NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Phenytoin, formerly known as diphenylhydantoin, is a potent anticonvulsant used to treat and prevent generalized grand mal seizures, complex partial seizures and status epilepticus. Phenytoin was formerly the most commonly used anticonvulsant agent but is now declining in use, having been replaced by more modern, better tolerated agents. Phenytoin is an uncommon but well known cause of acute idiosyncratic drug induced liver disease that can be severe and even fatal.
Background
Phenytoin (fen' i toyn) is a hydantoin derivative that has potent anti-seizure activity that is believed to be based upon stabilization of neuronal membranes caused by an increase in the efflux and decrease in the influx of sodium ions across GABA regulated sodium channels. A similar action in cardiac muscle may account for its activity against ventricular arrhythmias. Phenytoin was first approved for use in the United States in 1946, and currently more than 2 million prescriptions are filled yearly. Current indications for phenytoin are treatment and prevention of generalized tonic-clonic (grand mal) seizures and complex partial seizures and management of status epilepticus. Once the most commonly used anticonvulsant agent, phenytoin is currently less commonly used, largely because of its complex pharmacokinetics, serious adverse events and difficult drug interactions. It is also no longer used to stabilize cardiac arrhythmias not responding to lidocaine. Phenytoin is available generically in oral and parenteral formulations. Oral forms include tablets and capsules of 100 to 300 mg, including extended release formations for once daily dosing. Commercial names include Dilantin. Chewable tablets and oral suspensions are available for pediatric use. The recommended dose of phenytoin for chronic use is 300 mg daily. Common side effects include dizziness, ataxia, nausea, gum hyperplasia and rash (which can occur in 10% of patients). Phenytoin has major effects on metabolism of other medications, and patients should be provided specific advice about other medications that can be used during long term phenytoin therapy. Serious adverse events include suicidal thoughts and behaviors, agranulocytosis, aplastic anemia, severe cutaneous reactions, Stevens-Johnson syndrome, and embryo-fetal abnormalities.
Hepatotoxicity
Prospective studies indicate that a fairly high proportion of patients taking phenytoin have transient serum aminotransferase elevations. These elevations are usually benign, not associated with liver histological abnormalities and usually resolve even with drug continuation. In addition, a higher proportion of patients have mild-to-moderate elevations in gammaglutamyl transpeptidase (GGT) levels, which is indicative of hepatic enzyme induction rather than liver injury. Marked aminotransferase elevations (>3 fold elevated) occur rarely.
Importantly, however, phenytoin is one of the most common causes of clinically apparent drug induced liver disease and acute liver failure. More than 100 cases of liver injury due to phenytoin (diphenylhydantoin) have been published and a characteristic clinical pattern (signature) of injury has been described. The estimated frequency ranges from 1 per 1000 to 1 per 10,000 and probably varies by race and ethnicity. The typical case arises after 2 to 8 weeks of therapy with initial onset of fever, rash, facial edema and lymphadenopathy, followed in a few days by jaundice and dark urine. The serum enzyme elevations can be hepatocellular, although mixed patterns are probably more common and rare cases are cholestatic. Eosinophilia, increased white counts and atypical lymphocytosis are also common. Autoantibody formation is rare. The clinical symptoms and signs can mimic acute mononucleosis or even lymphoma (pseudo-lymphoma syndrome). Almost all cases of phenytoin hepatotoxicity occur in the context of a systemic hypersensitivity syndrome and it is referred to often as the anticonvulsant hypersensitivity syndrome (HDS) or drug rash with eosinophilia and systemic symptoms syndrome (DRESS). Other manifestations can be Stevens-Johnson syndrome, toxic epidermal necrolysis, aplastic anemia, thrombocytopenia, neutropenia, nephritis, and pneumonitis. Most cases of liver injury are self-limiting and resolve within 1 to 2 months of stopping phenytoin. However, the liver injury can be severe and many fatal instances have been reported, phenytoin usually appearing in the top 10 causes of drug induced acute liver failure. In the typical case, however, recovery is usually complete.
Likelihood score: A (well known cause of clinically apparent liver injury).
Mechanism of Injury
The liver injury caused by phenytoin appears to be due to a hypersensitivity reaction and resembles typical cases of immunoallergic hepatotoxicity. This syndrome is more common in blacks than whites, but few other risk factors have been established. In some populations, the risk of injury correlates with the presence of HLA-B*15:02. Phenytoin is metabolized by CYP 450 system to arene oxide, which may represent the toxic or immunogenic intermediate.
Outcome and Management
Acute phenytoin hepatitis with jaundice has a fatality rate of greater than 10%. For this reason, phenytoin should be stopped promptly if symptoms of liver disease or jaundice arise early during therapy. Monitoring of ALT levels during introduction of phenytoin is recommended by some expert groups, but minor ALT elevations are common and it is unclear at what level of elevation that therapy should be suspended. Chronic injury due to phenytoin hepatotoxicity is rare or nonexistent, but cases of prolonged jaundice resembling vanishing bile duct syndrome have been reported. Rechallenge leads to rapid and usually more severe recurrence which can be fatal and should be avoided. Cross sensitivity with other aromatic anticonvulsants (phenobarbital, carbamazepine, lamotrigine and ethosuximide) can occur but is not invariable. Nevertheless, it is prudent to avoid use of these other aromatic anticonvulsants and to switch to agents such as valproate, gabapentin, levetiracetam, topiramate or a benzodiazepine, which do not appear to induce the same hypersensitivity syndrome. Corticosteroid therapy is often used in patients with severe phenytoin hepatotoxicity and anecdotal reports suggest that responses are rapid and beneficial. If used, corticosteroid therapy should be limited in dose and duration.
Drug Class: Anticonvulsants
CASE REPORT
Case 1. Acute immunoallergic hepatitis 3 weeks after starting phenytoin.(1)
A 25 year old man was started on phenytoin (100 mg thrice daily) for new onset seizures and developed fever, morbilliform pruritic rash and fatigue 3 weeks later. He was treated symptomatically and maintained on anticonvulsant therapy until 2 weeks later when he was admitted for worsening rash and jaundice. He had no other medical illnesses, no history of liver disease, drank little alcohol and was taking no other medications except salicylates for fever. On examination, he had a generalized erythematous rash and facial edema. His temperature was 39.5 oC and he had mild cervical, axillary and inguinal adenopathy. He was jaundiced, but had no hepatomegaly or peripheral signs of liver disease. Laboratory tests showed elevations in serum enzymes and bilirubin (Table). The total white count was 18,200/μL with 13% eosinophils and 13% atypical lymphocytes. An abdominal ultrasound was normal without splenomegaly or biliary abnormalities. He tested negative for markers of hepatitis A, B and C as well as cytomegalovirus, Epstein-Barr and herpes simplex viruses. Serological tests for syphilis were negative. Phenytoin was stopped. Initially, the prothrombin time was prolonged (20.4 sec, control 11 sec), but it corrected in the following week (and after vitamin K injections). The skin rash became desquamative. A liver biopsy showed changes of acute hepatitis with some granulomata and moderate cholestasis. In follow up six weeks after onset, the rash had resolved, he was asymptomatic and all laboratory tests (white counts and liver tests) were normal.
Key Points
| Medication: | Phenytoin (100 mg three times daily) |
|---|---|
| Pattern: | Mixed (R=4.9) |
| Severity: | 4+ (hospitalization for jaundice, abnormal prothrombin time) |
| Latency: | 3 weeks to symptoms, 5 weeks to jaundice |
| Recovery: | Complete within 6 weeks |
| Other medications: | Salicylates for fever |
Laboratory Values
Comment
The patient developed a typical acute anticonvulsant hypersensitivity syndrome with fever, rash, facial edema and lymphadenopathy within 3 weeks of starting phenytoin. Signs and symptoms of hepatitis arose thereafter and phenytoin was stopped once jaundice appeared. This pattern of onset and association with immunoallergic manifestations is typical of phenytoin hepatic injury. The enzyme pattern was “mixed” hepatocellular-cholestatic pattern and the liver biopsy showed a similar pattern. The hepatic injury was severe but rapidly reversible once phenytoin was stopped, the reversal beginning ~7 days after stopping, which is typical of phenytoin hepatic injury.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Phenytoin – Generic, Dilantin®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
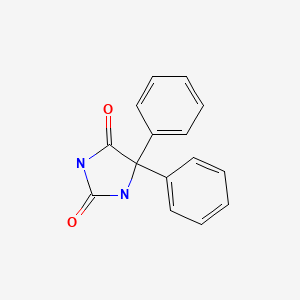
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Phenytoin | 57-41-0 | C15-H12-N2-O2 |
|
CITED REFERENCE
- 1.
- Gloria L, Serejo F, Cruz E, Freitas J, Costa A, Ramalho F, Batista A, de Moura MC. Diphenylhydantoin-induced hepatitis: a case report. Hepatogastroenterology. 1998;45:411–4. [PubMed: 9638415]
ANNOTATED BIBLIOGRAPHY
References updated: 30 July 2020
Abbreviations used: HHV, human herpes virus; DRESS, drug rash, eosinophilia and systemic signs; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis.
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999 which provides a concise description of the typical signature hepatic injury associated with phenytoin).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-442.(Review of anticonvulsant induced liver injury including phenytoin).
- Smith MD, Metcalf CS, Wilcox KS. Pharmacology of the epilepsies. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 303-26.(Textbook of pharmacology and therapeutics).
- Kazamatsuri H. Elevated serum alkaline phosphatase levels in epilepsy during diphenylhydantoin therapy. N Engl J Med. 1970;283:1411–2. [PubMed: 5481765](Among 61 patients with epilepsy treated with phenytoin, serum Alk P levels were abnormal in 60% compared to none of 49 controls).
- Pezzimenti JF, Hahn AL. Anicteric hepatitis induced by diphenylhydantoin. Arch Intern Med. 1970;125:118–20. [PubMed: 5410643](19 year old black man developed rash and fever 3 weeks after starting phenytoin with 6% eosinophils, rapid resolution, but immediate recurrence on one dose rechallenge with fever to 40 oC [eosinophils 19%, bilirubin 0.8 mg/dL, AST 226 U/L, Alk P normal, protime 18.5 sec], resolving in 3 weeks; 10 cases in the literature are reviewed, all had rash and fever, 2 fatal).
- Anthony JJ. Malignant lymphoma associated with hydantoin drugs. Arch Neurol. 1970;22:450–4. [PubMed: 5435667](Review of 100 cases of hydantoin associated lymphadenopathy in the literature, most onset in 1-2 months; 4 case reports of lymphoma/leukemia after long term phenytoin therapy; no consistent pattern but suspect an increase risk of lymphatic cancer with therapy).
- Andreasen PB, Lyngbye J, Trolle E. Abnormalities in liver function tests during long-term diphenylhydantoin therapy in epileptic outpatients. Acta Med Scand. 1973;194:261–4. [PubMed: 4749163](Cross sectional study of 55 “epileptics” and 250 controls for liver tests; 25% had ALT [all <3 times ULN] and 22% Alk P elevations [all <3 times ULN], mostly related to phenytoin).
- Jerina DM, Daly JW. Arene oxides: a new aspect of drug metabolism. Science. 1974;185:573–82. [PubMed: 4841570](Epoxidation of aromatic double bonds yields highly reactive arene oxides which react with nucleophiles including DNA, RNA and protein; molecular basis for cytotoxicity of aromatic hydrocarbons).
- Dhar GJ, Ahamed PN, Pierach CA, Howard RB. Diphenylhydantoin-induced hepatic necrosis. Postgrad Med. 1974;56:128–34. [PubMed: 4209753](20 year old woman developed fever and lymphadenopathy 15 days after starting phenytoin [2% eosinophils, AST 115 U/L, Alk P 210 U/L]; she restarted phenytoin during recovery and relapsed in 5 days [bilirubin 7.0 mg/dL, AST 113 U/L, Alk P 232 U/L], with fatal outcome; review of 17 cases in literature including 9 women, 8 men; 12 blacks; 7 died, 10 recovered).
- Weedon AP. Diphenylhydantoin sensitivity. A syndrome resembling infectious mononucleosis with a morbilliform rash and cholestatic hepatitis. Aust N Z J Med. 1975;5:561–3. [PubMed: 132924](10 year old boy developed fever, rash, sore throat and jaundice 4-5 weeks after starting phenytoin [8% eosinophils, bilirubin 11.1 mg/dL, AST 248 U/L, Alk P 625 U/L], with prolonged course of jaundice before recovery).
- Kleckner HB, Yakulis V, Heller P. Severe hypersensitivity to diphenylhydantoin with circulating antibodies to the drug. Ann Intern Med. 1975;83:522–3. [PubMed: 1166986](15 year old boy developed fever and rash, followed by dark urine arising 15 days after starting phenytoin [bilirubin 3.1 mg/dL, AST 1490 U/L, Alk P 74 U/L], with progressive worsening and apparent response to prednisone; anti-BSA-phenytoin antibody of 1:320; controls negative).
- Lee TJ, Carney CN, Lapis JL, Higgins T, Fallon HJ. Diphenylhydantoin-induced hepatic necrosis. A case study. Gastroenterology. 1976;70:422–4. [PubMed: 814030](21 year old woman developed fever and lymphadenopathy 18 months after starting phenytoin and 4 weeks after starting phenobarbital [WBC 20,000, 7% eosinophils, bilirubin 18.9 mg/dL, ALT 2048 U/L, Alk P 3 times ULN], with worsening hepatic failure and death, autopsy showing massive necrosis).
- Lapes MJ, Vivacqua RJ, Antoniades K. Immunoblastic lymphadenopathy associated with phenytoin (diphenylhydantoin). Lancet. 1976;1:198. [PubMed: 54713](78 year old black woman developed massive diffuse lymphadenopathy on phenytoin of unmentioned duration with atypical lymphocytosis and monoclonal IgG and IgA, responding to chemotherapy; no liver tests given).
- Jacobsen NO, Mosekilde L, Myhre-Jensen O, Pedersen E, Wildenhoff KE. Liver biopsies in epileptics during anticonvulsant therapy. Acta Med Scand. 1976;199:345–8. [PubMed: 1274671](Among 11 patients on long term phenytoin [10-35 years], 6 had mild ALT [37-46 U/L] and 4 Alk P elevations [282-471 U/L], but bilirubin levels were normal; all 11 had liver biopsy most showing nonspecific changes; none had fibrosis).
- Charlesworth EN. Phenytoin-induced pseudolymphoma syndrome. An immunologic study. Arch Derm. 1977;113:477–80. [PubMed: 848977](28 year old woman developed rash and lymphadenopathy 6 weeks after starting phenytoin therapy [24% eosinophils, bilirubin normal, AST 126 U/L, Alk P twice ULN], resolving rapidly on stopping; lymphocyte stimulation assays were negative).
- Campbell CB, McGuffie C, Weedon AP, Powell LW. Cholestatic liver disease associated with diphenylhydantoin therapy. Possible pathogenic importance of altered bile salt metabolism. Am J Dig Dis. 1977;22:255–62. [PubMed: 842535](10 year old boy developed rash, fever and jaundice 5 weeks after starting phenytoin [bilirubin 17.8 mg/dL, ALT ~450 U/L, Alk P above 2000 U/L], with marked and fluctuating cholestasis and high serum bile acid levels, resolving with corticosteroid therapy).
- Parker WA, Shearer CA. Phenytoin hepatotoxicity: a case report and review. Neurology. 1979;29:175–8. [PubMed: 571061](17 year old woman developed erythroderma and fever 4 weeks after starting phenytoin and phenobarbital [14% eosinophils, atypical lymphocytes, bilirubin 2.7 mg/dL, ALT 70 U/L, Alk P 210 U/L]; prednisone given but rash returned with tapering, switched to carbamazepine and phenobarbital).
- Haruda F. Phenytoin hypersensitivity. Thirty-eight cases. Neurology. 1979;29:1480–5. [PubMed: 574201](Review of admissions to Johns Hopkins between 1963-77 identified 38 cases of phenytoin hypersensitivity; onset in 1-35 days, but a few for longer: rash in 74%, fever 37%, abnormal liver tests in 29%, eosinophilia in 21%).
- Mullick FG, Ishak KG. Hepatic injury associated with diphenylhydantoin therapy: a clinicopathologic study of 20 cases. Am J Clin Pathol. 1980;74:442–52. [PubMed: 7424826](20 cases of phenytoin hepatotoxicity from Armed Forces Institute of Pathology files; onset of fever [75%], rash [62%], eosinophilia [89%] after 1-8 weeks of phenytoin; usually with mixed hepatocellular-cholestatic pattern of liver injury).
- Aiges HW, Daum F, Olson M, Kahn E, Teichberg S. The effects of phenobarbital and diphenylhydantoin on liver function and morphology. J Pediatr. 1980;97:22–6. [PubMed: 6103924](Cross sectional study of 63 children on phenobarbital and/or phenytoin for at least 12 months found 100% had GGT elevations [mean ~twice ULN], none had 5’NT, but 17% had AST and ALT elevations; 6 on phenytoin had persistent ALT elevations [72-122 U/L] and underwent liver biopsy: slight hepatocyte swelling, but no necrosis or inflammation, electron microscopy showed proliferation of endoplasmic reticulum, over time, ALT levels fell to normal but GGT remained elevated).
- Spielberg SP, Gordeon GB, Blake DA, Goldstein DA. Herlong HFl. Predisposition to phenytoin hepatotoxicity assessed in vitro. N Engl J Med. 1981;305:722–7. [PubMed: 6790991](3 women, ages 21 to 30 years, developed rash, fever and jaundice 1-4 weeks after starting phenytoin [bilirubin 7.8, 11.5 and 0.5 g/dL, AST 790, 2340 and 62 U/L, eosinophils 9%, 10% and 10%], all recovered; all had dose dependent cytotoxicity of lymphocytes exposed to microsomal metabolized [arene oxides] phenytoin).
- Cook IF, Shilkin KB, Reed WD. Phenytoin induced granulomatous hepatitis. Aust N Z J Med. 1981;11:539–41. [PubMed: 6948549](15 year old girl developed rash 10 days after starting carbamazepine; later started phenytoin and developed rash and fever after 6 weeks [bilirubin 2.4 mg/dL, AST 212 U/L, Alk P 639 U/L, 8% eosinophils], biopsy showed granulomas and hepatitis).
- Spechler SJ, Sperber H, Doos WG, Koff RS. Cholestasis and toxic epidermal necrolysis associated with phenytoin sodium ingestion: the role of bile duct injury. Ann Intern Med. 1981;95:455–6. [PubMed: 7283297](29 year old developed severe rash and jaundice 2 weeks after starting phenytoin [bilirubin 7.8 rising to 37.5 mg/dL, ALT 147 U/L, Alk P 201 rising to 2710 U/L], with prolonged cholestasis and recovery only after 8 months; liver biopsy showed intrahepatic cholestasis).
- Cacatian AA, Rando J. Diphenylhydantoin-induced pseudolymphoma syndrome. With severe thrombocytopenia. N Y State J Med. 1981;81:1085–7. [PubMed: 6942252](43 year old woman on phenytoin for ~20 years developed thrombocytopenia requiring splenectomy; unclear why case was considered pseudo-lymphoma or whether it was due to phenytoin, no fever or rash and minimal lymphadenopathy).
- Ting S, Dunsky EH. Diphenylhydantoin-induced hepatitis. Ann Allergy. 1982;48:331–2. [PubMed: 6212004](17 year old black male developed rash, fever and lymphadenopathy 2 weeks after starting phenytoin [bilirubin 3.2 mg/dL, ALT 780 U/L, Alk P 140 U/L, 18% eosinophils], treated with prednisone with rapid response; rechallenge led to rash in 6 hours but rapid recovery; had a positive lymphocyte stimulation test to phenytoin).
- Rosenthal CJ, Noguera CA, Coppola A, Kapelner SN. Pseudolymphoma with mycosis fungoides manifestations, hyperresponsiveness to diphenylhydantoin, and lymphocyte disregulation. Cancer. 1982;49:2305–14. [PubMed: 6978761](2 men and 1 woman, ages 18 to 47 years, developed severe exfoliative dermatitis, fever, lymphadenopathy and hepatomegaly at unspecified times after starting phenytoin [bilirubin not mentioned, ALT 72-430 U/L, Alk P 115-830 U/L, 13-23% eosinophilia], with Sezary cells and skin biopsy findings suggestive of mycosis fungoides, but which resolved when phenytoin was stopped).
- Tomsick RS. The phenytoin syndrome. Cutis. 1983;32:535–41. [PubMed: 6227459](Five case reports of African-Americans with onset after 1 to 4 weeks of phenytoin therapy of rash [erythroderma, morbilliform, toxic epidermal necrolysis [TEN], follicular papules and pustules], fever, eosinophilia [13-40%], and ALT abnormalities [37-1211 U/L]; 2 had jaundice, 2 died due to relapsing TEN and sepsis, 3 recovered within 2-4 weeks).
- Perucca E, Hedges A, Makki KA, Ruprah M, Wilson JF, Richens A. A comparative study on the relative enzyme inducing properties of anticonvulsant drugs in epileptic patients. Br J Clin Pharmacol. 1984;18:401–10. [PMC free article: PMC1463658] [PubMed: 6435654](Cross sectional study of antipyrine clearance in 122 patients with epilepsy, found dose dependent increase in clearance in those on phenytoin, phenobarbital and carbamazepine, but not on valproate).
- Taylor JW, Stein MN, Murphy MJ, Mitros FA. Cholestatic liver dysfunction after long-term phenytoin therapy. Arch Neurol. 1984;41:500–01. [PubMed: 6721716](64 year old woman treated with phenytoin for 41 years developed anorexia and jaundice with ascites [bilirubin 7.9 mg/dL, AST 74 U/L, Alk P 167 U/L], with biopsy showing intrahepatic cholestasis; rapid recovery but recurrence on restarting phenytoin [bilirubin 1.5 mg/dL, AST 450 U/L, Alk P 251 U/L]; no recurrence on switching to carbamazepine and mephobarbital).
- Kahn HD, Faguet GB, Agee JF, Middleton HM. Drug-induced liver injury. In vitro demonstration of hypersensitivity to both phenytoin and phenobarbital. Arch Intern Med. 1984;144:1677–9. [PubMed: 6466024](16 year old girl developed fever, facial edema, lymphadenopathy and rash 3 weeks after starting phenobarbital and phenytoin [bilirubin 0.3 mg/dL, AST 62 U/L, Alk P 123 U/L]; stopping phenytoin had no effect, but she improved with stopping phenobarbital and had positive lymphocyte stimulation tests to both drugs).
- Aaron JS, Bank S, Ackert G. Diphenylhydantoin-induced hepatotoxicity. Am J Gastroenterol. 1985;80:200–2. [PubMed: 3976639](64 year old black man developed rash and fever 3 weeks after starting phenytoin and was switched to phenobarbital, but 1 week later developed jaundice [bilirubin 3.4 mg/dL, ALT 456 U/L, Alk P 557 U/L, 19% eosinophils, 8% atypical lymphocytes], which worsened for a week, but ultimately resolved completely).
- Wolf R, Kahane E, Sandbank M. Mycosis fungoides-like lesions associated with phenytoin therapy. Arch Derm. 1985;121:1181–2. [PubMed: 4037845](78 year old lady developed 2 small mycosis fungoides like plaques on the skin and lymphadenopathy 11 months after starting phenytoin [6% eosinophils, but normal AST, Alk P and bilirubin], plaques remained for next 2 years while on phenytoin when biopsy showed mycosis fungoides; stopping phenytoin and switching to carbamazepine was followed by resolution of skin lesions and lymphadenopathy).
- Ratnaike RN, Schapel GJ, Purdie G, Rischbieth RH, Hoffmann S. Hyperammonaemia and hepatotoxicity during chronic valproate therapy: enhancement by combination with other antiepileptic drugs. Br J Clin Pharmacol. 1986;22:100–3. [PMC free article: PMC1401093] [PubMed: 3091053](Cross sectional analysis of ammonia levels and liver tests in 81 patients with epilepsy; higher average levels of ammonia in patients on valproate [37.1 vs 28.7 μmol], slightly higher rate of elevations in those receiving other agents [phenytoin, carbamazepine] as well).
- Riera Velasco JR, Rodrigo Saez LR, Perez Alvarez M, Gonzalez G. Hepatitis cronica activa por difenilhidantoinas. Med Clin (Barc). 1986;87:214. [PubMed: 3736251](49 year old man with a history of alcoholism developed evidence of liver disease, 2 years after starting phenytoin [bilirubin 0.4 mg/dL, ALT 113 U/L, Alk P 115 U/L], biopsy showing chronic hepatitis and ground-glass hepatocytes; likely chronic hepatitis C and cell-ER-activation by phenytoin rather than hepatotoxicity; only mild improvement upon stopping drug; report predates tests for anti-HCV).
- Brown M, Schubert T. Phenytoin hypersensitivity hepatitis and mononucleosis syndrome. J Clin Gastroenterol. 1986;8:469–77. [PubMed: 3093562](23 year old black woman with seizures developed fever, sore throat, rash and lymphadenopathy 5 weeks after starting phenytoin and 3 weeks after starting carbamazepine; drugs were stopped, and she was given iv phenytoin for a seizure, immediately redeveloping fever, severe exfoliative rash, facial edema and jaundice [bilirubin 5.6 mg/dL, ALT 1530 U/L, Alk P 267 U/L, protime 20.4 sec], responding slowly to corticosteroids: thorough review of rechallenge studies).
- Engel JN, Mellul VG, Goodman DBP. Phenytoin hypersensitivity: a case of severe acute rhabdomyolysis. Am J Med. 1986;81:928–30. [PubMed: 3776999](22 year old black man developed fever, rash, myalgias and facial edema 3 months after starting phenytoin [normal bilirubin ALT 90 U/L, AST 510 U/L, CPK 85,000 U/L, 9% eosinophils], responding to stopping drug and prednisone therapy).
- Deutsch J, Fritsch G, Golles J, Semmelrock HJ. Effects of anticonvulsive drugs on the activity of gammaglutamyltransferase and aminotransferases in serum. J Pediatr Gastroenterol Nutr. 1986;5:542–8. [PubMed: 2874203](Cross sectional study of 198 children on anticonvulsants; GGT levels were usually high and correlated with phenytoin levels, less with other agents; provided mean levels and not proportion with abnormalities).
- Sherertz EF, Jegasothy BV, Lazarus GS. Phenytoin hypersensitivity reaction presenting with toxic epidermal necrolysis and severe hepatitis. Report of a patient treated with corticosteroid “pulse therapy”. J Am Acad Dermatol. 1985;12:178–81. [PubMed: 3973116](49 year old black man developed fever and lymphadenopathy 3 weeks after starting phenytoin [bilirubin 2.5 mg/dL, AST 246 U/L, Alk P 168 U/L]; rechallenge with a single dose led to a recurrence with near fatal outcome, use of high dose corticosteroids was complicated by perforation of a duodenal ulcer).
- Keeffe EB, Sunderland MC, Gabourel JD. Serum gamma-glutamyltranspeptidase activity in patients receiving chronic phenytoin therapy. Dig Dis Sci. 1986;31:1056–61. [PubMed: 2875856](Among 58 patients started on phenytoin, GGT levels increased in 90%; levels were abnormal in 22% at baseline and in 74%, 63% and 64% at 6, 12 and 24 months, levels rising from mean of 45 to 136 U/L at 6 months, peak levels 400-500 U/L, higher in patients who drank alcohol and higher in patients than controls).
- Shear NH, Spielberg SP. Anticonvulsant hypersensitivity syndrome: in vitro assessment of risk. J Clin Invest. 1988;82:1826–32. [PMC free article: PMC442760] [PubMed: 3198757](Peripheral blood mononuclear cells cytotoxicity in response to drug metabolites from 53 patients with hypersensitivity to anticonvulsants including 35/36 to phenytoin, 22/27 to phenobarbital, and 25/27 to carbamazepine; 51% had hepatitis).
- Silverman AK, Fairley J, Wong RC. Cutaneous and immunologic reactions to phenytoin. J Am Acad Dermatol. 1988;18:721–41. [PubMed: 2967311](Extensive review of dermatologic complications of phenytoin therapy, including acne, exfoliative dermatitis, erythema multiforme, hyperpigmentation, maculopapular rashes, pseudolymphoma, toxic epidermal necrolysis, vasculitis, bullous reactions, gingival hyperplasia and coarsening of facial features).
- Aldenhövel HG. The influence of long-term anticonvulsant therapy with diphenylhydantoin and carbamazepine on serum gamma-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase. Eur Arch Psychiatry Neurol Sci. 1988;237:312–6. [PubMed: 2901959](Serum enzymes done on 54 patients on phenytoin and 56 on carbamazepine; GGT elevations [14-283 U/L] in 91% vs 64%, ALT [5-48 U/L] in 28% vs 9%, and Alk P [14 to 218 U/L] in 39% vs 14%, most elevations were modest; GGT elevations were dose related, but not the others).
- Roberts EA, Spielberg SP, Goldbach M, Phillips MJ. Phenobarbital hepatotoxicity in an 8-month-old infant. J Hepatol. 1990;10:235–9. [PubMed: 2332596](8 month old male child with seizures given phenobarbital for 2 weeks developed rash followed by fever with eosinophilia, atypical lymphocytes, ALT 430 U/L, Alk P 135 U/L, bilirubin 2.9 mg/dL, slow recovery, positive lymphocyte cytotoxicity test to phenobarbital, phenytoin and carbamazepine).
- Howard PA, Engen PL, Dunn MI. Phenytoin hypersensitivity syndrome: a case report. DICP. 1991;25:929–32. [PubMed: 1949968](27 year old black man developed rash 2 weeks and fatigue and fever 4 weeks after starting phenytoin [bilirubin 1.2 rising to 5.7 mg/dL, ALT 1050 U/L, Alk P 239 U/L, 15% eosinophils, 11% atypical lymphocytes], with subsequent severe course, but improvements starting 5 days after stopping phenytoin and ultimate full recovery).
- Kleier RS, Breneman DL, Boiko S. Generalized pustulation as a manifestation of the anticonvulsant hypersensitivity syndrome. Arch Dermatol. 1991;127:1361–4. [PubMed: 1832535](2 men, ages 21 and 49 years, developed rash after 3-4 weeks of phenytoin evolving into sterile pustules; eosinophilia and fever [bilirubin normal, ALT 57 and 660 U/L, Alk P 85 and 117 U/]L, worsening on switching to carbamazepine and phenobarbital, resolving after switching to clonazepam).
- Gennis MA, Vemuri R, Burns EA, Hill JV, Miller MA, Spielberg SP. Familial occurrence of hypersensitivity to phenytoin. Am J Med. 1991;91:631–4. [PubMed: 1750433](3 siblings, ages 23 to 32 years, developed fever, rash, facial edema, lymphadenopathy, eosinophilia and anicteric hepatitis 2-21 days after starting phenytoin, resolving rapidly; index case had in vitro evidence of toxicity of carbamazepine and phenytoin, but not phenobarbital and similar findings in siblings).
- Roy AK, Mahoney HC, Levine RA. Phenytoin-induced chronic hepatitis. Dig Dis Sci. 1993;38:740–3. [PubMed: 8462373](52 year old woman had chronic, mild ALT elevations on long term phenytoin [bilirubin and Alk P normal], biopsy showing mild inflammation without fibrosis, ALT elevations resolved rapidly with stopping and recurred within 5 days of rechallenge).
- Handfield-Jones SE, Jenkins RE, Whittaker SJ, Besse CP, McGibbon DH. The anticonvulsant hypersensitivity syndrome. Br J Dermatol. 1993;129:175–7. [PubMed: 7654579](2 women and 1 man, ages 20, 46 and 61 years developed fever and rash 3, 4 and 10 weeks after starting phenytoin or carbamazepine; two required corticosteroids; only 1 had hepatitis; one died of multiorgan failure, others switched to valproate or clobazam without recurrence).
- García-Samaniego J, Soriano V, Soto J, Muñoz F. An Med Interna. 1994;11:541–2. [Phenytoin hypersensitivity syndrome] [PubMed: 7654902](15 year old developed fever and oral ulcers 2 months after starting phenytoin, followed by rash, pruritus and adenopathy [bilirubin 8 mg/dL, ALT 780 U/L, Alk P 836 U/L and eosinophilia]; given corticosteroids and switched to valproate and recovered).
- Vittorio CC, Muglia JJ. Anticonvulsant hypersensitivity syndrome. Arch Intern Med. 1995;155:2285–90. [PubMed: 7487252](One 24 year old woman and a 38 year old man developed anticonvulsant hypersensitivity syndrome, 4 and 6 weeks after starting carbamazepine [bilirubin 0.6 and 4.1 mg/dL, ALT 402 and 1890 U/L, Alk P 184 and 487 U/L], both recovered but required corticosteroid therapy).
- Mauri-Hellweg D, Bettens F, Mauri D, Brander C, Hunziker T, Pichler WJ. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phenytoin, and carbamazepine. J Immunol. 1995;155:462–72. [PubMed: 7602118](In vitro lymphocyte studies on patients with drug allergy, found proliferation in response to specific drugs, including phenytoin, with proliferation of both CD4 and CD8 T cells and increase in CD-25 and HLA-DR expression and production of IL5 and IFNγ).
- de la Serna Higuera C, Gil Grande LA, Barcena Marugan R. Gastroenterol Hepatol. 1995;18:471–3. [Toxic cholestatic hepatitis due to phenytoin] [PubMed: 8521225](51 year old woman developed rash, fever and then jaundice 3 weeks after starting phenytoin [bilirubin 8.2 mg/dL, ALT 847 U/L, Alk P 1782 U/L, 15% eosinophilia], and slow biochemical recovery).
- Schneider S, Charles F, Chichmanian RM, Montoya ML, Rampal P. Gastroenterol Clin Biol. 1995;19:1064–5. [Acute hepatitis associated with microvesicular steatosis induced by Atrium] [PubMed: 8729422](37 year old woman developed right upper quadrant pain after 6 months of Atrium therapy [bilirubin rising to 2.0 mg/dL, ALT 9 times ULN, Alk P normal, ANA 1:1000], biopsy showed microvesicular fat; also on carbimazole and phenytoin).
- Wallace SJ. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf. 1996;15:378–93. [PubMed: 8968693](Systematic review; ALT elevations occur in 4% of children on phenytoin, 6% on valproate, 1% on carbamazepine, but not with gabapentin or phenobarbital; skin rash and Stevens-Johnson syndrome discussed related to phenobarbital).
- Chopra S, Levell NJ, Cowley G, Gilkes JJH. Systemic corticosteroids in the phenytoin hypersensitivity syndrome. Br J Dermatol. 1996;134:1109–12. [PubMed: 8763435](46 year old woman developed rash and fever, 8 weeks after starting phenytoin [bilirubin 1.0 mg/dL, AST 96 U/L, Alk P 642 U/L, 7% eosinophils], with rapid response to prednisone, relapse when stopped early).
- Conger LA Jr, Grabski WJ. Dilantin hypersensitivity reaction. Cutis. 1996;57:223–6. [PubMed: 8727770](41 year old woman developed fever, rash, lymphadenopathy and facial edema 6 weeks after starting phenytoin [no mention of bilirubin, ALT 63 U/L, Alk P 137 U/L, 13% eosinophils, 3% atypical lymphocytes], resolving rapidly upon stopping).
- Schlienger RG, Shear NH. Antiepileptic drug hypersensitivity syndrome. Epilepsia. 1998;39 Suppl 7:S3–7. [PubMed: 9798755](Review: onset 2-8 weeks after starting therapy with aromatic anticonvulsants presenting with high fever, rash, adenopathy and pharyngitis followed by organ involvement, most commonly the liver [~50%], but also hematologic, renal or pulmonary; eosinophilia, blood dyscrasias, nephritis; sometimes facial edema, oral ulcers, hepatosplenomegaly, myopathy, disseminated intravascular coagulation, atypical lymphocytosis; rash usually exanthema with pruritus, occasional follicular pustules or exfoliative dermatitis and erythroderma, erythema multiforme, Stevens-Johnson syndrome or toxic epidermal necrolysis).
- Gloria L, Serejo F, Cruz E, Freitas J, Costa A, Ramalho F, Batista A, Carneiro de Moura M. Diphenylhydantoin-induced hepatitis: a case report. Hepatogastroenterology. 1998;45:411–4. [PubMed: 9638415](25 year old man developed fever, rash, facial edema and malaise 3 weeks after starting phenytoin followed by jaundice [bilirubin 6.9 mg/dL, ALT 888 U/L, Alk P 570 U/L, 13% eosinophils], resolving within 6 weeks of stopping).
- Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: incidence, prevention and management. Drug Saf. 1999;21:489–501. [PubMed: 10612272](Classic review of anticonvulsant hypersensitivity syndrome: triad of fever, rash and internal organ injury occurring 1-8 weeks after exposure to anticonvulsant, liver being most common internal organ involved. Occurs in 1:1000-1:10,000 initial exposures to phenytoin, carbamazepine, phenobarbital or lamotrigine, unrelated to dose, perhaps predisposed by valproate; liver injury arises 1-4 weeks after onset of rash and ranges in severity from asymptomatic ALT elevations to icteric hepatitis to acute liver failure. High mortality rate with jaundice; other organs include muscle, kidney, brain, heart and lung. Pseudolymphoma syndrome and serum sickness like syndrome are separate complications of anticonvulsants. Role of corticosteroids uncertain; cross reactivity among the agents should be assumed).
- Kakar A, Byotra SP. Phenytoin induced severe agranulocytosis and hepatitis. J Assoc Physicians India. 1999;47:644. [PubMed: 10999171](45 year old woman developed fever one week after starting phenytoin [bilirubin not given, ALT 106 U/L, Alk P 913 U/L]).
- Hamer HM, Morris HH. Hypersensitivity syndrome to antiepileptic drugs: a review including new anticonvulsants. Cleve Clin J Med. 1999;66:239–45. [PubMed: 10199060](Clinical review of anticonvulsant hypersensitivity syndrome; occurs in 1-5/10,000 users, higher risk in African Americans and siblings of affected subjects; liver involvement common, but most cases anicteric; other manifestations are facial edema, lymphadenopathy, bone marrow aplasia, pseudolymphoma, thyroiditis, pneumonitis and interstitial nephritis).
- Hamer HM, Morris HH. Successful treatment with gabapentin in the presence of hypersensitivity syndrome to phenytoin and carbamazepine: a report of three cases. Seizure. 1999;8:190–2. [PubMed: 10356381](3 patients with onset of rash, fever, lymphadenopathy and eosinophilia 4-6 weeks after starting either phenytoin or carbamazepine, [bilirubin 0.5-1.8 mg/dL, ALT 866-1402 U/L, Alk P 69-364U/L], all 3 later tolerated gabapentin).
- Nashed MH, Liao L. Possible atypical cross-sensitivity between phenytoin and carbamazepine in the anticonvulsant hypersensitivity syndrome. Pharmacotherapy. 2001;21:502–5. [PubMed: 11310525](73 year old black woman developed rash, fever and confusion 3-4 weeks after starting phenytoin and failed to improve when switched to carbamazepine [peak bilirubin 0.7 mg/dL, ALT 77 U/L, Alk P 521, 19% eosinophils], with rapid resolution on stopping both).
- Güngör E, Alli N, Comoğlu S, Cömcüoğlu C. Phenytoin hypersensitivity syndrome. Neurol Sci. 2001;22:261–5. [PubMed: 11731881](5 cases from Turkey, with fever, rash, edema, eosinophils and increased white blood cell counts, 2 days to 3 months after starting phenytoin [bilirubin normal, ALT 34-97 U/L, Alk P 498-747 U/L], rapid recovery after stopping).
- Bessmertny O, Hatton RC, Gonzalez-Peralta RP. Antiepileptic hypersensitivity syndrome in children. Ann Pharmacother. 2001;35:533–8. [PubMed: 11346057](Among 14 children with anticonvulsant hypersensitivity syndrome, 58% were receiving phenytoin, 43% carbamazepine, and 29% phenobarbital; 63% had ALT and 36% bilirubin elevations and 1 died of hepatic failure).
- Kaur S, Sarkar R, Thami GP, Kanwar AJ. Anticonvulsant hypersensitivity syndrome. Pediatr Dermatol. 2002;19:142–5. [PubMed: 11994179](13 year old boy on phenytoin developed fever, rash and facial edema 1 month after starting carbamazepine [bilirubin 0.4 mg/dL, ALT 72 U/L, Alk P 551 U/L] and did not improve until both agents were stopped, subsequently remaining well on valproate).
- Galindo PA, Borja J, Gómez E, Mur P, Gudín M, García R, Encinas C, et al. Anticonvulsant drug hypersensitivity. J Investig Allergol Clin Immunol. 2002;12:299–304. [PubMed: 12926190](Patch testing on 15 patients with skin reactions to anticonvulsants; 12 were positive, often to several of the aromatic anticonvulsants).
- Altuntaş Y, Oztürk B, Erdem L, Günes G, Karul S, Uçak S, Sengül A. Phenytoin-induced toxic cholestatic hepatitis in a patient with skin lesions: case report. South Med J. 2003;96:201–3. [PubMed: 12630649](47 year old woman developed exfoliative rash 25 days after starting phenytoin with fever, leukocytosis, eosinophilia and hepatitis [bilirubin 6.4 mg/dL, ALT 441 U/L, Alk P 1431 U/L], rapid improvement on stopping).
- Choi TS, Doh KS, Kim SH, Jang MS, Suh KS, Kim ST. Clinicopathological and genotypic aspects of anticonvulsant-induced pseudolymphoma syndrome. Br J Dermatol. 2003;148:730–6. [PubMed: 12752131](8 patients from Korea with maculopapular rash, fever, lymphadenopathy and facial edema arising 3 to 24 [mean=7] weeks after starting an anticonvulsant, elevated liver tests in 5; largely dermatologic description).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants done in the United States between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, of which 10 were attributed to phenytoin, 10 to valproate and 1 to carbamazepine, but none to lamotrigine or other anticonvulsants).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](36 years of reporting to Swedish registry identified 103 cases of acute liver failure due to drugs, of which 1 was attributed to phenytoin, 1 to valproate and 1 to carbamazepine, but none to lamotrigine or other anticonvulsants ).
- Sierra NM, García B, Marco J, Plaza S, Hidalgo F, Bermejo T. Cross hypersensitivity syndrome between phenytoin and carbamazepine. Pharm World Sci. 2005;27:170–4. [PubMed: 16096883](Among 20 patients with anticonvulsant hypersensitivity syndrome due to carbamazepine or phenytoin who were restarted on the other opposite agent, 9 redeveloped hypersensitivity features, but 8 were subsequently able to tolerate valproate [4], vigabatrin [2] or phenobarbital [2] without problems).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–8. [PubMed: 16054882](In WHO database of fatal adverse drug reactions from 1968-2003 including 4690 reports of drug induced liver fatality, phenytoin ranked 12th [57 cases], the only anticonvulsant among the top 19 causes).
- Korem M, Hiller N, Ackerman Z, Chajek-Shaul T, Abramowitz Y. Spleen rupture secondary to anticonvulsant hypersensitivity syndrome. Eur J Intern Med. 2006;17:517–9. [PubMed: 17098601](23 year old woman developed rash, fever, edema and eosinophilia [27%] 2 months after starting phenytoin [Alk P 320 U/L, but no bilirubin or ALT values], suffered spontaneous splenic rupture, but was managed medically).
- Tohyama M, Hashimoto K, Yasukawa M, Kimura H, Horikawa T, Nakajima K, Urano Y, et al. Association of human herpes virus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. 2007;157:934–40. [PubMed: 17854362](Anti-HHV-6 testing of 100 patients with drug induced hypersensitivity syndrome [34% with hepatitis] found rise in IgG levels in 62 patients, largely in more severe cases; human herpes virus [HHV]-6 DNA detected in 18; drugs included carbamazepine, phenobarbital, phenytoin, allopurinol, sulfasalazine and mexiletine).
- Mansur AT, Pekcan Yaşar S, Göktay F. Anticonvulsant hypersensitivity syndrome: clinical and laboratory features. Int J Dermatol. 2008;47:1184–9. [PubMed: 18986457](Among 31 patients seen at a single Turkish medical center with anticonvulsant hypersensitivity syndrome, 48% had received carbamazepine, 36% phenytoin, 10 lamotrigine and 6% lamotrigine and valproate; onset 2-86 days after starting with ALT elevations in 71%, eosinophilia in 64%, only one death [TEN]).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand. 2008;118:281–90. [PubMed: 18341684](Review of all anticonvulsants; phenytoin usually causes liver injury as a part of the hypersensitivity syndrome, in 1:10,000 to 1:50,000 persons, 100 published cases, mean onset at 4 weeks, 13% mortality).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, valproate accounted for 6 cases, lamotrigine 5, phenytoin 5, gabapentin and topiramate 1 each).
- Ito S, Shioda M, Sasaki K, Imai K, Oguni H, Osawa M. Agranulocytosis following phenytoin-induced hypersensitivity syndrome. Brain Dev. 2009;31:449–51. [PubMed: 18774664](5 year old boy with hypersensitivity syndrome after 12 days of phenytoin with mild ALT elevations [154 U/L] but no jaundice, who suffered from transient neutropenia during recovery).
- Crespo Pérez L, Moreira Vicente V, Cano Ruiz A, Gobernado Serrano JM, Cobo Ibañez N, Milicua Salamero JM. Gastroenterol Hepatol. 2009;32:687–92. [Anticonvulsant hypersensitivity syndrome: an entity to be remembered] [PubMed: 19732994](15 year old girl developed fever, rash and adenopathy 6 weeks after starting carbamazepine and phenytoin [peak bilirubin 16.3 mg/dL, ALT 809 U/L, GGT 809 U/L, eosinophils 5%], resolving within a month of stopping both drugs).
- Franciotta D, Kwan P, Perucca E. Genetic basis for idiosyncratic reactions to antiepileptic drugs. Curr Opin Neurol. 2009;22:144–9. [PubMed: 19262378](Review of the genetic associations with hypersensitivity reactions to anticonvulsant medications; closest association has been with HLA-B*15:02 and Stevens-Johnson syndrome [SJS] after aromatic anticonvulsants).
- Newell BD, Moinfar M, Mancini AJ, Nopper AJ. Retrospective analysis of 32 pediatric patients with anticonvulsant hypersensitivity syndrome (ACHSS). Pediatr Dermatol. 2009;26:536–46. [PubMed: 19840307](Among 32 children with anticonvulsant hypersensitivity syndrome seen at two medical centers, 12 had been exposed to phenytoin, 13 carbamazepine, 5 phenobarbital, 5 lamotrigine and 1 primidone [who also received lamotrigine]; liver involved in 91%, but jaundice in just 18%, no deaths).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India; 21 [6.7%] were attributed to phenytoin, one of which was fatal).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 8 due to phenytoin, 3 to carbamazepine and 2 to valproate).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, phenytoin accounting for 57 cases [0.6%, ranking 20th] for an adjusted relative risk odds ratio of 3.0).
- Devarbhavi H, Karanth D, Prasanna KS, Adarsh CK, Patil M. Drug-Induced liver injury with hypersensitivity features has a better outcome: a single-center experience of 39 children and adolescents. Hepatology. 2011;54:1344–50. [PubMed: 21735470](Among 39 children with drug induced liver disease seen at a single referral center in India between 2005 and 2010, 10 cases were due to phenytoin and 6 to carbamazepine, all of whom survived in contrast to deaths of 12 of 23 children with hepatocellular injury without signs of hypersensitivity [largely due antituberculosis medications]).
- Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N., Drug-Induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN Prospective Study. J Pediatr Gastroenterol Nutr. 2011;53:182–9. [PMC free article: PMC3634369] [PubMed: 21788760](Among 30 children with drug induced liver injury enrolled in a prospective US database between 2004 and 2008, 8 were due to anticonvulsants [lamotrigine in 3, valproate in 3, phenytoin in 1 and carbamazepine in 1], none of which were fatal or led to chronic injury).
- Neuman MG, Cohen L, Nanau RM, Hwang PA. Genetic and immune predictors for hypersensitivity syndrome to antiepileptic drugs. Transl Res. 2012;159:397–406. [PubMed: 22500513](Study of 20 patients with hypersensitivity reactions to phenytoin or carbamazepine and 40 controls found positive lymphocyte toxicity assays in all patients with hypersensitivity and a correlation of HLA-B*15:02 with the allergic reactions in Han Chinese).
- Knowles SR, Dewhurst N, Shear NH. Anticonvulsant hypersensitivity syndrome: an update. Expert Opin Drug Saf. 2012;11:767–78. [PubMed: 22794330](Updated review of anticonvulsant hypersensitivity syndrome; associated with phenytoin, phenobarbital, lamotrigine and carbamazepine and rarely with zonisamide, valproate and oxcarbazepine).
- Drugs for epilepsy. Treat Guidel Med Lett. 2013;11:9–18. Erratum in Treat Guidel Med Lett 2013; 11: 112. [PubMed: 23348233](Concise review of indications and side effects of anticonvulsants mentions that phenytoin is no longer considered a drug of first choice because of its pharmacokinetics, side effects and drug-drug interactions, and that phenytoin can cause hypersensitivity reactions, Stevens-Johnson syndrome and fatal hepatitis).
- Gaeti WP, Obreli-Neto PR, Moliterno RA, Schiavon GB, Cuman RK. HLA typing in Brazilian boys with aromatic antiepileptic drug-induced DRESS. Int J Clin Pharm. 2013;35:319–22. [PubMed: 23575622](9 and 11 year old boys developed rash, fever, facial edema and eosinophilia [8-14%] 27 and 19 days after starting an anticonvulsant [carbamazepine and phenytoin], with elevations in ALT [631 and 222 U/L] and Alk P [835 and 439 U/L] without jaundice; HLA testing showed no commonality and absence of B*15:02]).
- Ghabril M, Fontana R, Rockey D, Jiezhun G, Chalasani N. Drug-induced liver injury caused by intravenously administered medications: The Drug-induced Liver Injury Network experience. J Clin Gastroenterol. 2013;47:553–8. [PMC free article: PMC3681898] [PubMed: 23388845](Among 524 cases of drug induced liver injury from the US, 32 were attributed to intravenous medications including 3 to phenytoin).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 1 attributed to phenytoin among only 410 persons receiving the drug in Iceland).
- Keel BR, Payne CL. Anticonvulsant hypersensitivity syndrome (AHS): a case report. Tenn Med. 2013;106:35–6, 38. [PubMed: 23617037](40 year old African-American woman developed a desquamating rash, fever and abdominal pain 3-4 weeks after starting phenytoin [bilirubin 0.3 mg/dL, ALT 245 rising to 690 U/L, Alk P 88, INR 1.2], which began to resolve 5 days after stopping).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 7 [4%] of which were attributed to anticonvulsants including 3 due to phenytoin, 2 of which were fatal).
- Velasco MJ, McDermott J. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome and hepatitis induced by phenytoin. Int J Dermatol. 2014;53:490–3. [PubMed: 23829691](33 year old man developed fever, rash, and eosinophilia 23 days after starting phenytoin for seizures [bilirubin 2.5 rising to 11.7 mg/dL, ALT 501 to 1703 U/L, Alk P 255 U/L] treated with corticosteroids with slow recovery).
- Devarbhavi H, Andrade RJ. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014;34:145–61. [PubMed: 24879980](Review of drug induced liver injury caused by several drug classes including antiepileptics, which account for 2-11% of all cases in various registries; a common presentation is with DRESS, particularly with the older agents such as carbamazepine, phenytoin and phenobarbital and often associated with reactivation of human herpes virus 6 or 7).
- Rolls S, Hyams C, Sheaff M, O'Shaughnessy TC. Is this still just sarcoidosis, or should we a-DRESS a different diagnosis? BMJ Case Rep. 2015;2015:bcr2014207778. [PMC free article: PMC4488709] [PubMed: 26123453](48 year old Afro-Caribbean woman with rheumatoid arthritis developed fevers and pneumonitis after starting therapy with methotrexate and sulfasalazine and then severe toxic epidermal necrolysis after starting phenytoin for seizures during her hospitalization, slowly responding to corticosteroid therapy).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were due to anticonvulsants including 12 due to phenytoin, 9 lamotrigine, 7 valproate, 4 carbamazepine, 3 gabapentin, 2 topiramate and 1 each for ethosuximide, fosphenytoin, and pregabalin).
- Trivedi BS, Darji NH, Malhotra SD, Patel PR. Antiepileptic drugs-induced Stevens-Johnson syndrome: a case series. J Basic Clin Pharm. 2016;8:42–4. [PMC free article: PMC5201065] [PubMed: 28104975](Among 9 cases of Stevens-Johnson syndrome seen at a large referral center in India, 5 were due to phenytoin, 2 carbamazepine and 2 oxcarbazepine; no mention of hepatic involvement).
- Chalasani N, Reddy KRK, Fontana RJ, Barnhart H, Gu J, Hayashi PH, Ahmad J, Stolz A, Navarro V, Hoofnagle JH. Idiosyncratic drug induced liver injury in African-Americans is associated with greater morbidity and mortality compared to Caucasians. Am J Gastroenterol. 2017;112:1382–8. [PMC free article: PMC5667647] [PubMed: 28762375](Among subjects enrolled in a US prospective database of drug induced liver injury, causes more frequent in African Americans than Caucasians were trimethoprim/sulfamethoxazole, methyldopa, phenytoin and allopurinol; in addition, African Americans were more likely to have severe skin adverse events [2.1% vs 0.4%], fatal or transplantation outcomes [10% vs 6%] as well as chronic injury [24% vs 16%]).
- Vidaurre J, Gedela S, Yarosz S. Antiepileptic drugs and liver disease. Pediatr Neurol. 2017;77:23–36. [PubMed: 29097018](Review of the use of anticonvulsants in patients with liver disease recommends use of agents that have little hepatic metabolism such as levetiracetam, lacosamide, topiramate, gabapentin and pregabalin, levetiracetam being an "ideal" first line therapy for patients with liver disease because of its safety and lack of pharmacokinetic interactions).
- Drugs for epilepsy. Med Lett Drugs Ther. 2017;59(1526):121–30. [PubMed: 28746301](Concise review of the drugs available for therapy of epilepsy lists phenytoin as effective for partial and generalized but no longer the drug of choice because of its complicated pharmacokinetics, adverse events and drug interactions; among side effects, rash, fever, hepatotoxicity and Stevens-Johnson syndrome are mentioned).
- Curry B, Mican L, Smith TL. Phenytoin-induced chronic liver enzyme elevation and hepatic fibrosis: A case report. Ment Health Clin. 2018;8:184–7. [PMC free article: PMC6063461] [PubMed: 30155393](50 year old African American female developed mild elevations in serum enzymes while on phenytoin [ALT rising from 11 to 139 U/L and Alk P from 40 to 180 U/L], persisting for several years and not decreasing with stopping valproate and atorvastatin but promptly falling into the normal range when phenytoin was stopped; presence of fibrosis was suggested by abdominal ultrasound but liver biopsy was not done).
- Borrelli EP, Lee EY, Descoteaux AM, Kogut SJ, Caffrey AR. Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs: An analysis of the US Food and Drug Administration Adverse Event Reporting System. Epilepsia. 2018;59:2318–24. [PMC free article: PMC6420776] [PubMed: 30395352](Review of adverse event reports to the FDA between 2014 and 2018 identified ~2.9 million reports, 1034 for SJS/TEN, the most common class of drugs being anticonvulsants with 17 of 34 having at least one report, those most frequently linked being lamotrigine [n=106], carbamazepine [22], levetiracetam [14], phenytoin [14], valproate [9], clonazepam [8] and zonisamide [7]; no mention of accompanying liver injury or whether attribution was as a single agent or one of several).
- Han XD, Koh MJ, Wong SMY. Drug reaction with eosinophilia and systemic symptoms in a cohort of Asian children. Pediatr Dermatol. 2019;36:324–9. [PubMed: 30920020](Among 10 children with DRESS syndrome seen at a single, Singapore referral center between 2006 and 2016, 3 cases were attributed to sulfamethoxazole/trimethoprim, 2 to carbamazepine, 1 sulfasalazine, 2 phenobarbital and 1 to levetiracetam [latency 28 days] but none to phenytoin; all had ALT elevations [88 to 1172 U/L], bilirubin was elevated in 7, but none had acute liver failure and none were fatal).
- Cano-Paniagua A, Amariles P, Angulo N, Restrepo-Garay M. Epidemiology of drug-induced liver injury in a University Hospital from Colombia: Updated RUCAM being used for prospective causality assessment. Ann Hepatol. 2019;18:501–7. [PubMed: 31053545](Among 286 patients with liver test abnormalities seen in a single hospital in Colombia over a 1 year period, 17 were diagnosed with drug induced liver injury, the most common cause being antituberculosis therapy [n=6] followed by anticonvulsants [n=3, 1 each due to phenytoin, gabapentin and valproate]).
- Wang YH, Chen CB, Tassaneeyakul W, Saito Y, Aihara M, Choon SE, Lee HY, et al. Asian Severe Cutaneous Adverse Reaction Consortium. The medication risk of Stevens-Johnson Syndrome and toxic epidermal necrolysis in Asians: the major drug causality and comparison with the US FDA label. Clin Pharmacol Ther. 2019;105:112–20. [PubMed: 29569740](Among 1028 cases of SJS/TEN reported to registries in 8 Asian countries, the most frequently implicated class of drugs was anticonvulsants including carbamazepine [26%], phenytoin [13%], lamotrigine [10%], phenobarbital [2%] and oxcarbazepine [1.7%]; non-anticonvulsant causes included allopurinol [20%] and sulfamethoxazole [8%]).
- Kim HK, Kim DY, Bae EK, Kim DW. Adverse skin reactions with antiepileptic drugs using Korea adverse event reporting system database, 2008-2017. J Korean Med Sci. 2020;35:e17. [PMC free article: PMC6995813] [PubMed: 31997613](Among 2942 reports of cutaneous drug reactions made to a Korean pharmacovigilance registry between 2008 and 2017, 241 [8%] were for severe cutaneous reactions [DRESS in 109, SJS in 106 and TEN in 25], the most common causes of which were carbamazepine [24%], lamotrigine [24%], valproate [8%], phenytoin [6%] and oxcarbazepine [5%]; no mention of accompanying ALT elevations or hepatotoxicity ).
- Low EXS, Zheng Q, Chan E, Lim SG. Drug induced liver injury: East versus West - a systematic review and meta-analysis. Clin Mol Hepatol. 2020;26:142–54. [PMC free article: PMC7160354] [PubMed: 31816676](Systematic review of surveys of drug induced liver injury comparing 16 studies from Western countries with 26,069 cases and 12 studies from Eastern countries with 33,294 cases; phenytoin was the second most common cause of liver injury in studies from the East [25 cases: 3.5%]).
- Sridharan K, Daylami AA, Ajjawi R, Ajooz HAMA. Drug-induced liver injury in critically ill children taking antiepileptic drugs: a retrospective study. Curr Ther Res Clin Exp. 2020;92:100580. [PMC free article: PMC7138958] [PubMed: 32280391](Among 41 children admitted to a pediatric intensive care unit who received anticonvulsants for seizure control, 5 of 9 receiving phenobarbital alone, 9 of 12 receiving phenytoin alone but none of six receiving valproate developed liver test abnormalities (all hepatocellular), the timing, height and outcome of which were not given).
- Vignesh V, Rameshkumar R, Mahadevan S. Comparison of phenytoin, valproate and levetiracetam in pediatric convulsive status epilepticus: a randomized double-blind controlled clinical trial. Indian Pediatr. 2020;57:222–7. [PubMed: 32198861](Among 110 children with status epilepticus not responding to intravenous benzodiazepine who were treated with intravenous infusions of phenytoin, valproate or levetiracetam, the rate of seizure control was similar in the 3 groups and adverse events were rare; no mention of liver injury or ALT elevations).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Anticonvulsant therapy for status epilepticus.[Cochrane Database Syst Rev. 2014]Review Anticonvulsant therapy for status epilepticus.Prasad M, Krishnan PR, Sequeira R, Al-Roomi K. Cochrane Database Syst Rev. 2014 Sep 10; 2014(9):CD003723. Epub 2014 Sep 10.
- Review Cutaneous and immunologic reactions to phenytoin.[J Am Acad Dermatol. 1988]Review Cutaneous and immunologic reactions to phenytoin.Silverman AK, Fairley J, Wong RC. J Am Acad Dermatol. 1988 Apr; 18(4 Pt 1):721-41.
- [Grand mal status and grand mal series--causes, therapy and course].[Anasth Intensivther Notfallmed...][Grand mal status and grand mal series--causes, therapy and course].Fröscher W, Ansmann EB. Anasth Intensivther Notfallmed. 1985 Feb; 20(1):12-8.
- Review Fosphenytoin and phenytoin in patients with status epilepticus: improved tolerability versus increased costs.[Drug Saf. 2000]Review Fosphenytoin and phenytoin in patients with status epilepticus: improved tolerability versus increased costs.DeToledo JC, Ramsay RE. Drug Saf. 2000 Jun; 22(6):459-66.
- [TREATMENT OF EPILEPSY WITH 3-ETHOXYCARBO-5,5-DIPHENYLHYDANTOIN (P-6127), A NEW ANTICONVULSANT].[No To Shinkei. 1964][TREATMENT OF EPILEPSY WITH 3-ETHOXYCARBO-5,5-DIPHENYLHYDANTOIN (P-6127), A NEW ANTICONVULSANT].KISHI Y, MANMARU S, FUKUI S, AOKI Y. No To Shinkei. 1964 Nov; 16:982-93.
- Phenytoin - LiverToxPhenytoin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...