syndrome [mean bilirubin 10.9 mg/dL, ALT 2950 U/L, Alk P all less than 3 times elevated], 3 with acute liver failure and 2 undergoing emergency liver transplantation).
- García-Cortés M, Robles-Díaz M, Ortega-Alonso A, Medina-Caliz I, Andrade RJ. Hepatotoxicity by dietary supplements: a tabular listing and clinical characteristics. Int J Mol Sci. 2016;17(4):E537. pii. [PMC free article: PMC4848993] [PubMed: 27070596](Listing of published cases of liver injury from HDS products including more than 50 attributed to OxyELITE Pro, all since 2014).
- Chatham-Stephens K, Taylor E, Chang A, Peterson A, Daniel J, Martin C, Deuster P, et al. Hepatotoxicity associated with weight loss or sports dietary supplements, including OxyELITE Pro™ -- United States, 2013. Drug Test Anal. 2017;9:68–74. [PMC free article: PMC5579712] [PubMed: 27367536](Search from several databases for instances of acute liver injury following use of muscle building or weight reducing dietary supplements occurring between April and December 2013, identified 40 cases, 27 of which were linked to use of OxyElite Pro).
- Teschke R, Eickhoff A. Suspected liver injury and the dilemma of causality. Dig Dis Sci. 2017;62:1095–1098. [PubMed: 28210906](Letter to the editor in response to Heidemann [2016] questioning the causality assessment as being “vague, unscored, not quantitative and not transparent” invented by a “close-knit US based group”).
- Teschke R, Wolff A, Eickhoff A, Danan G. Is obesity rather than the dietary supplement used for weight reduction the cause of liver injury? JGH Open. 2018;2:152–157. [PMC free article: PMC6152465] [PubMed: 30483581](Review of reports of liver injury attributed to dietary supplements used for weight loss [such as green tea extract, Hydroxycut, Herbalife products and OxyELITE Pro] found that nonalcoholic liver disease was rarely considered responsible for the liver injury rather than the dietary supplement).
- Roytman MM, Poerzgen P, Navarro V. Botanicals and hepatotoxicity. Clin Pharmacol Ther. 2018;104:458–469. [PubMed: 29920648](Review of the hepatotoxicity of botanical products and the difficulty in their regulation, uses OxyELITE Pro as an example of a weight loss product that was eventually linked to at least 97 cases of acute liver injury including several fatalities and emergency liver transplants).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Hepatotoxicity associated with weight loss or sports dietary supplements, including OxyELITE Pro™ - United States, 2013.[Drug Test Anal. 2017]Hepatotoxicity associated with weight loss or sports dietary supplements, including OxyELITE Pro™ - United States, 2013.Chatham-Stephens K, Taylor E, Chang A, Peterson A, Daniel J, Martin C, Deuster P, Noe R, Kieszak S, Schier J, et al. Drug Test Anal. 2017 Jan; 9(1):68-74. Epub 2016 Aug 4.
- The Role of Adverse Event Reporting in the FDA Response to a Multistate Outbreak of Liver Disease Associated with a Dietary Supplement.[Public Health Rep. 2015]The Role of Adverse Event Reporting in the FDA Response to a Multistate Outbreak of Liver Disease Associated with a Dietary Supplement.Klontz KC, DeBeck HJ, LeBlanc P, Mogen KM, Wolpert BJ, Sabo JL, Salter M, Seelman SL, Lance SE, Monahan C, et al. Public Health Rep. 2015 Sep-Oct; 130(5):526-32.
- Severe Acute Hepatocellular Injury Attributed to OxyELITE Pro: A Case Series.[Dig Dis Sci. 2016]Severe Acute Hepatocellular Injury Attributed to OxyELITE Pro: A Case Series.Heidemann LA, Navarro VJ, Ahmad J, Hayashi PH, Stolz A, Kleiner DE, Fontana RJ. Dig Dis Sci. 2016 Sep; 61(9):2741-8. Epub 2016 May 3.
- Review Hydroxycut.[LiverTox: Clinical and Researc...]Review Hydroxycut.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Liver Injury from Herbal, Dietary, and Weight Loss Supplements: a Review.[J Clin Transl Hepatol. 2015]Review Liver Injury from Herbal, Dietary, and Weight Loss Supplements: a Review.Zheng EX, Navarro VJ. J Clin Transl Hepatol. 2015 Jun 28; 3(2):93-8. Epub 2015 Jun 15.
- OxyELITE Pro - LiverToxOxyELITE Pro - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...