NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Prochlorperazine is a phenothiazine used primarily as an antiemetic agent. In rare instances, prochlorperazine can cause clinically apparent acute and chronic cholestatic liver injury.
Background
Prochlorperazine (proe" klor per' a zeen) is a tricyclic aliphatic phenothiazine which acts by postsynaptic inhibition of dopamine receptors. Prochlorperazine has other peripheral and central nervous system effects, producing both alpha adrenergic stimulation and blocking histamine- and serotonin-mediated effects. Prochlorperazine is indicated primarily for the therapy of nausea and vomiting. Prochlorperazine also has antianxiety and antipsychotic effects, but is used less commonly for these indications compared to the major phenothiazines such as chlorpromazine, fluphenazine, perphenazine, thioridazine and trifluoperazine. Prochlorperazine was approved for use in the United States in 1956 and is still widely used in therapy of nausea and vomiting. Prochlorperazine is available in generic forms as tablets of 5, 10 and 25 mg, in long acting capsules of 15 mg, as an oral solution of 5 mg/ 5 mL, as suppositories of 2.5, 5 and 25 mg, and in parenteral forms. Prochlorperazine is also available under the brand names of Compazine and Compro. Typical doses for nausea are 5 to 10 mg three to four times daily. Common side effects are similar to other phenothiazines and include drowsiness, dizziness, headache, blurred vision, dry mouth, constipation, tremor, restlessness, muscle spasms and weight gain. Rare but potentially severe adverse events (which apply to most antipsychotic agents) include an increased risk for death in the elderly with dementia-related psychosis, neuroleptic malignant syndrome, tardive dyskinesia and orthostatic hypotension.
Hepatotoxicity
Liver test abnormalities are uncommon during prochlorperazine therapy, perhaps because it is rarely given long term or in high doses chronically. Aminotransferase elevations can occur during therapy, but they are usually mild, asymptomatic and transient and reversible even with continuation of medication. Rare instances of clinically apparent acute liver injury have been reported due to prochlorperazine which resemble the cholestatic liver injury associated with chlorpromazine. The onset of jaundice is usually within 1 to 4 weeks, and the pattern of serum enzyme elevations is typically cholestatic or mixed. Immunoallergic features (fever and eosinophilia) occur in some cases, but they are usually mild and self-limited; autoantibodies are rare. Liver biopsy typically shows a cholestatic hepatitis. Importantly, prochlorperazine jaundice can be prolonged and has been associated with rare cases of vanishing bile duct syndrome (Case 1) that can be fatal or ultimately require liver transplantation.
Likelihood score: B (uncommon but likely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which prochlorperazine causes serum aminotransferase elevations is not known but is likely shared with other phenothiazines. Several features of the clinical presentation of prochlorperazine hepatotoxicity (short latency period, fever, eosinophilia) suggest a hypersensitivity reaction, and rechallenge typically causes a rapid recurrence of injury. Prochlorperazine is extensively metabolized by the liver via sulfoxidation and oxidation, and some instances of serum aminotransferase elevations as well as more clinically apparent liver injury may be caused by production of a toxic intermediate of its metabolism.
Outcome and Management
The serum aminotransferase elevations that occur on prochlorperazine therapy are usually transient and do not require dose modification or discontinuation of therapy. The acute cholestatic hepatitis caused by prochlorperazine is typically self-limited and benign but should prompt immediate discontinuation. A small proportion of cases are followed by prolonged jaundice and cholestasis and features of vanishing bile duct syndrome. Many patients with chronic cholestasis eventually improve, but they may have persistent enzyme elevations and biliary cirrhosis. Fatalities from prochlorperazine jaundice have been reported. Rechallenge with phenothiazines usually causes a prompt recurrence of the liver injury and should be avoided.
Drug Class: Gastrointestinal Agents; Antipsychotic Agents
Other Drugs in the Subclass, Phenothiazines: Chlorpromazine, Fluphenazine, Perphenazine, Thioridazine, Trifluoperazine
CASE REPORT
Case 1. Prolonged cholestatic liver injury due to prochlorperazine.(1)
A 68 year old man was treated with trimethoprim/sulfamethoxazole (800/400 mg three times daily) for one week and prochlorperazine (10 mg daily) for 4 weeks for suspected otitis media. A few weeks after stopping prochlorperazine, he developed jaundice and pruritus. When first seen, one month after stopping medications, serum bilirubin was 18.4 mg/dL, alkaline phosphatase was 1.5 times normal and ALT was minimally elevated (Table). Tests for viral hepatitis and abdominal ultrasound were normal. After persistence of jaundice for 3 months, a liver biopsy was done which showed centrilobular cholestasis with minimal hepatocyte necrosis or portal inflammation. The intralobular bile ducts were normal. He continued to have jaundice and severe pruritus and developed skin hyperpigmentation. Tests for hepatitis B and mitochondrial antibody were negative. Endoscopic retrograde cholangiopancreatography was normal. Serum bilirubin levels peaked 4 months after presentation and then began to decline, not becoming normal until one year later. Pruritus and hyperpigmentation also resolved, but serum alkaline phosphatase and GGT levels remained elevated. A repeat liver biopsy, done two years after onset and 9 months after resolution of jaundice and symptoms, showed minimal cholestasis but bridging hepatic fibrosis and paucity of intralobular bile ducts. Two-and-a-half years after onset, serum alkaline phosphatase levels were still abnormal but he had no symptoms.
Key Points
| Medication: | Prochlorperazine (10 mg daily for 4 weeks) |
|---|---|
| Pattern: | Cholestatic |
| Severity: | 4+ (prolonged jaundice and hepatic fibrosis) |
| Latency: | 3 weeks |
| Recovery: | Incomplete after 2 years |
| Other medications: | Trimethoprim/sulfamethoxazole |
Laboratory Values
| Time After Starting | Time After Stopping | ALT* (U/L) | Alk P* (U/L) | Bilirubin* (mg/dL) | Comments |
|---|---|---|---|---|---|
| Chlorpromazine (50 mg daily) given for nausea for 4 weeks | |||||
| 1 month | 0 | 49 | 116 | 18.4 | |
| 3 months | 80 | 210 | 26.2 | Biopsy #1 | |
| 4 months | 88 | 219 | 19.3 | ERCP normal | |
| 6 months | 85 | 365 | 11.2 | ||
| 10 months | 65 | 430 | 2.6 | ||
| 1 year | 12 months | 60 | 410 | 1.5 | |
| 2 years | 22 months | 85 | 445 | 1.0 | Biopsy #2 |
| Normal Values | <42 | <90 | <1.2 | ||
- *
Some values estimated from Figure 1 and bilirubin converted from μmol/L to mg/dL.
Comment
A typical example of the evolution of an acute cholestatic hepatitis to prolonged cholestasis and vanishing bile duct syndrome. The patient eventually improved and was asymptomatic, but alkaline phosphatase levels were persistently elevated and liver biopsy showed paucity of intralobular bile ducts and bridging hepatic fibrosis. Not all cases of vanishing bile duct syndrome progress to hepatic failure and eventual clinical improvement is common. Long term follow up on such cases usually demonstrates well compensated and nonprogressive cirrhosis and persistence of mild elevations in serum alkaline phosphatase.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Prochlorperazine – Generic, Compazine®, Compro®
DRUG CLASS
Gastrointestinal Agents; Antipsychotic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Prochlorperazine | 58-38-8 | C20-H24-Cl-N3-S |
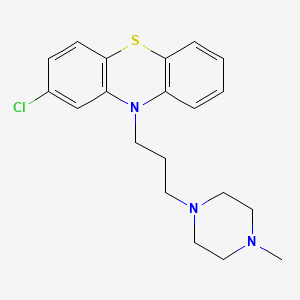
|
CITED REFERENCE
- 1.
- Lok AS, Ng IO. Prochlorperazine-induced chronic cholestasis. J Hepatol. 1988;6:369–73. [PubMed: 3392386]
ANNOTATED BIBLIOGRAPHY
References updated: 01 July 2020
- Zimmerman HJ. Neuroleptic drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 483-91.(Expert review of hepatotoxicity of neuroleptic drugs including chlorpromazine published in 1999; several hundred cases of chlorpromazine jaundice have been reported, usually cholestatic, arising after 1-5 weeks, often with fever and eosinophilia, sometimes causing vanishing bile duct syndrome; other phenothiazines have only rarely been linked to liver injury, except for prochlorperazine).
- Larry D, Ripault MP. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 447-62.(Review of phenothiazine hepatotoxicity mentions that liver enzyme elevations arise in up to 40% of patients, and hundreds of cases of chlorpromazine jaundice have been published with a frequency of 0.5-1%, onset within 2-5 weeks and usually presenting with acute cholestatic hepatitis with jaundice and pruritus and a prolonged course in 7%; other phenothiazines have been linked to liver injury similar to that of chlorpromazine, “but with a lower frequency” ).
- Meyer JM. Pharmacotherapy of psychosis and mania. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 279-302.(Textbook of pharmacology and therapeutics).
- Mechanic RC, Meyers L. Chlorpromazine-type cholangitis; report of a case occurring after the administration of prochlorperazine. N Engl J Med. 1958;259:778–80. [PubMed: 13590448](68 year old woman developed jaundice within 10 days of starting once daily prochlorperazine for nausea [peak bilirubin 26.8 mg/dL, AST 77 U/L, Alk P ~3 fold elevated], resolving slowly after stopping and undergoing laparotomy for suspected extrahepatic biliary obstruction).
- Milne HB, Berliner F. A clinical trial of Stemetil (prochlorperazine). J Ment Sci. 1958;104:873–9. [PubMed: 13588349]
- Solomon FA Jr, Campagna FA. Jaundice due to prochlorperazine (Compazine). Am J Med. 1959;27:840–3. [PubMed: 13832654](50 year old female developed jaundice 5 days after restarting prochlorperazine for nausea [bilirubin 19.2 mg/dL, Alk P ~20 times ULN]; biopsy showed intrahepatic cholestasis similar to chlorpromazine jaundice).
- Crandell A, Ma JY. Jaundice precipitated by prochlorperazine (Compazine) in the treatment of alcoholic psychiatric disturbance. J Med Soc N J. 1959;56:553–4. [PubMed: 13812634](26 year old man with alcoholism and tuberculosis developed fever and jaundice 24 days after starting a third course of prochlorperazine; serum enzyme and bilirubin levels were not provided).
- Weinstein A, Alper B, Dade J. Cholestasis due to prochlorperazine. J Am Med Assoc. 1959;170:1663–4. [PubMed: 13672755](49 year old woman developed nausea and pruritus 3 weeks after starting prochlorperazine [bilirubin 4.7 mg/dL, AST 75 U/L, Alk P ~2x ULN], biopsy showing intrahepatic cholestasis and resolving within 3-4 weeks of stopping).
- Salde H, Wallindr J. Svenska Lakartidningen. 1961;58:188–97. [Prochlorperazine for chronic psychosis] [PubMed: 13745578](Analysis of 40 patients treated with prochlorperazine for psychosis; major side effects were sedation and extrapyramidal symptoms; no jaundice reported).
- McQueen EG. Toxic effects of phenothiazine tranquillizers. N Z Med J. 1963;62:460–2. [PubMed: 14073060](Review of the phenothiazines and their side effects; “Jaundice has occurred in about 1% of patients taking chlorpromazine, and also, although less frequently, in patients taking one of the more recently developed analogues”).
- McFarland RB. Fatal drug reaction associated with prochlorperazine (Compazine). Report of a case characterized by jaundice, thrombocytopenia, and agranulocytosis. Am J Clin Pathol. 1963;40:284–90. [PubMed: 14063694](73 year old woman hospitalized for suspect myocardial infarction was given prochlorperazine and developed fever 31 days later with thrombocytopenia [bilirubin 2.8 mg/dL, AST 1480 U/L]; prochlorperazine was stopped, but she worsened and died of multiorgan failure 2 weeks later, autopsy showed centrolobular necrosis).
- Cook GC, Sherlock S. Jaundice and its relation to therapeutic agents. Lancet. 1965;1:175–9. [PubMed: 14238042](Summary of cases of drug induced liver disease seen at Royal Free Hospital between 1959-65; 11 cases of acute liver failure including 3 due to iproniazid, 2 phenelzine, 2 phenoxypropazine, 1 prochlorperazine and 3 halogenated anesthetics; 20 cases of cholestatic hepatitis including 18 due to chlorpromazine, 1 perphenazine and 1 nitrofurantoin).
- Walker CO, Combes B. Biliary cirrhosis induced by chlorpromazine. Gastroenterology. 1966;51:631–40. [PubMed: 5926937](Two patients, a 32 year old woman and a 31 year old man, developed persistent jaundice [>4 years], cholestasis and liver fibrosis 3 and 4 weeks after starting chlorpromazine; acute cholestatic hepatitis evolving into chronic form with biopsies showing cirrhosis and complications of portal hypertension).
- Case records of the Massachusetts General Hospital. Case 32-1967. N Engl J Med. 1967;277:255–62. [PubMed: 6029314](86 year old woman with complicated course after cholecystectomy resulting in multiorgan failure; autopsy showing “toxic hepatitis” and prochlorperazine considered a possible cause).
- Ishak KG, Irey NS. Hepatic injury associated with the phenothiazines. Clinicopathologic and follow-up study of 36 patients. Arch Pathol. 1972;93:283–304. [PubMed: 5017281](Review of 36 liver biopsies of phenothiazine induced hepatotoxicity from the files of the Armed Forces Institute of Pathology; 33 due to chlorpromazine, 3 prochlorperazine; mean onset 15 days, eosinophilia in 73%, mean bilirubin 12.4 mg/dL, Alk P ~8 fold elevated, ALT 146 U/L; 6 [17%] had prolonged course for 10-16 months).
- Døssing M, Andreasen PB. Drug-induced liver disease in Denmark. An analysis of 572 cases of hepatotoxicity reported to the Danish Board of Adverse Reactions to Drugs. Scand J Gastroenterol. 1982;17:205–11. [PubMed: 6982502](Among 572 cases of drug induced liver disease seen between 1968-78 in Denmark, 51 were attributed to chlorpromazine [9%, ranking 2nd behind halothane], latency averaging 14 days [range 11-46]; 5 deaths; no other phenothiazines mentioned).
- Kaplowitz N, Aw TY, Simon FR, Stolz A. Drug-induced hepatotoxicity. Ann Intern Med. 1986;104:826–39. [PubMed: 3518564](Review of drug induced hepatotoxicity including phenothiazine jaundice).
- Munyon WH, Salo R, Briones DF. Cytotoxic effects of neuroleptic drugs. Psychopharmacology (Berl). 1987;91:182–8. [PubMed: 2883697](In vitro assay for cytotoxicity of 8 neuroleptic drugs found that chlorpromazine was more toxic than haloperidol and loxapine, but similar to other phenothiazines).
- Regal RE, Bili JE, Glazer HM. Phenothiazine-induced cholestatic jaundice. Clin Pharm. 1987;6:787–94. [PubMed: 2905941](Review of phenothiazine induced liver injury; cross sensitivity is rare “but does exist”).
- Lok AS, Ng IO. Prochlorperazine-induced chronic cholestasis. J Hepatol. 1988;6:369–73. [PubMed: 3392386](68 year old man developed jaundice 4 weeks after starting prochlorperazine [peak bilirubin 26 mg/dL, ALT 50-90 U/L, Alk P 120-500 U/L], jaundice and pruritus persisting for more than a year, but then gradual clinical improvement but with persistent enzyme elevations, and biopsy 22 months after onset showed fibrosis and paucity of bile ducts: Case 1).
- Bach N, Thung SN, Schaffner F, Tobias H. Exaggerated cholestasis and hepatic fibrosis following simultaneous administration of chlorpromazine and sodium valproate. Dig Dis Sci. 1989;34:1303–7. [PubMed: 2502367](45 year old man developed fatigue and fever 12 days after starting chlorpromazine for hiccups [bilirubin 21.5 mg/dL, ALT 1312 U/L, Alk P 617 U/L], with persistent jaundice and pruritus for several years and eventual presence of cirrhosis and varices; paucity of bile ducts on biopsy).
- Pillans PI. Drug associated hepatic reactions in New Zealand: 21 years experience. N Z Med J. 1996;109:315–9. [PubMed: 8816722](Over 21 year period in New Zealand, there were 943 official reports of liver injury involving 205 drugs; chlorpromazine was in the top 20 drugs implicated accounting for 2.7% of cases; prochlorperazine was cause of 4 cases, but other phenothiazines not mentioned).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Among 126 cases of drug induced liver injury seen in Spain between 1993 and 2000, 3 were due to chlorpromazine with relative risk of 613: frequency of 261 per 100,000 person year exposures; no other phenothiazine mentioned).
- Mindikoglu AL, Anantharaju A, Hartman GG, Li SD, Villanueva J, Van Thiel DH. Prochlorperazine-induced cholestasis in a patient with alpha-1 antitrypsin deficiency. Hepatogastroenterology. 2003;50:1338–40. [PubMed: 14571732](58 year old woman 9 years after lung transplant for alpha-1-antitrypsin deficiency on multiple medications including prochlorperazine for 27 months developed jaundice and chronic allograft rejection [peak bilirubin 38.6 m/dL, ALT 71 U/L, Alk P 362 U/L]; drug stopped, but only 3 days of follow up provided).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were due to phenothiazines).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury including 4 due to psychotropic agents; one each for quetiapine, nefazodone, fluoxetine and venlafaxine, but none to phenothiazines).
- Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N., Drug-induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN prospective study. J Pediatr Gastroenterol Nutr. 2011;53:182–9. [PMC free article: PMC3634369] [PubMed: 21788760](Among 30 children with suspected drug induced liver injury, half [n=15] were due to antimicrobials [minocycline 4, INH 3, azithromycin 3] and the rest largely due to CNS agents and anticonvulsants; one case was attributed to perphenazine but none to prochlorperazine).
- Marwick KF, Taylor M, Walker SW. Antipsychotics and abnormal liver function tests: systematic review. Clin Neuropharmacol. 2012;35:244–53. [PubMed: 22986798](Systematic review of the literature found rates of any serum enzyme elevation during antipsychotic therapy to range from 5-78% and "clinically significant' elevations in 0-15%; lists 12 reports of clinically apparent liver injury due to prochlorperazine, one of which was fatal [McFarland 1963]).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, only one of which was attributed to chlorpromazine, the only antipsychotic medication listed).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 5 cases [0.6%] were attributed to antipsychotic agents, including 3 due to quetiapine and 2 to olanzapine, but none to prochlorperazine or other phenothiazines]).
- Drugs for psychotic disorders. Med Lett Drugs Ther. 2016;58(1510):160–4. [PubMed: 27960194](Concise review of medications available in the US for therapy of psychotic disorders; the phenothiazines are discussed but not prochlorperazine).
- Schreiner NM, Windham S, Barker A. Atypical neuroleptic malignant syndrome: diagnosis and proposal for an expanded treatment algorithm: a case report. A A Case Rep. 2017;9:339–43. [PubMed: 28767476](48 year old man with bipolar disorder and NASH underwent liver transplantation and developed confusion, dyskinesia, rigidity, hyperthermia and tachycardia/tachypnea postoperatively having been given lithium, lamotrigine, promethazine and ziprasidone, responding to therapy of neuroleptic malignant syndrome with benzodiazepines and propofol).
- Baeza I, de la Serna E, Calvo-Escalona R, Merchán-Naranjo J, Rodríguez-Latorre P, Martínez-Cantarero MC, Andrés P, et al. One-year prospective study of liver function tests in children and adolescents on second-generation antipsychotics: is there a link with metabolic syndrome? J Child Adolesc Psychopharmacol. 2018;28:463–73. [PubMed: 29975563](Among 216 children and adolescents starting atypical antipsychotics, mean weight gain at 6 months was 6.5 kg and mean ALT levels increased by 8.6 U/L, while among 37 taking olanzapine mean weight gain was 10.3 kg and ALT increase 2.6 U/L; increases in ALT associated with development of the metabolic syndrome, mean ALT increasing by 27.8 U/L at 6 months).
- Ouellette L, Judge B, Zamarripa A, McFadden P, Jones J. Safety and effectiveness of intravenous prochlorperazine for intractable vomiting in children with gastroenteritis. Am J Emerg Med. 2019;37:1982–3. [PubMed: 31005395](Among 390 children with acute gastroenteritis given a single infusion of prochlorperazine, 92% had relief of nausea within 3 hours and only 24 [6%] required a second dose; adverse reactions included akathisia/dystonia in 3% to 4%, no mention of ALT elevations or hepatotoxicity).
- Golikhatir I, Cheraghmakani H, Bozorgi F, Jahanian F, Sazgar M, Montazer SH. The efficacy and safety of prochlorperazine in patients with acute migraine: a systematic review and meta-analysis. Headache. 2019;59:682–700. [PubMed: 30990883](In a systematic review of 11 controlled trials in patients with migraine headaches, prochlorperazine was more effective than placebo and other comparators, and adverse events included akathisia, dystonia, drowsiness and orthostatic hypotension; no mention of ALT elevations or hepatotoxicity).
- Haldipur D, Krishnamurthy N, A B. The real-world safety and effectiveness of prochlorperazine in Indian patients with dizziness. J Assoc Physicians India. 2020;68:61–6. [PubMed: 32009365](Among 500 patients treated with prochlorperazine [5 mg three times daily for 7 days], dizziness improved in most and only three patients reported adverse events: somnolence, headache and fatigue).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Acute oculogyric crisis after administration of prochlorperazine.[South Med J. 1991]Acute oculogyric crisis after administration of prochlorperazine.Schumock GT, Martinez E. South Med J. 1991 Mar; 84(3):407-8.
- Complications following the use of prochlorperazine (compazine) as an antiemetic.[AMA J Dis Child. 1958]Complications following the use of prochlorperazine (compazine) as an antiemetic.CLEVELAND WW, SMITH GF. AMA J Dis Child. 1958 Sep; 96(3):284-7.
- Prochlorperazine as an antiemetic in the severely retarded child.[AMA J Dis Child. 1958]Prochlorperazine as an antiemetic in the severely retarded child.BERMAN HH, LAZAR M, NOE O. AMA J Dis Child. 1958 Feb; 95(2):146-9.
- Review Indomethacin/prochlorperazine/caffeine: a review of its use in the acute treatment of migraine and in the treatment of episodic tension-type headache.[CNS Drugs. 2011]Review Indomethacin/prochlorperazine/caffeine: a review of its use in the acute treatment of migraine and in the treatment of episodic tension-type headache.Hoy SM, Scott LJ. CNS Drugs. 2011 Apr; 25(4):343-58.
- Review Thioridazine.[LiverTox: Clinical and Researc...]Review Thioridazine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Prochlorperazine - LiverToxProchlorperazine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...