NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Paclitaxel is an antineoplastic agent which acts by inhibitor of cellular mitosis and which currently plays a central role in the therapy of ovarian, breast, and lung cancer. Therapy with paclitaxel has been associated with a low rate of serum enzyme elevations, but has not been clearly linked to cases of clinically apparent acute liver injury.
Background
Paclitaxel (pak" li tax' el) is a complex diterpenoid molecule that contains a central 8-member taxane ring. Paclitaxel was initially isolated from the bark of the Western Yew tree (Taxus breviflora) and found to have antitumor activity in high throughput assays. It is a potent antineoplastic agent and its mechanism of action appears to be mediated by its binding to microtubulin, which is important in the mitotic phase of cell division. The binding of paclitaxel prevents the disassembly of the cytoskeletal microtubules, preventing cell division and leading to cell death. Paclitaxel was approved for use in the United States in 1992 and it remains an important agent in the therapy of several cancers. Paclitaxel is considered a first line treatment for advanced ovarian carcinoma and is also used in breast cancer and advanced non-small cell lung cancer and AIDS-related Kaposi's sarcoma. Paclitaxel is available in solution for injection (6 mg/mL) generically and under the brand names Taxol and Onxol. Paclitaxel is also available as protein bound particles in a lyophilized powder for injection under the brand name Abraxane. Paclitaxel is administered intravenously, typically as 3 to 24 hour infusions every three weeks in cycles in combination with other antineoplastic agents. The dose varies by indication and body weight and is reduced in persons with preexisting liver disease. Side effects are common and include diarrhea, nausea, vomiting, mucositis, fatigue, myalgias, skin rash, alopecia, phlebitis, bone marrow suppression, fluid retention, cardiomyopathy, peripheral neuropathy and hypersensitivity reactions. Severe adverse reactions include acute hypersensitivity reactions which occur in up to 2% of patients, typically with the first few infusions, and which are marked by urticaria, rash, fever, facial edema, hypotension, dyspnea and shock which can lead to multiorgan failure and death.
Hepatotoxicity
Paclitaxel has been associated with serum aminotransferase elevations in 7% to 26% of patients, but values greater than 5 times the upper limit of normal (ULN) in only 2% of those receiving the highest doses. Similar rates of alkaline phosphatase elevations and occasional mild bilirubin elevations also occur. The abnormalities are usually asymptomatic, mild and self-limited, rarely requiring dose modification or discontinuation. Paclitaxel has not been linked convincingly to instances of delayed, idiosyncratic clinically apparent liver injury with jaundice. However, the hypersensitivity reactions that occur with infusions of paclitaxel can be severe and accompanied by acute hepatic necrosis. The liver injury may be relatively mild and anicteric (Case 1), but can also be severe with rapid onset of multiorgan failure and death. At least one instance of acute liver failure following a hypersensitivity reaction to paclitaxel has been published in the literature and recent modifications of the product labels for paclitaxel and docetaxel mention the occurrence of toxic deaths following severe infusion reactions. Because paclitaxel is often given with other antineoplastic agents, liver injury arising during therapy cannot always be reliably attributed to paclitaxel rather than to other specific agents. Furthermore, paclitaxel in combination with other anticancer agents may be associated with reactivation of hepatitis B, increased risk of opportunistic viral infections, sinusoidal obstruction syndrome or sepsis, any of which can cause liver test abnormalities or clinically apparent liver injury.
Likelihood score: D (possible cause of acute hepatic necrosis associated with a hypersensitivity reaction to the initial infusions).
Mechanism of Injury
The mild liver injury that arises during therapy is probably due to a direct effect of paclitaxel in inhibiting microtubular function. Paclitaxel is metabolized by the cytochrome P450 system, largely CYP 2C8 and to a lesser extent 3A4. The severe liver injury that accompanies infusion reactions to paclitaxel is likely due to hypersensitivity and acute direct hepatic injury, perhaps due to hypotension and ischemia.
Outcome and Management
The serum aminotransferase elevations that occur on paclitaxel therapy are usually self-limited and do not require dose modification or discontinuation of therapy. Patients with an acute hypersensitivity reaction to paclitaxel should have the infusion stopped and not be reexposed to paclitaxel or to docetaxel, a closely related taxane (Case 1). The acute hepatic necrosis that accompanies rare instances of hypersensitivity reactions to paclitaxel is usually treated with corticosteroids which may have an effect on the signs and symptoms of hypersensitivity, but may not affect the course of the acute liver injury.
Drug Class: Antineoplastic Agents, Taxanes
Other Drugs in the Subclass, Taxanes: Cabazitaxel, Docetaxel
CASE REPORT
Case 1. Acute hepatocellular injury after an infusion of docetaxel.(1)
A 31 year old woman with endometrial cancer developed abdominal pain and serum aminotransferase elevations the week following an initial infusion of docetaxel and carboplatin. She had no history of liver disease, drug allergies, alcohol abuse or risk factors for viral hepatitis. Her other medical conditions included seasonal allergies, reactive airway disease and dyspepsia. Her endometrial carcinoma was initially treated surgically with hysterectomy and bilateral oophorectomy followed by pelvic irradiation and intravenous cisplatin which was poorly tolerated. She then started paclitaxel and carboplatin but had an immediate hypersensitivity reaction during the infusion manifested by chest tightness with arm and facial flushing despite premedication with antihistamines and dexamethasone. The following week her chemotherapy regimen was switched to docetaxel and carboplatin with intravenous dexamethasone followed by oral dexamethasone. She had mild symptoms of hypersensitivity during the infusions, but during the ensuing week she developed fatigue and abdominal pain. At the time of the next planned infusion, serum ALT was found to be 649 U/L, AST 211 U/L and alkaline phosphatase 161 U/L (R=11.7: hepatocellular). Serum bilirubin and albumin were normal and INR 0.9. (Table). Therapy was held. The following day, serum ALT levels had decreased. Serologic markers for acute hepatitis A, B and C were negative. Serum ANA was weakly positive (1:80) but SMA and AMA were negative. Imaging of the liver was normal without evidence of biliary or portal venous obstruction. Her symptoms resolved and over the next few weeks her serum aminotransferase levels fell to near normal levels. She was restarted on carboplatin without docetaxel and serum enzymes remained normal or minimally elevated
Key Points
| Medication: | Docetaxel (124 mg iv once) |
|---|---|
| Pattern: | Hepatocellular (R=11.7) |
| Severity: | 1+ symptomatic (no jaundice) |
| Latency: | 1 week |
| Recovery: | 5 weeks |
| Other medications: | Carboplatin, dexamethasone |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre (-2 mo) | Pre | 25 | 73 | 0.2 | |
| Pre (-1 wk) | Pre | 35 | 66 | 0.3 | Docetaxel and carboplatin |
| 7 days | 0 | 649 | 161 | 0.2 | Therapy held |
| 8 days | 1 day | 545 | 148 | 0.3 | INR 0.9 |
| 14 days | 7 days | 295 | 92 | 0.3 | |
| 19 days | 12 days | 126 | 78 | 0.2 | Carboplatin restarted |
| 26 days | 19 days | 64 | 87 | 0.3 | |
| 35 days | 28 days | 94 | 95 | 0.3 | |
| 44 days | 37 days | 30 | 84 | 0.2 | |
| Normal Values | <40 | <125 | <1.2 | ||
Comment
This young woman with endometrial cancer and history of season allergies, had a mild hypersensitivity reaction during an infusion of paclitaxel and significant hepatic injury with a subsequent single infusion of docetaxel. The injury was symptomatic but without jaundice or evidence of hepatic synthetic dysfunction. The rapid rise and equally rapid fall in serum aminotransferase levels suggests direct hepatic injury and acute hepatic necrosis. In this case the injury was mild and self-limited injury, but paclitaxel hypersensitivity reactions can be severe and associated with jaundice and hepatic failure. This patient appeared to have cross sensitivity between paclitaxel and docetaxel.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Paclitaxel – Generic, Onxol®, Taxol®
Paclitaxel (Powder for Inj.) – Generic, Abraxane®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Paclitaxel | 33069-62-4 | C47-H51-N-O14 |
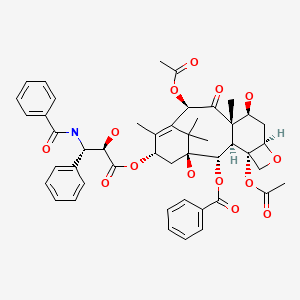
|
CITED REFERENCE
- 1.
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159]
ANNOTATED BIBLIOGRAPHY
References updated: 07 September 2020
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp.673-708.(Textbook of hepatotoxicity published in 1999; paclitaxel is not mentioned).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 549-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; the taxanes are not specifically discussed).
- Wellstein A, Giaccone G, Atkins MB, Sausille EA. Taxanes. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1187-9.(Textbook of pharmacology and therapeutics).
- Onetto N, Canetta R, Winograd B, Catane R, Dougan M, Grechko J, Burroughs J, Rozencweig M. Overview of Taxol safety. J Natl Cancer Inst Monogr. 1993;(15):131–9. [PubMed: 7912519](Among 655 patients treated with paclitaxel, dose limiting toxicities included bone marrow suppression [especially neutropenia], mucositis, neuropathy and rarely cardiomyopathy; hypersensitivity reactions can be controlled with premedication; liver test abnormalities were dose dependent with any AST elevations in 7-26% of patients and values >5 times ULN in only 2% of those receiving the highest dose).
- Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol). Semin Oncol. 1993;20(4) Suppl 3:1–15. [PubMed: 8102012](Extensive review of toxicities of paclitaxel; according to data on file with the sponsor, elevations of AST, Alk P and bilirubin >5 times the ULN occurred in <1% of 402 patients in phase II and III trials).
- Horikoshi N, Inoue K, Aiba K, Mukaiyama T, Ogihara A, Sumida T, Akatsuka Y, et al. Gan To Kagaku Ryoho. 1994;21:2407–14. [Phase I study of paclitaxel] Japanese. [PubMed: 7944484]
- Von Hoff DD. The taxoids: same roots, different drugs. Semin Oncol. 1997;24(4) Suppl 13:S13–3. [PubMed: 9335511](Review of the development of paclitaxel and docetaxel, clinical use, efficacy and toxicity stressing the differences between the two taxoids, which are due largely to differences in pharmacokinetics).
- Fumoleau P. Efficacy and safety of docetaxel in clinical trials. Am J Health Syst Pharm. 1997;54(24) Suppl 2:S19–24. [PubMed: 9435929](Overall response rates to docetaxel and paclitaxel in advanced breast cancer ranged from 29-68% of patients; major dose related toxicities included neutropenia, mucositis, cardiomyopathy and fluid retention; hepatotoxicity was not mentioned).
- Colomer R, Llombart A, Lluch A, Ojeda B, Barnadas A, Carana V, Fernandez Y, et al. Paclitaxel/gemcitabine administered every two weeks in advanced breast cancer: preliminary results of a phase II trial. Semin Oncol. 2000;27(1) Suppl 2:20–4. [PubMed: 10697032](Among 43 women with metastatic breast cancer treated with paclitaxel and gemcitabine for up to 8 cycles, 41% had serum aminotransferase elevations which were above 5 times ULN in 5%).
- De Pas T, de Braud F, Danesi R, Sessa C, Catania C, Curigliano G, Fogli S, et al. Phase I and pharmacologic study of weekly gemcitabine and paclitaxel in chemo-naive patients with advanced non-small-cell lung cancer. Ann Oncol. 2000;11:821–7. [PubMed: 10997809](Among 35 patients with lung cancer given escalating doses of gemcitabine and paclitaxel, 33% of first and 20% of all cycles were associated with aminotransferase elevations >5 times ULN, but all were asymptomatic and reversible, although some led to dose reduction).
- Patnaik A, Warner E, Michael M, Egorin MJ, Moore MJ, Siu LL, Fracasso PM, et al. Phase I dose-finding and pharmacokinetic study of paclitaxel and carboplatin with oral valspodar in patients with advanced solid tumors. J Clin Oncol. 2000;18:3677–89. [PubMed: 11054441](Among 58 patients with advanced cancers treated with cycles of paclitaxel, carboplatin and valspodar, 3 had aminotransferase elevations >5 times ULN, two of which were dose limiting).
- Douillard JY, Lerouge D, Monnier A, Bennouna J, Haller AM, Sun XS, Assouline D, et al. Combined paclitaxel and gemcitabine as first-line treatment in metastatic non-small cell lung cancer: a multicentre phase II study. Br J Cancer. 2001;84:1179–84. [PMC free article: PMC2363882] [PubMed: 11336467](Among 54 patients with advanced lung cancer treated with cycles of paclitaxel and gemcitabine, 98% had some elevation in liver tests and 9% had ALT elevations of >5 times ULN, but all were asymptomatic and self-limited).
- Markman M, Zanotti K, Webster K, Belinson J, Rose P. Toxicity associated with carboplatin/paclitaxel/Irinotecan use in advanced ovarian cancer: preliminary analysis. Oncology (Williston Park). 2003;17(5) Suppl 5:34–5. [PubMed: 12800604](Among 26 women with advanced ovarian cancer given paclitaxel, carboplatin and irinotecan, 1[4%] developed transient ALT elevations >5 times ULN).
- Harries M, O'Donnell A, Scurr M, Reade S, Cole C, Judson I, Greystoke A, et al. Phase I/II study of DHA-paclitaxel in combination with carboplatin in patients with advanced malignant solid tumours. Br J Cancer. 2004;91:1651–5. [PMC free article: PMC2410023] [PubMed: 15494716](Among 15 patients with advanced solid cancers given carboplatin and paclitaxel conjugated to fatty acids, 5 [33%] developed transient ALT elevations >5 times ULN).
- Ohlmann CH, Kohlmorgen S, Sahi D, Engelmann U, Heidenreich A. Urologe A. 2007;46:1425–7. [Lethal course after chemotherapy with docetaxel. Acute liver failure with accompanying erythema multiforme major] German. [PubMed: 17563866](67 year old man with prostate cancer developed Stevens-Johnson Syndrome, neutropenia and thrombocytopenia after 5 weekly infusions of docetaxel, with subsequent rise in liver tests and jaundice that progressed to hepatic failure and death; few details provided).
- Bailey HH, Alberti DB, Thomas JP, Mulkerin DL, Binger KA, Gottardis MM, Martell RE, et al. Phase I trial of weekly paclitaxel and BMS-214662 in patients with advanced solid tumors. Clin Cancer Res. 2007;13:3623–9. [PubMed: 17510207](Among 26 patients with advanced cancer treated with paclitaxel and an experimental farnesyl transferase inhibitor, 3 developed febrile neutropenia and raised ALT levels 1 to 3 days after the infusions, not attributed to paclitaxel).
- Pignata S, Breda E, Scambia G, Pisano C, Zagonel V, Lorusso D, Greggi S, et al. A phase II study of weekly carboplatin and paclitaxel as first-line treatment of elderly patients with advanced ovarian cancer. A Multicentre Italian Trial in Ovarian cancer (MITO-5) study. Crit Rev Oncol Hematol. 2008;66:229–36. [PubMed: 18243011](Among 27 elderly women with advanced ovarian cancer treated with weekly infusions of carboplatin and paclitaxel, one had ALT increase above 5 times ULN during first course, but without jaundice, and she subsequently tolerated 5 more courses).
- Campone M, Levy V, Bourbouloux E, Berton Rigaud D, Bootle D, Dutreix C, Zoellner U, et al. Safety and pharmacokinetics of paclitaxel and the oral mTOR inhibitor everolimus in advanced solid tumours. Br J Cancer. 2009;100:315–21. [PMC free article: PMC2634724] [PubMed: 19127256](Among 16 patients with advanced malignancies treated with paclitaxel and oral everolimus, one developed transient ALT elevations >5 times ULN).
- Sun Q, Liu C, Zhong H, Zhong B, Xu H, Shen W, Wang D. Multi-center phase II trial of weekly paclitaxel plus cisplatin combination chemotherapy in patients with advanced gastric and gastro-esophageal cancer. Jpn J Clin Oncol. 2009;39:237–43. [PubMed: 19264768](Among 49 patients with gastrointestinal cancers treated with cyclic regimens of paclitaxel and cisplatin, 9 [18%] developed transient ALT elevations which were greater than 5 times ULN in 1 [2%], but none had jaundice).
- Oshita F, Saito H, Murakami S, Kondo T, Yamada K. Phase II study of paclitaxel and irinotecan with intercalated gefitinib in patients with advanced non-small-cell lung cancer. Am J Clin Oncol. 2010;33:66–9. [PubMed: 19786849](Among 16 patients with advanced non-small cell lung cancer treated with paclitaxel, irinotecan and gefitinib, 8 had ALT elevations [only one >5 times ULN], but none developed jaundice).
- Mandaliya H, Baghi P, Prawira A, George MK. A rare case of paclitaxel and/or trastuzumab induced acute hepatic necrosis. Case Rep Oncol Med. 2015;2015:825603. [PMC free article: PMC4641929] [PubMed: 26605100](62 year old woman with metastatic breast cancer received 4 cycles of doxorubicin and cyclophosphamide and developed pulmonary edema and respiratory failure within 12 hours of the initial infusions of paclitaxel and trastuzumab, dying one day later and autopsy showing acute hepatic necrosis; no liver test results provided).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 [5.5%] were attributed to antineoplastic agents of which only 1 was due to a taxane [docetaxel]).
- Fushida S, Kinoshita J, Kaji M, Oyama K, Hirono Y, Tsukada T, Fujimura T, et al. Paclitaxel plus valproic acid versus paclitaxel alone as second- or third-line therapy for advanced gastric cancer: a randomized Phase II trial. Drug Des Devel Ther. 2016;10:2353–8. [PMC free article: PMC4966651] [PubMed: 27524882](Among 66 patients with advanced gastric cancer treated with paclitaxel with or without valproic acid, overall and progression free survival was similar in the two groups; one patient had acute liver injury, but no details provided).
- Kümmel S, Paepke S, Huober J, Schem C, Untch M, Blohmer JU, Eiermann W, et al. Randomised, open-label, phase II study comparing the efficacy and the safety of cabazitaxel versus weekly paclitaxel given as neoadjuvant treatment in patients with operable triple-negative or luminal B/HER2-negative breast cancer (GENEVIEVE). Eur J Cancer. 2017;84:1–8. [PubMed: 28768217](Among 333 women with breast cancer given neoadjuvant therapy with cabazitaxel [every 3 weeks] or paclitaxel [weekly] for 12 weeks before definitive surgery, pathologic complete responses were lower with cabazitaxel [1% vs 11%] while serious adverse events were higher [25% vs 10%], ALT elevations occurring at similar rates [40% vs 45%] which were above 5 times ULN in only 1.2% vs 0.6%).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Taxanes.[LiverTox: Clinical and Researc...]Review Taxanes.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Docetaxel.[LiverTox: Clinical and Researc...]Review Docetaxel.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Exposure to low intensity ultrasound removes paclitaxel cytotoxicity in breast and ovarian cancer cells.[BMC Cancer. 2021]Exposure to low intensity ultrasound removes paclitaxel cytotoxicity in breast and ovarian cancer cells.Amaya C, Luo S, Baigorri J, Baucells R, Smith ER, Xu XX. BMC Cancer. 2021 Sep 1; 21(1):981. Epub 2021 Sep 1.
- Protein kinase RNA-activated controls mitotic progression and determines paclitaxel chemosensitivity through B-cell lymphoma 2 in ovarian cancer.[Oncogene. 2021]Protein kinase RNA-activated controls mitotic progression and determines paclitaxel chemosensitivity through B-cell lymphoma 2 in ovarian cancer.Yin L, Zeng Y, Zeng R, Chen Y, Wang TL, Rodabaugh KJ, Yu F, Natarajan A, Karpf AR, Dong J. Oncogene. 2021 Dec; 40(50):6772-6785. Epub 2021 Nov 19.
- Review Gemcitabine.[LiverTox: Clinical and Researc...]Review Gemcitabine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Paclitaxel - LiverToxPaclitaxel - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...