NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsDesogestrel, Dydrogesterone, Levonorgestrel, Medroxyprogesterone, Megestrol, Norethindrone, Norgestrel, Norgestimate, Progesterone
OVERVIEW
Introduction
Progesterone is the naturally occurring hormone that is actively secreted by the ovary and interacts with progesterone receptors in the reproductive tract, mammary gland and central nervous system. Progesterone and the progestins have been used alone or in combination with estrogens in oral contraceptives, as therapy of postmenopausal symptoms, for secondary amenorrhea, abnormal uterine bleeding, endometriosis and progesterone sensitive cancers. High doses of progestins can cause liver test abnormalities and can occasionally lead to clinical apparent acute liver injury.
Background
Progesterone (proe jes' ter one) is the naturally occurring progestin which is secreted by the ovary and has a multitude of actions on many organs, but predominantly on the reproductive tract, mammary glands and central nervous system. Large amounts of progesterone are produced in women by the corpus luteum during the second half of the menstrual cycle, which inhibits the effects of estrogen on endometrial proliferation and results in a secretory status of the endometrium in preparation for implantation of a fertilized egg. If pregnancy does not occur, the corpus luteum regresses and levels of progesterone fall, triggering menstruation and resetting of the ovarian cycle. Progesterone also affects mammary glands and is required for their development and maintenance and, in the central nervous system, increases body temperature and ventilator responses. Progesterone also has androgenic and antiestrogenic effects and causes an increase in basal insulin levels, enhances fat deposition and decreases bone turnover. Modifications of the progesterone molecule can produce compounds with better absorption and pharmacokinetics and more focused and specific activities.
Progestins are compounds with biological activities similar to progesterone. Progestins developed for clinical use include desogestrel, dydrogesterone, levonorgestrel, medroxyprogesterone, megestrol, 19-nortestosterone, norethindrone, norgestrel and norgestimate, among others. Many of these progestins are used in combination with estrogens in oral contraceptives. In addition, some are used alone as contraceptive agents and to treat secondary amenorrhea, abnormal uterine bleeding, endometriosis, infertility and premature labor. Progestins have also been used to treat progesterone sensitive cancers (endometrial, renal, breast), and as therapy of anorexia and cachexia due to cancer chemotherapy or the acquired immunodeficiency syndrome (AIDS). Progestins used without estrogens include (with common brand names and year of approval in the United States): medroxyprogesterone (Provera and others: 1959), megestrol (Megase: 1971) and norethindrone (Camila, Errin, Micronor, Aygestin: 1973). Common side effects of progestin therapies include nausea, headaches, anxiety, weight gain, edema and breast tenderness and engorgement.
Hepatotoxicity
High doses of progestins can cause liver enzyme elevations that generally rise after 1 to 2 weeks of treatment and consist largely of serum aminotransferase elevations without changes in alkaline phosphatase or bilirubin. These abnormalities are generally short lived and resolve rapidly with dose modification or discontinuation. These elevations may be more frequent when progestins are administered with high doses of estrogens or tamoxifen. Isolated case reports of symptoms with serum aminotransferase elevations and even jaundice during progestin therapy have been published, but the relationship to the hormonal therapy has not always been clear. In some instances, the liver injury was attributed to a progestin releasing intrauterine device (IUD). Finally, rare instances of cholestasis with bland hepatic injury that is typical of estrogen induced liver injury have been reported with use of progestins alone. The fact that progesterone derivatives can be metabolized to estrogenic compounds makes it possible that these cases of cholestatic jaundice are actually due to estrogens rather than the progestin molecules.
Likelihood score: A (well established cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which progesterone therapy leads to liver injury is not known. Semi-synthetic progesterones may be metabolized to estrogenic compounds that might be responsible for the rare instances of cholestatic jaundice reported with progesterone only therapy.
Outcome and Management
The rare instances of liver injury reported during progestin therapy have invariably been self-limiting and have resolved completely with stopping the progesterone preparation. Clearly documented fatal cases have not been published. Cross sensitivity to estrogen therapy has not been demonstrated in patients developing jaundice during progesterone therapy, but might be expected.
References to the safety and hepatic injury associated with progesterone and the progestins are given together after this introductory section. The safety and hepatic injury due to estrogenic agents and estrogen containing birth control pills are described in the overview section on Estrogens and Oral Contraceptives.
Drug Class: Obstetrical and Gynecological Agents; Hormonal Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Medroxyprogesterone – Generic, Provera® [Oral], Depo-Provera® [Injection]
DRUG CLASS
Hormonal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Progesterone | 57-83-0 | C21-H30-O2 |
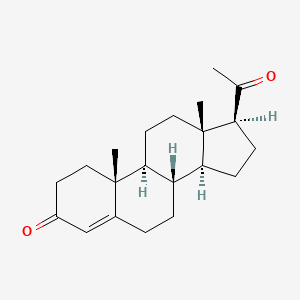
|
| Desogestrel | 54024-22-5 | C22-H30-O |
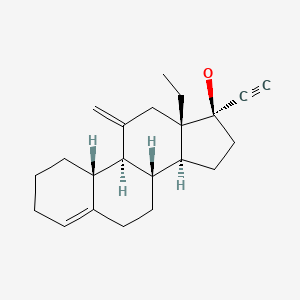
|
| Dydrogesterone | 152-62-5 | C21-H28-O2 |
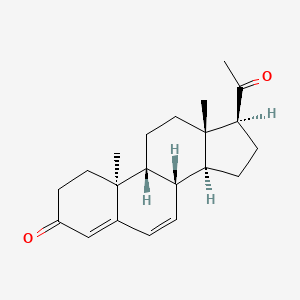
|
| Levonorgestrel | 797-63-7 | C21-H28-O2 |
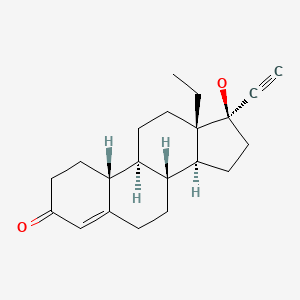
|
| Medroxyprogesterone | 520-85-4 | C22-H32-O3 |
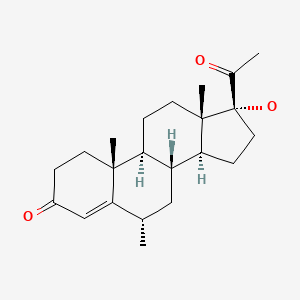
|
| Megestrol | 3562-63-8 | C22-H20-O3 C22-H30-O3 |
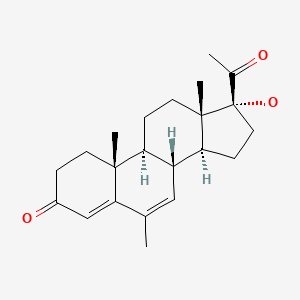
|
| Norethindrone | 68-22-4 | C20-H26-O2 |
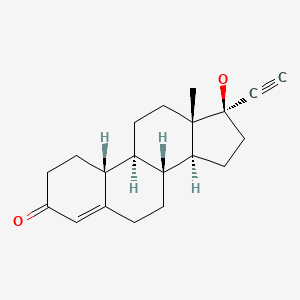
|
| Norgestrel | 6533-00-2 | C21-H28-O2 |
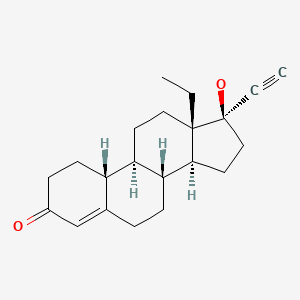
|
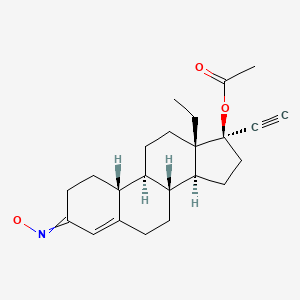
| Norgestimate | 35189-28-7 | C23-H31-N-O3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 01 June 2020
Abbreviations: OCC, oral contraceptive; IUD, intrauterine device.
- Zimmerman HJ. Hormonal derivatives and related drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 555-88.(Expert review of hepatotoxicity published in 1999; while progesterone has no demonstrable adverse effects on hepatic function, synthetic progestins have been linked to cases of cholestatic jaundice, particularly when used in higher than contraceptive doses).
- Chitturi S, Farrell GC. Adverse effects of hormones and hormone antagonists on the liver. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007, pp. 707-22.(Review of hepatotoxicity of female sex hormones published in 2007 states that progestin-only contraceptives or high dose progestin regimens used in breast cancer may rarely produce cholestasis).
- Levin ER, Vitek WS, Hammes SR. Progestins: Estrogens, progestins and the female reproductive tract. In, Brunton LL, Hilal-Dandan R, Knollman B, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 803-32.(Textbook of pharmacology and therapeutics).
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137–8. [PubMed: 13942388](35 year old woman developed jaundice 1 year after starting norethindrone and shortly after dose escalation [bilirubin 6.5 mg/dL], liver biopsy showing cholestasis, resolving after stopping).
- Routier G, Corette L, Dannas P. J Sc Med Lille. 1967;85:229–34. [Severe hepatitis and synthetic progestins] [PubMed: 5619096]
- Werner T. Z Gastroenterol. 1969;7:186–7. [Jaundice with blockage after therapy with norethisterone acetate](63 year old woman with metastatic breast cancer developed jaundice 7 weeks after starting high doses of norethisterone [bilirubin 10.4 mg/dL, ALT 33 IE, Alk P 2 times ULN], liver biopsy showing bland cholestasis, resolving slowly within 4 months of stopping).
- Adlercreutz H, Tenhunen R. Some aspects of the interaction between natural and synthetic female sex hormones and the liver. Am J Med. 1970;49:630–48. [PubMed: 4924590](Review of the hepatic effects of synthetic estrogens and progestins states that "intrahepatic cholestasis and jaundice... have been reported only for progestogens with the 19-norsteroid configuration").
- Metreau JM, Dhumeaux D, Berthelot P. Oral contraceptives and the liver. Digestion. 1972;7:318–35. [PubMed: 4602326](Review of the hepatic effects of oral contraceptives).
- Langlands AO, Martin WM. Jaundice associated with norethisterone-acetate treatment of breast cancer. Lancet. 1975;1(7906):584–5. [PubMed: 47070](Among 107 patients with advanced breast cancer treated with norethisterone, 6 developed jaundice after 6-57 weeks of treatment [bilirubin 1.8-7.6 mg/dL, ALT 192-287 U/L, Alk P 12-80 KAU], resolving after stopping; literature review suggests that jaundice arises in 2-3% of progesterone treated patients).
- Kreek MJ. Female sex steroids and cholestasis. Semin Liver Dis. 1987;7:8–23. [PubMed: 3296217](Review of cholestasis of pregnancy and estrogens: "many synthetic progestins are metabolized to estrogenic compounds").
- Meijers WH, Willemse PHB, Sleijfer D, Mulder NH, Grond J. Hepatocellular damage by cyproterone acetate. Eur J Cancer Clin Oncol. 1986;22:1121–2. [PubMed: 2946585](Among 20 women with advanced breast cancer treated with cyproterone [an antiandrogen with progestative effects], 3 developed serum enzyme elevations at least 10 times ULN [GGT and AST] after 12 weeks, which resolved rapidly upon stopping).
- Riippa P, Kauppila A. Sundströ, Vihko R. Hepatic impairment during simultaneous administration of medroxyprogesterone acetate and tamoxifen in the treatment of endometrial and ovarian carcinoma. Anticancer Res. 1984;4:109–12. [PubMed: 6235770](In a trial of the combination of tamoxifen and high doses of medroxyprogesterone, 4 of 30 patients developed marked increases in ALT [80-600 IU/L] within 1-3 months, resolving within 1-2 months of stopping without jaundice and with minimal symptoms).
- Foitl DR, Hyman G, Lefkowitch JH. Jaundice and intrahepatic cholestasis following high-dose megestrol acetate for breast cancer. Cancer. 1989;63:438–9. [PubMed: 2912522](Woman with metastatic breast cancer was treated with high doses of megestrol [800-1200 mg/day] intermittently and presented 1 month later with liver test abnormalities [bilirubin 1.0 rising to 11.2 mg/dL, ALT 160 U/L, Alk P 324 U/L], with fluctuating levels of bilirubin and fever until her death 26 days after admission).
- Hannaford PC, Kay CR, Vessey MP, Painter R, Mant J. Combined oral contraceptives and liver disease. Contraception. 1997;55:145–51. [PubMed: 9115002](Analysis of two large prospective studies of OCC use from the UK [>200,000 women-years] found low rate of liver disease and no association with OCC use; in one study, a possible increased risk for mild liver disease during first 4 years of use ).
- Altintaş E, Oğuz D, Kaçar S, Ozderin Y, Sezgin O, Zengin NI. Dydrogesterone-induced hepatitis and autoimmune hemolytic anemia. Turk J Gastroenterol. 2004;15:49–52. [PubMed: 15264122](26 year old woman had recurrent episodes of hepatitis occurring after taking dydrogesterone for 18 and 15 days [bilirubin 31 rising to 51.1 mg/dL, ALT 204 to 704 U/L, Alk P 144, ANA 1:40], resolving within 1 month of stopping).
- Anand V, Gorard DA. Norethisterone-induced cholestasis. QJM. 2005;98:232–4. [PubMed: 15728406](Two cases of cholestatic jaundice during norethisterone therapy: 18 and 34 year old women on norethisterone for 2 weeks and 2 years developed jaundice and itching [bilirubin 2.5 and 1.9 mg/dL, ALT 540 and 179, Alk P 102 and 532 U/L], with recovery after stopping, one treated with prednisone).
- Faddah LM, Al-Rehany MA, Abdel-Hamid NM, Bakeet AA. Oxidative stress, lipid profile and liver functions in average Egyptian long term depo medroxy progesterone acetate (DMPA) users. Molecules. 2005;10:1145–52. [PMC free article: PMC6147702] [PubMed: 18007380](Among 80 women on medroxyprogesterone [given by depo injection every 3 months] for 1-4 years, there were no consistent differences in mean ALT [15-20 U/L], Alk P [64-71 U/L] or bilirubin [0.49-0.54 mg/dL] levels compared to controls).
- Molinari M, Watt KD, Kruszyna T, Nelson R, Walsh M, Huang WY, Nashan B, Peltekian K. Acute liver failure induced by green tea extracts: case report and review of the literature. Liver Transpl. 2006;12:1892–5. [PubMed: 17133573](44 year old woman developed jaundice 4 months after starting green tea extract [720 mg/day] for weight loss [bilirubin 13.1 rising to 43.2 mg/dL, ALT 3583 U/L, GGT 112 U/L], undergoing liver transplantation 17 days after admission; patient was also on progesterone injections for contraception).
- Jimenez-Saenz M, Martinez-Sanchez C. Green tea extracts and acute liver failure: the need for caution in their use and diagnostic assessment. Liver Transpl. 2007;13:1067. [PubMed: 17600357](Letter in response to Molinari [2006] indicating that progesterone may have contributed to the injury and describing a 22 year old woman who developed jaundice 2 weeks after starting progesterone [bilirubin 13.5 mg/dL ALT 1584 U/L], with resolution on stopping).
- Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, Nakanishi T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–803. [PubMed: 17602085](Among 45 women with endometrial hyperplasia or carcinoma treated with medroxyprogesterone for up to 3 years, 5 developed liver enzyme elevations, but few details provided).
- Bigrigg A, Evans M, Gbolade B, Newton J, Pollard L, Szarewski A, Thomas C, et al. Depo Provera. Position paper on clinical use, effectiveness and side effects. Br J Fam Plann. 1999;25:69–76. [PubMed: 10454658](Expert review of medroxyprogesterone given im every 12 weeks as a form of progesterone only birth control claims that it is "the most effective reversible contraceptive available" and that major side effects are menstrual irregularity [70%], weight gain [in 70%, averaging 2 kg per year] and mood changes; no mention of hepatotoxicity or jaundice).
- Tuomikoski P, Aittomä K, Mikkola TS, Ropponen A, Ylikorkala O. Effect of oral and transdermal hormone therapy on hyaluronic acid in women with and without a history of intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol. 2008;198:375.e1–5. [PubMed: 18279829](20 women with and 20 without a history of cholestasis of pregnancy were treated with transdermal contraceptive estrogens and progestins; no patient developed liver test abnormalities, jaundice or itching).
- Bakry S, Merhi ZO, Scalise TJ, Mahmoud MS, Fadiel A, Naftolin F. Depot-medroxyprogesterone acetate: an update. Arch Gynecol Obstet. 2008;278:1–12. [PubMed: 18470526](Review of the chemistry, mechanism of action, clinical efficacy and safety of medroxyprogesterone states: "Very few studies have evaluated the possibility of hepatotoxicity using Depo-Provera").
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, 2 cases were attributed to an estrogen containing preparation).
- Franco Hidalgo S, Prieto de Paula JM, Salado Valdivieso I. Gastroenterol Hepatol. 2009;32:72–3. [Toxic hepatitis associated with the use of medroxyprogesterone] Spanish. [PubMed: 19174109](24 year old woman developed ALT elevations without symptoms 8 days after starting medroxyprogesterone [ALT 1062 U/L, bilirubin and Alk P not provided], which fell to normal within 3 weeks of stopping; identified 13 cases of hepatotoxicity from progesterone in French and Spanish registries, characterized mostly as aminotransferase elevations without jaundice).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, ethinylestradiol with levonorgestrel accounted for 43 cases [ranking 27th], with an adjusted odds ratio of 1.9 compared to controls).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](Among 313 cases of drug induced liver injury seen between 1997 and 2008 at a large hospital in Bangalore, India, 1 case was attributed to an OCC).
- Elouni B, Ben Salem C, Zamy M, Ganne N, Beaugrand M, Bouraoui K, Biour M. Cytolytic hepatitis possibly related to levonorgestrel/ethinylestradiol oral contraceptive use: 2 case reports. Ann Pharmacother. 2010;44:2035–7. [PubMed: 21119102](Two women, ages 28 and 29, developed marked ALT elevations with no jaundice or Alk P elevations 11 and 8 months after starting OCCs, ALT levels increasing [from 7 to 31 times ULN and from 2 to 23 times ULN] as long as OCCs were continued, and resolving within 3-4 weeks once stopped).
- Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, Secura GM. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998–2007. [PubMed: 22621627](Among 7486 women receiving reversible contraception followed for up to 5 years, there were 334 unintended pregnancies [4.55 per 100 women-years] and rates were lower with IUDs or implants than with OCC, patch or ring; adverse events were not discussed).
- Nelson A, Apter D, Hauck B, Schmelter T, Rybowski S, Rosen K, Gemzell-Danielsson K. Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstet Gynecol. 2013;122:1205–13. [PubMed: 24240244](Among 2,885 women randomized to receive one of two levonorgestrel releasing IUDs, pregnancy rates were similar [0.33 vs 0.31] as were adverse events; no mention of ALT elevations, jaundice or hepatotoxicity).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none of the 96 were attributed to estrogens or OCCs).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino RA, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Summary of 176 published cases of drug induced liver injury from 9 South American countries implicated a total of 53 drugs, 4 cases being due to estrogens, progestins or birth control pills, all of which were from Chile).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 3 [0.3%] were attributed to oral contraceptives and one to estrogen).
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD., ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92:10–6. [PubMed: 25934164](Among 1751 women given a levonorgestrel-releasing IUD the accumulative pregnancy rate was 0.55 [6 pregnancies, 4 ectopic] and adverse events most commonly resulting in discontinuation were expulsion, bleeding, acne and mood swings; no mention of ALT elevations or hepatotoxicity).
- Loulergue P, Coriat R, Mir O. Recurrent transaminitis induced by oral contraceptives during HIV infection. Ann Pharmacother. 2015;49:258–9. [PubMed: 25583940](45 year old woman with HIV infection on a successful antiretroviral regimen developed abnormal liver tests 1 month after starting OCC [levonorgestrel/ethinyl estradiol: 150/30 mcg], with normal bilirubin and Alk P but ALT 17 times ULN, resolving within a month of stopping and 2 years later developed a similar syndrome [ALT 12 times ULN] 2 weeks after starting another OCC [norgestrel/ethinyl estradiol: 500/50 mcg], resolving within 1 month of stopping).
- Choudhary NS, Bodh V, Chaudhari S, Saraf N, Saigal S. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266–8. [PMC free article: PMC5620103] [PubMed: 28970715](3 women with vaginal bleeding, ages 27, 43 and 52 years, were found to have marked serum aminotransferase elevations without symptoms or jaundice 3 weeks to 2 months after starting norethisterone [bilirubin 0.2-1.0 mg/dL, ALT 614-1122 U/L, Alk P 89-151 U/L], improving promptly with discontinuation and recurring with rechallenge in one patient).
- Cheema HI, Gupta A. Levonorgestrel-releasing intrauterine device-related acute liver injury. Case Rep Gastroenterol. 2017;11:742–7. [PMC free article: PMC5803674] [PubMed: 29430227](24 year old woman developed abdominal pain 2 months after insertion of levonorgestrel-releasing IUD [bilirubin 11.2 mg/dL, ALT 3,243 U/L, Alk P 166 U/L], which resolved within 60 days of withdrawal of the IUD).
- Choice of contraceptives. Med Lett Drugs Ther. 2018;60(1557):161–8. [PubMed: 30335731](Concise review of the efficacy and tolerance of currently available of contraceptives concludes that intrauterine devices and etonogestrel implants are the most effective reversible contraceptive methods; no discussion of hepatic adverse events).
- Stannov SU, Ries A, Bang UC. Hepatotoxicity induced by a second-generation combined oral contraceptive: case report and review of the literature. Eur J Contracept Reprod Health Care. 2019;24(4):322–4. [PubMed: 30983430](24 year old woman developed fatigue and elevated liver tests having been on ethinyl estradiol/levonorgestrel [30/150 mcg] OCC for 2 years, ALT rising from 154 U/L to ~680 U/L with continued OCC use, falling with temporary stopping and rising with restarting, finally falling to normal after discontinuation).
- Teal SB, Turok DK, Chen BA, Kimble T, Olariu AI, Creinin MD. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63–70. [PMC free article: PMC6319579] [PubMed: 30531565](Among 1751 women [ages 16 to 45 years] given levonorgestrel intrauterine device, successful placement was achieved in 1714 [98%] and the pregnancy yearly rate was 0.15 to 0.20%, while adverse events included vaginal bacterial and fungal infections, acne, nausea and vomiting, dyspareunia, headache, pelvic pain, breast tenderness, anxiety, depression, mood changes, weight gain and bleeding; no mention of ALT elevations, jaundice or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Evaluation of the carcinogenic effects of estrogens, progestins and oral contraceptives on cervix, uterus and ovary of animals and man.[Arch Toxicol Suppl. 1979]Evaluation of the carcinogenic effects of estrogens, progestins and oral contraceptives on cervix, uterus and ovary of animals and man.Drill VA. Arch Toxicol Suppl. 1979; (2):59-84.
- Review Biology of female sex hormone action in relation to contraceptive agents and neoplasia.[Contraception. 1991]Review Biology of female sex hormone action in relation to contraceptive agents and neoplasia.King RJ. Contraception. 1991 Jun; 43(6):527-42.
- Review In vivo characterization of progestins with reduced non-genomic activity in vitro.[Ernst Schering Found Symp Proc...]Review In vivo characterization of progestins with reduced non-genomic activity in vitro.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Altmann H, Fritzemeier KH. Ernst Schering Found Symp Proc. 2007; (1):151-70.
- Present status of oral contraceptives: 1. effectiveness; basis for selection; side effects; metabolic changes.[Drug Ther (NY). 1971]Present status of oral contraceptives: 1. effectiveness; basis for selection; side effects; metabolic changes.Kistner RW. Drug Ther (NY). 1971 Dec; 1(12):14-29.
- THE ORAL PROGESTATIONAL AND ANTI-OVULATORY PROPERTIES OF MEGESTROL ACETATE AND ITS THERAPEUTIC USE IN GYNAECOLOGICAL DISORDERS.[J Obstet Gynaecol Br Commonw. ...]THE ORAL PROGESTATIONAL AND ANTI-OVULATORY PROPERTIES OF MEGESTROL ACETATE AND ITS THERAPEUTIC USE IN GYNAECOLOGICAL DISORDERS.OSTERGAARD E. J Obstet Gynaecol Br Commonw. 1965 Feb; 72:45-58.
- Progestins - LiverToxProgestins - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...