NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Oxybate is a small, neuroactive molecule (gamma-hydroxybutyrate) that is used to treat catalepsy and daytime sleepiness in patients with narcolepsy. Oxybate has been reported to cause serum enzyme elevations during therapy, but has not been implicated in instances of clinically apparent acute liver injury.
Background
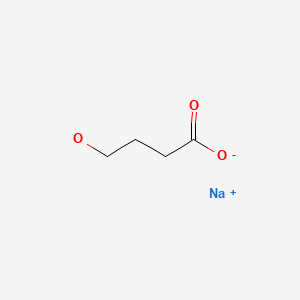
Oxybate (ox' i bate) is a simple amino acid-like molecule (sodium 4-hydroxybutyrate) that has mild neuroactivity which acts to induce normal sleep patterns. Its mechanism of action is unclear, but it is a derivative of gamma-aminobutyric acid (GABA) and appears to be an agonist at the GABA-B receptor. In prospective, randomized controlled trials, oxybate was effective in alleviating symptoms of daytime sleepiness and decreasing episodes of cataplexy in patients with narcolepsy. Oxybate was approved for use in the United States in 2002 as therapy for cataplexy in patients with narcolepsy. These indications were broadened in 2005 to include improvement in the quality of nighttime sleep and decrease in daytime sleepiness in patients with narcolepsy. Although evaluated and reported to be partially beneficial in other conditions (fibromyalgia, chronic fatigue, alcohol abstinence), oxybate is not approved for these indications. In addition, oxybate is a Schedule III agent, indicating that it has a mild-to-moderate potential for abuse and dependence. For these reasons, the availability of oxybate is restricted, and it can only be prescribed as a part of a risk evaluation and mitigation strategy (REMS) program. Oxybate sodium is available as an oral solution in 180 mL bottles of 500 mg/mL under the brand name Xyrem. In addition, a low-sodium formulation has been developed consisting of calcium, magnesium, potassium and sodium salts of oxybate and is available in a similar oral solution of 500 mg/mL under the brand name Xywav. The recommended starting dose of both formulations of oxybate is 4.5 g at nighttime, which can be increased or decreased at two week intervals in increments of 1.5 g, not to exceed 9 g daily. Common side effects include nausea, dizziness, headaches, mental confusion, paresthesia and enuresis (bed wetting). Uncommon, but potentially severe adverse reactions (usually associated with excessive doses) include hallucinations, mental confusion, abnormal thinking, disturbed sleep and depression. Instances of abuse and dependence as well as withdrawal symptoms have been described, but are rare. Hydroxybutyrate has been used as a “date rape” drug and it has been implicated in rare instances of acute psychosis, traffic accidents and suicidal overdose.
Hepatotoxicity
In preregistration clinical trials, serum enzyme elevations were reported in small numbers of treated patients, but no instance of clinically apparent liver injury was reported. Since the approval and more widespread use of oxybate, there have been no published cases of liver injury due to oxybate, and in postmarketing overviews of adverse events hepatotoxicity was not listed. Thus, despite use in high doses (3 to 9 g daily), acute liver injury from oxybate must be very rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which oxybate might cause liver injury is not known. Hydroxybutyrate is an endogenous derivative of GABA and thus is unlikely to be inherently hepatotoxic or immunogenic.
Drug Class: CNS Stimulants, Narcolepsy Agents
Other Drugs in the Subclass, Narcolepsy Agents: Amphetamines, Armodafinil, Dextroamphetamine, Modafinil, Methylphenidate, Pitolisant, Solriamfetol
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Sodium Oxybate – Xyrem®
DRUG CLASS
Narcolepsy Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
ANNOTATED BIBLIOGRAPHY
References updated: 18 August 2021
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Textbook of hepatotoxicity published in 1999, before the availability of oxybate).
- Westfall TC, Macarthur H, Westfall DP. Narcolepsy and sleep/wake imbalance. Adrenergic agonists and antagonists. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 207.(Textbook of pharmacology and therapeutics).
- Gamma hydroxy butyrate poisoning. Med Lett Drugs Ther. 1991;33(836):8. [PubMed: 1986210](Hydroxybutyrate is promoted and sold in health food stores illegally for sleep and weight control and for its euphoric effects and has been linked to cases of abrupt unconsciousness, coma, respiratory depression, seizures, and abnormal behavior).
- A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep. 2002;25:42–9. [PubMed: 11833860](Among 136 patients with narcolepsy and episodes of cataplexy who were treated with oxybate [3, 6 or 9 g daily] or placebo, the highest dose of oxybate was associated with decrease in episodes of cataplexy and decrease in daytime sleepiness, and adverse events included nausea, diarrhea, dizziness and enuresis but there was “no measurable effect on…hepatic or renal function”).
- Gamma hydroxybutyrate (Xyrem) for narcolepsy. Med Lett Drugs Ther. 2002;44(1145):103–5. [PubMed: 12473959](Concise review of the mechanism of action, pharmacology, efficacy, adverse effects, and costs of gamma hydroxybutyrate [oxybate] shortly after its approval as therapy for narcolepsy in the US; mentions its neuropsychiatric side effects, but not ALT elevations or hepatotoxicity).
- Scharf MB, Baumann M, Berkowitz DV. The effects of sodium oxybate on clinical symptoms and sleep patterns in patients with fibromyalgia. J Rheumatol. 2003;30:1070–4. [PubMed: 12734908](Among 24 women with fibromyalgia treated with oxybate or placebo for 1 month in a crossover trial, pain and fatigue were improved in 29-33% of oxybate- vs only 6-10% of placebo-recipients and side effects included gastrointestinal upset, anxiety, headache and paresthesias; there were no serious adverse events and no mention of ALT elevations or hepatotoxicity).
- U.S. Xyrem Multicenter Study Group. A 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsy. Sleep. 2003;26:31–5. [PubMed: 12627729](Among 118 patients with narcolepsy who had participated in a placebo controlled trial [U.S. Xyrem, 2002] who were treated with open label oxybate [3-9 g nightly] for 12 months, episodes of cataplexy decreased and daytime sleepiness decreased and “adverse events were generally mild” while “only a mild increase in SGPT was determined to be possibly related to the study medication” and no patient discontinued therapy for this reason).
- U.S. Xyrem Multicenter Study Group. Sodium oxybate demonstrates long-term efficacy for the treatment of cataplexy in patients with narcolepsy. Sleep Med. 2004;5:119–23. [PubMed: 15033130](Among 55 patients with narcolepsy treated with oxybate for 7-44 months who were then randomized to continue or stop oxybate for 2 weeks, the frequency of cataleptic episodes increased with stopping [median increase 21 vs 0]).
- Xyrem International Study Group. A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J Clin Sleep Med. 2005;1:391–7. [PubMed: 17564408](Among 228 patients with narcolepsy and cataplexy treated with oxybate [4.5, 6 or 9 g nightly] or placebo for 8 weeks, those on the two highest doses had improvement in wakefulness; side effects included nausea, dizziness, enuresis, disorientation, dyspnea, snoring and muscle pains; 1 patient developed elevated levels of ALT and AST that gradually resolved after stopping).
- A new indication for gamma hydroxybutyrate (Xyrem) in narcolepsy. Med Lett Drugs Ther. 2006;48(1227):11–2. [PubMed: 16444137](Concise review of narcolepsy and the mechanism of action, clinical efficacy, and adverse effects of oxybate, shortly after the broadening of its indications to include excessive daytime sleepiness in patients with narcolepsy; no mention of serum enzyme elevations or hepatotoxicity).
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med. 2009;5:365–71. [PMC free article: PMC2725257] [PubMed: 19968016](Analysis of the postmarketing adverse event surveillance, including 928 reports from an estimated 26,000 patients taking oxybate over a 6 year period mentions rare instances of abuse [n=10], dependence [4], withdrawal symptoms [8], overdose [8], sexual assault [2], traffic accidents [3] and one death thought to be related; no mention of liver related effects among the 20 most common adverse events).
- Russell IJ, Perkins AT, Michalek JE., Oxybate SXB-26 Fibromyalgia Syndrome Study Group. Sodium oxybate relieves pain and improves function in fibromyalgia syndrome: a randomized, double-blind, placebo-controlled, multicenter clinical trial. Arthritis Rheum. 2009;60:299–309. [PubMed: 19116896](Among 188 patients with fibromyalgia treated with oxybate [4.5 or 6 g nightly] or placebo for 8 weeks, oxybate was associated with improvements in pain scores and side effects included nausea, dizziness, paresthesia, headache and enuresis, but “there were no clinically important changes in…laboratory measures”).
- Spaeth M, Alegre C, Perrot S, Wang Y, Guinta DR, Alvarez-Horine S, Russell I., Sodium Oxybate Fibromyalgia Study Group. Long-term tolerability and maintenance of therapeutic response to sodium oxybate in an open-label extension study in patients with fibromyalgia. Arthritis Res Ther. 2013;15:R185. [PMC free article: PMC3978755] [PubMed: 24286114](Among 560 women with fibromyalgia who had participated in short controlled trials of oxybate and were then offered open label extended therapy [4.5-9 g nightly], symptom improvement was maintained and side effects were similar to those in short term studies; there were no liver related serious adverse events and no mention of ALT elevations).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to oxybate or other therapies for narcolepsy or excessive sleepiness).
- Mamelak M, Swick T, Emsellem H, Montplaisir J, Lai C, Black J. A 12-week open-label, multicenter study evaluating the safety and patient-reported efficacy of sodium oxybate in patients with narcolepsy and cataplexy. Sleep Med. 2015;16:52–8. [PubMed: 25533539](Among 202 patients with narcolepsy with cataplexy treated with open label, titrated doses of oxybate [3-9 g nightly] for 12 weeks, 90% were considered to have had a clinical response and side effects included nausea [10%], headache [7%], dizziness [5%] and enuresis [2%], while “overall changes in laboratory tests were minimal”).
- Drakatos P, Lykouras D, D'Ancona G, Higgins S, Gildeh N, Macavei R, Rosenzweig I, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med. 2017;35:80–4. [PMC free article: PMC5727622] [PubMed: 28619187](Among 90 patients with narcolepsy started on oxybate at a single medical center between 2009 and 2015 [3116 patient-months of exposure], sleepiness scores and cataplexy events improved in doses of 4.5-9 g nightly, but 27% of patients stopped therapy because of side effects [mostly nausea, mood swings and enuresis]; in long list of side effects there was no mention of ALT elevations or hepatotoxicity).
- Plazzi G, Ruoff C, Lecendreux M, Dauvilliers Y, Rosen CL, Black J, Parvataneni R, et al. Treatment of paediatric narcolepsy with sodium oxybate: a double-blind, placebo-controlled, randomised-withdrawal multicentre study and open-label investigation. Lancet Child Adolesc Health. 2018;2:483–94. [PubMed: 30169321](Among 63 children [ages 7 to 16 years] with narcolepsy treated with oxybate or placebo, daytime sleepiness scores and cataplexy events decreased with oxybate therapy, while adverse events included enuresis [21%], nausea [22%], vomiting [21%], headache [18%], weight loss [15%], and dizziness [5%] while “no notable findings of clinical relevance were observed in clinical laboratory values”).
- Mayer G, Plazzi G, Iranzo Á, Ortega-Albás J, Quinnell T, Pesch H, Serralheiro P, et al. Long-term compliance, safety, and tolerability of sodium oxybate treatment in patients with narcolepsy type 1: a postauthorization, noninterventional surveillance study. Sleep. 2018;41(9):zsy128. [PubMed: 29986085](Among 640 adults with narcolepsy started on oxybate in 41 medical centers in Europe between 2006 and 2016, side effects were reported in 67% and led to discontinuation in 9%, usually for nausea, depression or dizziness; no liver related serious adverse events or discontinuations and no mention of ALT elevations or hepatotoxicity).
- Addolorato G, Lesch OM, Maremmani I, Walter H, Nava F, Raffaillac Q, Caputo F. Post-marketing and clinical safety experience with sodium oxybate for the treatment of alcohol withdrawal syndrome and maintenance of abstinence in alcohol-dependent subjects. Expert Opin Drug Saf. 2020;19:159–66. [PubMed: 31876433](Systematic review of safety of oxybate therapy for alcohol withdrawal syndrome identified 3 controlled trials [520 patients], 43 pilot studies [2547 patients] and a pharmacovigilance safety information database [299,013 patients], reported most common adverse events to be dizziness [11%], headache [4%], fatigue 3.5%], and nausea [8%] with serious adverse events of depression, suicidal ideation, overdose, and epilepsy; no mention of ALT elevations or hepatotoxicity).
- Husain AM, Bujanover S, Ryan R, Scheckner B, Black J, Profant J. Incidence and duration of common, early-onset adverse events occurring during 2 randomized, placebo-controlled, phase 3 studies of sodium oxybate in participants with narcolepsy. J Clin Sleep Med. 2020;16:1469–74. [PMC free article: PMC7970612] [PubMed: 32356515](Post-hoc analysis of two placebo controlled trials of oxybate in adults with narcolepsy found that side effects were greatest at week one [dizziness, headache and nausea] and decreased thereafter; no mention of ALT elevations or hepatotoxicity).
- Thorpy MJ. Recently approved and upcoming treatments for narcolepsy. CNS Drugs. 2020;34:9–27. [PMC free article: PMC6982634] [PubMed: 31953791](Review of the mechanism of action, pharmacology, drug-drug interactions, clinical efficacy and safety of newly approved medications for narcolepsy including pitolisant and solriamfetol: no mention of ALT elevations or hepatotoxicity).
- Bogan RK, Thorpy MJ, Dauvilliers Y, Partinen M, Del Rio Villegas R, Foldvary-Schaefer N, et al. Efficacy and safety of calcium, magnesium, potassium, and sodium oxybates (lower-sodium oxybate [LXB]; JZP-258) in a placebo-controlled, double-blind, randomized withdrawal study in adults with narcolepsy with cataplexy. Sleep. 2021;44(3):zsaa206. [PMC free article: PMC7953213] [PubMed: 33184650](Among 201 adults with narcolepsy and cataplexy switched to are treated with lower-sodium oxybate or placebo, those switched to placebo had worsening of numbers of cataplexy events and sleepiness scores while those switched from high to low sodium oxybate were stable and adverse events were typical of oxybate; no mention of ALT elevations or hepatotoxicity).
- Guiraud J, Addolorato G, Aubin HJ, Batel P, de Bejczy A, Caputo F, Goudriaan AE, et al. SMO032 study group. Treating alcohol dependence with an abuse and misuse deterrent formulation of sodium oxybate: Results of a randomised, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2021;52:18–30. [PubMed: 34237655](Among 509 adults with alcohol dependence undergoing withdrawal and treated with oxybate [0.75, 1.25, 1.75 or 2.25 g] or placebo three times daily for 12 weeks, there were no differences in rates of abstinence between the 5 groups [~71% to 73%] and side effects more frequent with oxybate include dizziness, vertigo, nausea, fatigue and somnolence; no mention of ALT levels or hepatotoxicity).
- Kushida CA, Shapiro CM, Roth T, Thorpy MJ, Corser BC, Ajayi AO, Rosenberg R, et al. Once-nightly sodium oxybate (FT218) demonstrated improvement of symptoms in a phase 3 randomized clinical trial in patients with narcolepsy. Sleep 2021 Aug 6: ePub ahead of print. [PMC free article: PMC9189976] [PubMed: 34358324](Among 212 patients with narcolepsy treated with once nightly oxybate [4.5, 6, 7.5 and 9 g] or placebo, maintenance of wakefulness, excessive sleepiness and cataplexy were improved by oxybate and common adverse events were nausea, vomiting, headache, dizziness, and enuresis, while there were “no clinically meaningful changes from baseline in clinical laboratory values”).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- EFNS guidelines on management of narcolepsy.[Eur J Neurol. 2006]EFNS guidelines on management of narcolepsy.Billiard M, Bassetti C, Dauvilliers Y, Dolenc-Groselj L, Lammers GJ, Mayer G, Pollmächer T, Reading P, Sonka K, EFNS Task Force. Eur J Neurol. 2006 Oct; 13(10):1035-48.
- Sodium oxybate improves excessive daytime sleepiness in narcolepsy.[Sleep. 2006]Sodium oxybate improves excessive daytime sleepiness in narcolepsy.Black J, Houghton WC. Sleep. 2006 Jul; 29(7):939-46.
- Review An evaluation of sodium oxybate as a treatment option for narcolepsy.[Expert Opin Pharmacother. 2019]Review An evaluation of sodium oxybate as a treatment option for narcolepsy.Abad VC. Expert Opin Pharmacother. 2019 Jul; 20(10):1189-1199. Epub 2019 May 28.
- A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy.[J Clin Sleep Med. 2005]A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy.Xyrem International Study Group. J Clin Sleep Med. 2005 Oct 15; 1(4):391-7.
- Review Sodium oxybate: efficacy, safety and tolerability in the treatment of narcolepsy with or without cataplexy.[Drugs Today (Barc). 2008]Review Sodium oxybate: efficacy, safety and tolerability in the treatment of narcolepsy with or without cataplexy.Owen RT. Drugs Today (Barc). 2008 Mar; 44(3):197-204.
- Oxybate - LiverToxOxybate - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...