NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Prostacyclin is a small lipid eicosanoid molecule also known as prostaglandin I-2 (PGI-2) that is produced by endothelial cells and acts locally, inhibiting platelet activation and causing vasodilation. Analogs of prostacyclin have been developed as therapies of pulmonary arterial hypertension (PAH).
Background
Prostacyclin is synthesized in endothelial cells and released in response to local factors such as inflammation and injury. Prostacyclin acts locally on receptors present on endothelial cells and platelets activating adenyl cyclase and thereby increasing intracellular cyclic adenine monophosphate (cAMP), a signaling molecule that activates multiple downstream pathways. In platelets, cAMP inhibits their activation, thus limiting the formation of platelet clots and decreasing hemostasis. In endothelial cells, intracellular cAMP activate pathways to induce smooth muscle relaxation and vasodilation. Prostacyclin is a paracrine signaling molecule, acting locally and with a very short half-life. When used as a drug, it is referred to as epoprostenol which is a potent vasodilator, particularly of the pulmonary vascular bed. Because of its short half-life and relative instability, epoprostenol requires continuous intravenous infusion and special handling. Several analogs of prostacyclin have been produced that have a longer half-life and greater stability, resulting in more favorable pharmacokinetics and bioavailability allowing administration by subcutaneous and oral routes or by inhalation. Prostacyclin analogs in clinical use in the United States include epoprostenol, iloprost and treprostinil. All three are used to treat pulmonary arterial hypertension (PAH), a severe and progressive disease marked by vasoconstriction and smooth muscle cell proliferation of the pulmonary arterioles. Importantly, PAH is associated with a relative increase in mediators of vasoconstriction (endothelin-1, thromboxane A) and a relative decrease in mediators of vasodilation (prostacyclin, nitric oxide). Modern therapy of PAH often combines antagonists of vasoconstrictors with agonists of the vasodilatory mediators. All three of the vasodilatory prostacyclin analogs require careful and monitored dose adjustments and should be administered only by physicians and health care providers trained and experienced in the management of PAH. None of the prostacyclin analogs have been convincingly linked to instances of clinically significant acute liver injury.
Epoprostenol (e" poe pros' ten nol) is the pharmaceutical name for prostacyclin, a naturally occurring metabolite of arachidonic acid that causes pulmonary arterial vasodilation and inhibits platelet activation. Epoprostenol must be given parenterally and has a short half-life (~6 minutes), for which reasons it is administered as a continuous intravenous infusion. In multiple clinical trials in patients with PAH, prolonged epoprostenol infusions led to an increase in exercise capacity (6 minute walk distance), improvement in symptoms and delay in clinical progression in patients with idiopathic and inheritable forms of PAH (WHO Group 1) and connective tissue disease-associated PAH. Epoprostenol was approved for use in the United States in 1995 and remains available generically and under the brand name Flolan. Epoprostenol is provided as a sterile powder in single use vials of 0.5 or 1.5 mg. The typical dose is 2 ng per kg body weight per minute administered via an implanted central venous catheter. The dose can be increased in increments of 1 to 2 ng/kg/min based upon clinical response and tolerance. Common side effects include dizziness, jaw pain, headache, muscle pain, gastrointestinal upset, nausea and vomiting, effects commonly associated with vasodilatory therapies. Uncommon, but potentially severe adverse events include pulmonary edema, hypotension, syncope, and bleeding. Sudden discontinuation can lead to rebound pulmonary hypertension. Side effects of the intravenous catheter required for administration include local pain, swelling and infection at the site of injection and catheter-associated bacterial infections that can be severe and result in fatality. Epoprostenol has not been associated with serum enzyme elevations above the rate in control subjects and has not been linked to instances of clinically apparent acute liver injury.
Likelihood score: E (unlikely to cause clinically apparent liver injury).
Iloprost (eye' loe prost) is a synthetic prostacyclin analog that is more stable and has a longer half-life than epoprostenol and can be given by inhalation. Chronic inhalation therapy using iloprost has been shown to improve symptoms, exercise capacity and pulmonary function in patients with PAH, WHO type 1. Iloprost was approved for use in the United States in 2004 and is usually given in combination with other agents for PAH. Iloprost is available in ampules of 10 or 20 µg/mL under the brand name Ventavis and is administered via a nebulizer in doses of 2.5 to 5.0 µg, 6 to 9 times daily. Because of its short half-life, iloprost must be administered frequently (minimum of 2 hours between doses), a requirement that limits its acceptability. Common side effects include flushing, cough, headache, dizziness, insomnia, palpitations, jaw pain, muscle pain, palpitations, nausea and vomiting. Uncommon, but potentially severe adverse events include hypotension, hemoptysis, pulmonary edema, bronchospasm and pneumonia. Iloprost has not been associated with serum enzyme elevations above the rate in control subjects and has not been linked to instances of clinically apparent acute liver injury.
Likelihood score: E (unlikely to cause clinically apparent liver injury).
Treprostinil (tre pros’ ti nil) is a tricyclic benzidine analog of prostacyclin that can be administered orally, by inhalation, subcutaneously or by intravenous infusion. It has a longer half-life (3 to 4 hours) than epoprostenol and iloprost and is stable at room temperature and neutral pH, making it more convenient to administer when given parenterally. Intravenous and subcutaneous forms of treprostinil have been found to be better accepted than other intravenously administered prostacyclin analogs and to have similar long term efficacy. Treprostinil was initially approved for use in PAH (WHO Group 1) in the United States in 2002 and is available in solution for injection in vials of 1.0, 2.5, 5.0 and 10.0 mg/mL under the brand name Remodulin, the typical dose being 1.25 to 2.5 ng/kg per minute. Treprostinil is also available as a solution for inhalation under the brand name Tyvaso in 2.9 mL ampules with 1.74 mg/ampule, the usual dose being 3 to 9 breaths four times daily. Finally, oral treprostinil tablets of 0.125, 0.25, 1 and 2.5 mg are available under the brand name Orenitram, the initial dose being 0.125 three times daily or 0.25 twice daily, with careful adjustment of dose thereafter based upon tolerance. Side effects of treprostinil are similar to those of iloprost and epoprostenol and include headache, diarrhea, nausea and vomiting, flushing, dizziness, paresthesia, muscle and jaw pain. Sudden withdrawal of therapy can cause rebound worsening of pulmonary hypertension. Inhalation therapy can cause cough. Chronic intravenous therapy with treprostinil is associated with a high rate of infusion site pain and erythema and with catheter-associated bacterial infections that can be severe and have resulted in death in rare instances. The oral form of treprostinil has limited efficacy and is considered a second line therapy of PAH generally given with other oral agents for PAH or to replace intravenous or subcutaneous treprostinil in patients with poor tolerance of the parenteral therapies. Treprostinil therapy has not been associated with serum enzyme elevations above the rate in control subjects and has not been linked to instances of clinically apparent acute liver injury.
Likelihood score: E (unlikely to cause clinically apparent liver injury).
Mechanism of Injury
The mechanism by which prostacyclin analogs might cause liver injury is not known. All three are eicosanoid molecules that have a relatively rapid half-life and are metabolized locally. Furthermore, the prostacyclin analogs are typically administered in low daily doses (<1 mg), doses at which direct and even idiosyncratic liver injury are rare. The prostacyclin analogs have little or no effect on cytochrome P450 enzymes and are not prone to causing drug-drug interactions. On the other hand, they are often administered with other drugs used to treat PAH such as the endothelin-1 receptor antagonists (ambrisentan, bosentan, macitentan), guanylate cyclase simulators (riociguat) or prostacyclin receptor agonists (selexipag), some of which have been linked to idiosyncratic, symptomatic acute liver injury.
Drug Class: Pulmonary Arterial Hypertension Agents
Other Drugs in the Class: Ambrisentan, Bosentan, Macitentan, Riociguat, Selexipag
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Epoprostenol – Generic, Flolan®, Veletri®
Iloprost – Ventavis®
Treprostinil – Orenitram®, Remodulin®, Tyvaso®
DRUG CLASS
Pulmonary Arterial Hypertension Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Epoprostenol [Prostacyclin] | 35121-78-9 | C20-H32-O5 |
![Epoprostenol [Prostacyclin] chemical structure](/books/NBK548146/bin/Epoprostenol_structure.jpg) |
| Iloprost | 78919-13-8 | C22-H32-O4 |
 |
| Treprostinil | 81846-19-7 | C23-H34-O5 |
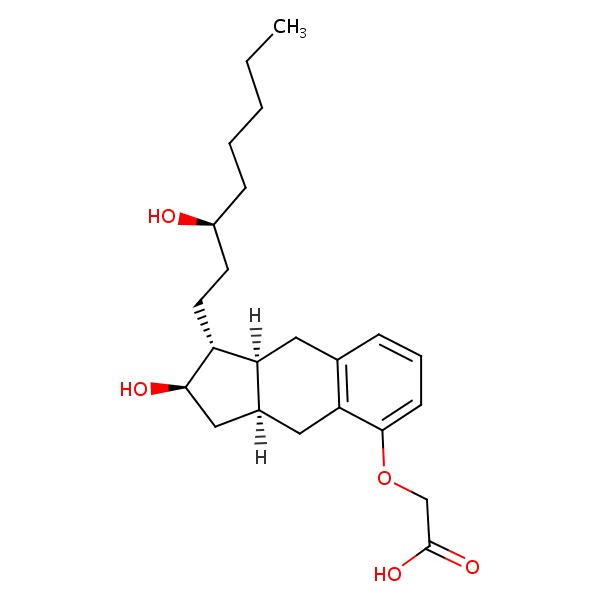 |
ANNOTATED BIBLIOGRAPHY
References updated: 25 November 2016
Abbreviations used: PAH, pulmonary arterial hypertension; sc, subcutaneous; iv, intravenous.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Textbook of hepatotoxicity published in 1999; does not discuss epoprostenol, iloprost or treprostinil).
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Textbook on drug induced liver injury; does not discuss the prostacyclin analogs).
- Barnes PJ. Pharmacotherapy of pulmonary arterial hypertension. Pulmonary Pharmacology. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1059-61.(Textbook of pharmacology and therapeutics).
- Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, et al.; Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996; 334: 296-301. [PubMed: 8532025](Among 81 patients with pulmonary arterial hypertension [PAH] treated with continuous infusions of epoprostenol vs conventional therapy for 12 weeks found improvements in exercise capacity and pulmonary hemodynamics; side effects included nausea, headache, dizziness, jaw pain, diarrhea and flushing and 4 cases of catheter sepsis, none fatal; no mention of enzyme elevations or hepatotoxicity).
- Epoprostenol for primary pulmonary hypertension. Med Lett Drugs Ther 1996; 38 (968): 14-5. [PubMed: 8592477](Concise summary of the mechanism of action, clinical efficacy, side effects and costs of epoprostenol shortly after its approval in the US, does not mention ALT elevations or hepatotoxicity).
- Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, Darius H, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST). Am Heart J 1997; 134: 44-54. [PubMed: 9266782](Among 471 patients with congestive heart failure treated with intravenous (iv) epoprostenol or standard care for up to 1.5 years, hemodynamics improved but not symptoms, exercise capacity or clinical status and patients treated with epoprostenol had an excess in early mortality largely due to worsening cardiac status; no mention of serum enzyme elevations or hepatotoxicity).
- Hoeper MM, Schwarze M, Ehlerding S, Adler-Schuermeyer A, Spiekerkoetter E, Niedermeyer J, Hamm M, et al. Long-term treatment of primary pulmonary hypertension with aerosolized iloprost, a prostacyclin analogue. N Engl J Med 2000; 342: 1866-70. [PubMed: 10861321](Among 24 patients with PAH treated with aerosolized iloprost for 1 year, exercise capacity and hemodynamics improved, while side effects tended to be mild and liver tests “remained stable in all patients”).
- Treprostinil (Remodulin) for pulmonary arterial hypertension. Med Lett Drugs Ther 2002; 44: 80-2. [PubMed: 12237619](Concise review of the mechanism of action, efficacy, safety and cost of subcutaneous (sc) treprostinil shortly after its approval for use in PAH in the US; mentions side effects of infusion site reactions, pump complications as well as diarrhea, jaw pain, flushing, edema and gastrointestinal hemorrhage, but does not mention hepatotoxicity).
- Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, Keogh A, et al.; Treprostinil Study Group. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002; 165: 800-4. [PubMed: 11897647](Among 470 patients with PAH treated with continuous sc treprostinil or placebo for 12 weeks, exercise capacity, symptoms and hemodynamics improved with treprostinil while adverse events included infusion site pain [85% vs 27%], headache, diarrhea, nausea, jaw pain and edema and 3 patients had gastrointestinal bleeding; no mention of ALT elevations or hepatotoxicity).
- Olschewski H, Simonneau G, Galiè N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, et al.; Aerosolized Iloprost Randomized Study Group. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002; 347: 322-9. [PubMed: 12151469](Among 203 patients with PAH treated with inhaled iloprost [6-9 times daily] or placebo for at least 12 weeks, side effects included cough, headache, flushing and jaw pain, but serum enzyme elevations and hepatotoxicity were not mentioned).
- McLaughlin VV, Gaine SP, Barst RJ, Oudiz RJ, Bourge RC, Frost A, Robbins IM, et al.; Treprostinil Study Group. Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. J Cardiovasc Pharmacol 2003; 41: 293-9. [PubMed: 12548091](In 3 trials of iv or sc treprostinil in comparison to iv epoprostenol or placebo in a total of 65 patients with PAH, both iv and sc treprostinil had similar efficacy to epoprostenol and was superior to placebo in improving exercise capacity and hemodynamics, while side effects included infusion site pain, erythema or induration in most patients receiving treprostinil [88% to 94%]; no mention of ALT elevations or hepatotoxicity).
- Leuchte HH, Behr J. Iloprost for idiopathic pulmonary arterial hypertension. Expert Rev Cardiovasc Ther 2005; 3: 215-23. [PubMed: 15853595](Review of the structure, mechanism of action, acute hemodynamic effects, clinical efficacy and safety of aerosolized iloprost as therapy of PAH does not mention serum enzyme elevations or hepatotoxicity).
- Gomberg-Maitland M, Preston IR. Prostacyclin therapy for pulmonary arterial hypertension: new directions. Semin Respir Crit Care Med 2005; 26: 394-401. [PubMed: 16121316](Review of prostacyclin analog therapy of PAH including iv epoprostenol, iv and sc treprostinil and inhaled iloprost; no mention of serum enzyme elevations during therapy or hepatotoxicity).
- Barst RJ, Galie N, Naeije R, Simonneau G, Jeffs R, Arneson C, Rubin LJ. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J 2006; 28: 1195-203. [PubMed: 16899485](Among 860 patients with PAH treated with sc treprostinil for up to 4 years, survival was 68-87%, much higher than the predicted rates of 38-69% and side effects were similar to those reported in short term trials; “all mean chemistry…values were within normal ranges throughout the 4 years of treatment”).
- McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, Badesch DB, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006; 174: 1257-63. [PubMed: 16946127](Among 67 patients with PAH on bosentan who were treated with inhaled iloprost or placebo for 12 weeks, iloprost improved exercise capacity and delayed time to clinical worsening and “no patient had significant liver function tests elevations”).
- Voswinckel R, Enke B, Reichenberger F, Kohstall M, Kreckel A, Krick S, Gall H, et al. Favorable effects of inhaled treprostinil in severe pulmonary hypertension: results from randomized controlled pilot studies. J Am Coll Cardiol 2006; 48: 1672-81. [PubMed: 17045906](Among 123 patients with PAH enrolled in 3 studies of inhaled treprostinil, pulmonary vascular resistance declined in each and tolerability was considered “excellent”).
- Lang I, Gomez-Sanchez M, Kneussl M, Naeije R, Escribano P, Skoro-Sajer N, Vachiery JL. Efficacy of long-term subcutaneous treprostinil sodium therapy in pulmonary hypertension. Chest 2006; 129: 1636-43. [PubMed: 16778286](Among 122 patients with PAH treated with sc treprostinil for up to 3 years, symptom relief was maintained and side effects included injection site pain; no mention of serum enzyme elevations or hepatotoxicity).
- Tapson VF, Gomberg-Maitland M, McLaughlin VV, Benza RL, Widlitz AC, Krichman A, Barst RJ. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trial. Chest 2006; 129: 683-8. [PubMed: 16537868](Among 16 patients with PAH treated with iv treprostinil for 12 weeks, exercise capacity and hemodynamics improved and side effects included jaw and extremity pain, nausea, headache, dizziness, diarrhea and flushing; no mention of serum enzyme elevations or hepatotoxicity).
- Centers for Disease Control and Prevention (CDC). Bloodstream infections among patients treated with intravenous epoprostenol or intravenous treprostinil for pulmonary arterial hypertension--seven sites, United States, 2003-2006. MMWR Morb Mortal Wkly Rep 2007; 56: 170-2. [PubMed: 17332729](In a retrospective review of bloodstream infections at 7 centers in the US between 2003 and 2006, 144 cases [including 2 deaths] were identified in patients with PAH receiving infusions of prostacyclin analogs, rates being higher for treprostinil than epoprostenol).
- Benza RL, Rayburn BK, Tallaj JA, Pamboukian SV, Bourge RC. Treprostinil-based therapy in the treatment of moderate-to-severe pulmonary arterial hypertension: long-term efficacy and combination with bosentan. Chest 2008; 134: 139-45. [PubMed: 18403673](Among 38 patients with PAH treated with sc infusions of treprostinil for up to 5 years, exercise capacity and hemodynamics were maintained and bosentan could be added “without serious adverse events”).
- Walsh M, Hanna BD. A pediatric case study of treprostinil overdose. J Heart Lung Transplant 2009; 28: 297-8. [PubMed: 19285625](A 10 year old girl with PAH mistakenly received an overdose of iv treprostinil with immediate symptoms of tingling, blurred vision and poor motor control with rapid improvement on stopping the infusion and no apparent long lasting effects; no mention of liver injury or results of serum enzymes).
- Olschewski H. Inhaled iloprost for the treatment of pulmonary hypertension. Eur Respir Rev 2009; 18: 29-34. [PubMed: 20956120](Review of the clinical efficacy and safety of inhaled iloprost in PAH mentions that side effects are generally mild and similar to those with other prostacyclin analogs).
- Olschewski H, Hoeper MM, Behr J, Ewert R, Meyer A, Borst MM, Winkler J, et al. Long-term therapy with inhaled iloprost in patients with pulmonary hypertension. Respir Med 2010; 104: 731-40. [PubMed: 20153158](Among 63 patients with PAH treated with inhaled iloprost [6-9 times daily] for up to 2 years, most [95%] had at least one adverse event, most commonly cough, headache, infection, dizziness, nausea, edema and throat pain, but there were no hepatic serious adverse effects or drug discontinuations for liver related events).
- Kingman MS, Tankersley MA, Lombardi S, Spence S, Torres F, Chin KS; Prostacyclin Safety Group. Prostacyclin administration errors in pulmonary arterial hypertension patients admitted to hospitals in the United States: a national survey. J Heart Lung Transplant 2010; 29: 841-6. [PMC free article: PMC5592093] [PubMed: 20430649](Surveys of physicians and nurses specializing in PAH care found that prostacyclin pump or dosing errors in epoprostenol and treprostinil administration were common and potentially serious, and were thought to have contributed to 9 patient deaths).
- Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, Badesch DB, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest 2012; 142: 1383-90. [PubMed: 22628490](Among 350 patients with PAH on stable therapy with an endothelin receptor antagonist or phosphodiesterase 5 inhibitor who were treated with oral treprostinil or placebo for 16 weeks, symptoms and exercise capacity improved with treprostinil and side effects included headache, nausea, diarrhea, flushing, jaw pain and myalgia, but there were no changes in “laboratory parameters” and no discontinuations for liver related events).
- de Jesus Perez VA, Rosenzweig E, Rubin LJ, Poch D, Bajwa A, Park M, Jain M, et al. Safety and efficacy of transition from systemic prostanoids to inhaled treprostinil in pulmonary arterial hypertension. Am J Cardiol 2012; 110: 1546-50. [PubMed: 22853986](Among 18 patients with PAH who were switched from parenteral to inhaled treprostinil, most had no worsening of symptoms and side effects improved).
- Kitterman N, Poms A, Miller DP, Lombardi S, Farber HW, Barst RJ. Bloodstream infections in patients with pulmonary arterial hypertension treated with intravenous prostanoids: insights from the REVEAL REGISTRY®. Mayo Clin Proc 2012; 87: 825-34. [PMC free article: PMC3498408] [PubMed: 22883740](Analysis of data from a prospective registry on PAH therapy identified 166 episodes of bloodstream infection in 123 patients among 1146 who were receiving iv prostacyclin analog therapy, use of treprostinil being associated with a 3-fold increased risk).
- Sadushi-Kolici R, Skoro-Sajer N, Zimmer D, Bonderman D, Schemper M, Klepetko W, Glatz J, et al. Long-term treatment, tolerability, and survival with sub-cutaneous treprostinil for severe pulmonary hypertension. J Heart Lung Transplant 2012; 31: 735-43. [PubMed: 22480725](Among 111 patients with PAH treated with sc treprostinil for up to 10 years, 12% stopped because of adverse effects including infusion site reactions and pain and diarrhea; no mention of serum enzyme elevations or hepatotoxicity).
- Tapson VF, Jing ZC, Xu KF, Pan L, Feldman J, Kiely DG, Kotlyar E, et al.; FREEDOM-C2 Study Team. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest 2013; 144: 952-8. [PubMed: 23669822](Among 310 patients with PAH on stable therapy with oral agents who were treated with oral treprostinil or placebo, exercise tolerance and symptoms did not improve with treprostinil, while side effects included headache [71%], diarrhea [55%], nausea [46%], flushing [35%], and jaw pain [25%]; there were no liver related serious adverse events).
- Sammut D, Elliot CA, Kiely DG, Armstrong IJ, Martin L, Wilkinson J, Sephton P, Jones J, Hamilton N, Hurdman J, McLellan E, Sabroe I, Condliffe R. Central venous catheter-related blood stream infections in patients receiving intravenous iloprost for pulmonary hypertension. Eur J Clin Microbiol Infect Dis 2013; 32: 883-9. [PubMed: 23388830](Among 59 patients with PAH receiving iv iloprost over a 5 year period, 15 episodes of catheter related blood stream infections occurred in 11 patients for a rate of 0.65 per 1000 treatment days; there were no deaths).
- Jing ZC, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, Torbicki A, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 2013; 127: 624-33. [PubMed: 23307827](Among 349 patients with PAH on no therapy who were treated with oral treprostinil or placebo for 12 weeks, exercise capacity improved, but there were no changes in symptoms or clinical status and side effects were common; there were no liver related serious adverse events or deaths).
- Chin KM, Badesch DB, Robbins IM, Tapson VF, Palevsky HI, Kim NH, Kawut SM, et al. Two formulations of epoprostenol sodium in the treatment of pulmonary arterial hypertension: EPITOME-1 (epoprostenol for injection in pulmonary arterial hypertension), a phase IV, open-label, randomized study. Am Heart J 2014; 167: 218-225.e1. [PubMed: 24439983](Among 30 patients with PAH treated with 1 of 2 formulations [arginine- vs glycine-mannitol excipients] of iv epoprostenol for 28 days, exercise capacity and hemodynamics improved similarly in both groups and side effect rates were not different).
- Hill NS, Badesch D, Benza RL, D'Eletto TA, Farber HW, Gomberg-Maitland M, Hassoun PM, et al. Perspectives on oral pulmonary hypertension therapies recently approved by the U.S. Food and Drug Administration. Ann Am Thorac Soc 2015; 12: 269-73. [PubMed: 25590376](Review of recently approved drugs for PAH including macitentan, riociguat and oral treprostinil mentions that dosing of oral treprostinil is complex and can result in suboptimal therapeutic levels, making the oral form of treprostinil not recommended as a first line agent for PAH).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one was attributed to bosentan, but none to other agents used primarily to treat pulmonary artery hypertension).
- Das A, Shabbir A, Sehgal S, Highland KB. Cutaneous hypersensitivity and eosinophilia associated with treprostinil. Pulm Pharmacol Ther 2015; 35: 17-8. [PubMed: 26407925](59 year old woman with scleroderma and PAH on sildenafil and macitentan was treated with iv followed by oral treprostinil for 3 months when she developed rash and eosinophilia [22%], that did not improve on switching back to the iv formulation, but resolved promptly when treprostinil was stopped; no mention of systemic signs or liver test abnormalities).
- Bourge RC, Waxman AB, Gomberg-Maitland M, Shapiro SM, Tarver JH 3rd, Zwicke DL, Feldman JP, et al. Treprostinil administered to treat pulmonary arterial hypertension using a fully implantable programmable intravascular delivery system: results of the DelIVery for PAH Trial. Chest 2016; 150: 27-34. [PubMed: 27396777](Among 60 patients with severe PAH treated with iv treprostinil using a fully implantable deliver device for 12 months, 6 catheter complications arose, but no bloodstream infections or thromboses; no mention of serum enzyme elevations or hepatotoxicity).
- O'Connell C, Amar D, Boucly A, Savale L, Jaïs X, Chaumais MC, Montani D, et al. Comparative safety and tolerability of prostacyclins in pulmonary hypertension. Drug Saf 2016; 39: 287-94. [PubMed: 26748508](Review of the clinical efficacy and safety of the prostacyclin analogs used to treat PAH including iloprost, epoprostenol and treprostinil; no mention of serum enzyme elevations occurring during therapy or hepatotoxicity).
- Parikh KS, Rajagopal S, Fortin T, Tapson VF, Poms AD. Safety and tolerability of high-dose inhaled treprostinil in pulmonary hypertension. J Cardiovasc Pharmacol 2016; 67: 322-5. PubMed Citation. [PMC free article: PMC4824653] [PubMed: 26828324](Among 80 patients treated with inhaled treprostinil [>9 breaths, 4 times daily] for an average of 20 months, chronic side effects included cough, headache, flushing, nausea, throat irritation, jaw pain, diarrhea, dizziness, syncope and hypotension; no mention of serum enzyme elevations or hepatotoxicity).
- Grünig E, Benjamin N, Lange TJ, Krueger U, Klose H, Neurohr C, Wilkens H, et al. Safety, tolerability and clinical effects of a rapid dose titration of subcutaneous treprostinil therapy in pulmonary arterial hypertension: a prospective multi-centre trial. Respiration 2016; 92: 362-70. [PubMed: 27784026](Among 39 patients with PAH on stable doses of oral therapies, addition of sc treprostinil in a rapid dose titration was associated with improved exercise capacity and hemodynamics and “a good overall safety profile”, although all patients had adverse events including infusion site pain in 97%, diarrhea in 77% and headache in 69%; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Prostanoid therapies in the management of pulmonary arterial hypertension.[Ther Clin Risk Manag. 2015]Review Prostanoid therapies in the management of pulmonary arterial hypertension.LeVarge BL. Ther Clin Risk Manag. 2015; 11:535-47. Epub 2015 Mar 31.
- Review The prostacyclin pathway in pulmonary arterial hypertension: a clinical review.[Expert Rev Respir Med. 2017]Review The prostacyclin pathway in pulmonary arterial hypertension: a clinical review.Del Pozo R, Hernandez Gonzalez I, Escribano-Subias P. Expert Rev Respir Med. 2017 Jun; 11(6):491-503. Epub 2017 Apr 24.
- Review Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn.[Pharmacol Rev. 2012]Review Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn.Majed BH, Khalil RA. Pharmacol Rev. 2012 Jul; 64(3):540-82. Epub 2012 Jun 7.
- Review Prostacyclin among prostanoids.[Pharmacol Rep. 2008]Review Prostacyclin among prostanoids.Gryglewski RJ. Pharmacol Rep. 2008 Jan-Feb; 60(1):3-11.
- Review Prostaglandin and prostaglandin receptors: present and future promising therapeutic targets for pulmonary arterial hypertension.[Respir Res. 2023]Review Prostaglandin and prostaglandin receptors: present and future promising therapeutic targets for pulmonary arterial hypertension.Zeng C, Liu J, Zheng X, Hu X, He Y. Respir Res. 2023 Nov 1; 24(1):263. Epub 2023 Nov 1.
- Prostacyclin Analogs - LiverToxProstacyclin Analogs - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...