NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Propylthiouracil is an antithyroid medication used in the therapy of hyperthyroidism and Graves disease. Propylthiouracil has been linked to serum aminotransferase elevations during therapy as well as to a clinically apparent, idiosyncratic liver injury that can be severe and even fatal.
Background
Propylthiouracil (proe" pil thye" oh ure' a sil) is a thioamide and a thyroid hormone antagonist which acts by inhibiting the incorporation of iodine into tyrosyl residues of thyroglobulin and thus lowering thyroid hormone levels. Propylthiouracil may also partially inhibit the peripheral deiodination of thyroxine [T4] to the active form [T3] and, in addition, may have immunosuppressive activities. Propylthiouracil was introduced into use in the 1940s and is still used for the temporary amelioration of hyperthyroidism in Graves disease in patients with mild or self-limited hyperthyroidism. However, because of the problems with hepatotoxicity including fatal cases of hepatitis, propylthiouracil is now considered a second line drug for hyperthyroidism, methimazole being preferred. Thus, propylthiouracil is currently used largely in patients who are intolerant to methimazole in preparation of definitive therapy or who wish to avoid thyroidectomy or radioactive iodine therapy. Propylthiouracil is also the preferred agent for medical therapy of hyperthyroidism in pregnancy, during the last trimester. Generic forms of propylthiouracil are available in 50 mg tablets. The usual dose in adults is 300 to 450 mg daily in divided doses until the patient is euthyroid, followed by a maintenance dose of 100 to 150 mg daily. Common side effects include gastrointestinal upset and rash. Rare complications of propylthiouracil (<1%) include agranulocytosis, aplastic anemia, nephritis and hepatitis.
Hepatotoxicity
Propylthiouracil has been associated with transient, asymptomatic elevations in serum aminotransferase levels, typically during the first 3 months of therapy. These elevations are rarely clinically significant and usually resolve even with continuation of therapy. Propylthiouracil is also capable of causing clinically apparent, idiosyncratic liver injury at a rate estimated to be at least 1 per 1000 persons exposed. The onset of hepatotoxicity is usually within 2 to 12 weeks of starting, and the pattern of enzyme elevations is typically hepatocellular, although cholestatic and mixed patterns have also been described (Cases 1 to 3). In some cases, onset is months or more than a year after initiating therapy, sometimes shortly after dose modification. Rash, fever and eosinophilia are not uncommon, but are rarely prominent or severe. Some cases of propylthiouracil hepatotoxicity present with autoimmune features including antinuclear antibody and hyperglobulinemia, perhaps because of the background of autoimmune thyroid disease. While most patients start to improve with stopping therapy, many cases are severe and some have been associated with acute liver failure with death or need for emergency liver transplantation (Case 4). Liver injury from propylthiouracil appears to be more common and perhaps more severe in children and adolescents than in adults. Rates of acute liver failure due to propylthiouracil are estimated to be 1:10,000 in adults and as high as 1:2000 in children.
Complicating the assessment of the role of methimazole or propylthiouracil in causing liver injury is the fact that hyperthyroidism by itself can cause liver test abnormalities and even jaundice. Indeed, more than half of patients with untreated hyperthyroidism have serum enzyme abnormalities (usually less than 5 times the upper limit of the normal range) and a small proportion are jaundiced and present with cholestatic hepatitis. The liver test elevations are most frequent in patients with high output heart failure. The abnormalities resolve rapidly with treatment of hyperthyroidism either with surgery, radioactive iodine or antithyroid medications.
Likelihood score: A (well known cause of clinically apparent liver injury including acute liver failure)
Mechanism of Injury
The mechanism by which propylthiouracil causes acute liver injury is unknown, but is likely due to an immunological reaction to a metabolic product of its metabolism. In several instances, positive results of lymphocyte stimulation to propylthiouracil have been reported. Complicating factors in assessing liver injury due to antithyroid medications is that hyperthyroidism itself can be associated with serum enzyme elevations and mild jaundice.
Outcome and Management
The severity of propylthiouracil induced liver injury varies from mild, transient serum aminotransferase elevations to severe hepatitis, hepatic failure and death or need for liver transplantation. Fatal cases of hepatocellular injury arising months after starting therapy have been described, many in children. Some cases have features of autoimmunity or immunoallergic hepatitis and have been treated with corticosteroids, but without proven evidence of benefit. In most instances, however, recovery is rapid once propylthiouracil is stopped and the first priority should be immediate discontinuation of antithyroid therapy at the first sign of clinically apparent liver disease. The presence of hyperthyroidism may play a role in worsening liver function and temporary management with beta-blockers or other approaches may be necessary, even during the course of the acute liver injury. In some instances, patients with propylthiouracil induced liver injury have been switched to methimazole without evidence of recurrence. In severe cases, however, more definitive therapy of the hyperthyroidism with radioactive iodine or surgery is more appropriate.
Drug Class: Antithyroid Agents
Other Drugs in the Class: Methimazole
CASE REPORTS
Case 1. Mixed cholestatic-hepatocellular injury due to propylthiouracil.(1)
A 29 year old woman with Graves disease developed itching 4 weeks after starting propylthiouracil. On admission two weeks later, she was jaundiced with bilirubin 6.0 mg/dL, ALT 246 U/L and alkaline phosphatase 245 U/L (Table). Once propylthiouracil was stopped, she began to improve promptly.
Key Points
| Medication: | Propylthiouracil (300 mg daily) |
|---|---|
| Pattern: | Mixed (R=2.9) |
| Severity: | 3+ jaundice and hospitalization) |
| Latency: | 4 weeks |
| Recovery: | 4 weeks |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | Pre | 25 | 80 | 0.8 | |
| 2 weeks | 42 | 95 | 0.7 | ||
| 4 weeks | 0 | Propylthiouracil stopped | Itching and rash | ||
| 6 weeks | 2 weeks | 246 | 245 | 6.0 | |
| 7 weeks | 3 weeks | 250 | 145 | 5.3 | |
| 8 weeks | 4 weeks | 215 | 102 | 2.8 | |
| 10 weeks | 8 weeks | 150 | 105 | 0.9 | |
| 12 weeks | 20 weeks | Normal | Normal | Normal | |
| Normal Values | <40 | <117 | <1.2 | ||
Comment
Typical, mild acute drug induced liver injury with a mixed pattern of serum enzyme elevations. Symptoms and liver test abnormalities worsened for 1 to 2 weeks after stopping therapy and then rapidly improved.
Case 2. Acute hepatocellular injury due to propylthiouracil.(2)
A 22 year old woman with Graves disease developed jaundice, anorexia and pruritus 5 months after starting propylthiouracil [150 mg five times daily]. She had become pregnant shortly after starting propylthiouracil. She was taking no other medications and had no history of liver disease or risk factors for hepatitis. She denied fever and rash. On examination, she was jaundiced and had mild right upper quadrant tenderness. She had fullness in the thyroid area, a slight exophthalmic stare, mild tremor and hyperreflexia. Ultrasound confirmed the history of a 20 week pregnancy. Laboratory tests showed normal blood counts and coagulation tests. Her total serum bilirubin was 10.8 mg/dL (8.5 direct), AST 1385 U/L and Alk P 385 U/L. Propylthiouracil was stopped and her hyperthyroidism managed with propranolol. Liver test abnormalities rapidly improved (Table). Tests for hepatitis A and B were negative and abdominal ultrasound showed no evidence of biliary obstruction. Serum ALT and bilirubin levels fell to normal within 3 weeks. She tolerated switching to methimazole without recurrence. Lymphocyte stimulation tests were positive in response to propylthiouracil, but negative to methimazole during the acute illness.
Key Points
Laboratory Values
Comment
An example of acute hepatocellular injury due to propylthiouracil, with rapid recovery with stopping the medication and no recurrence on switching to methimazole. Management of hyperthyroidism during acute liver injury is problematic and generally requires control of the symptoms with beta blockers and delaying definitive therapy until there is some improvement in the liver injury.
Case 3. Acute cholestatic hepatitis due to a short course of propylthiouracil.(3)
A 53 year old woman with hyperthyroidism and Graves disease was treated with propylthiouracil [50 mg three times daily] for 3 days and rapidly developed nausea, diarrhea, pruritus, and abdominal pain followed by dark urine and jaundice. Although propylthiouracil was stopped promptly, she continued to be symptomatic and liver tests worsened (Table). On admission 9 days after starting and 6 days after stopping propylthiouracil, she was afebrile but deeply icteric. She had a mild tremor, bilateral exophthalmos and a small diffuse goiter which were unchanged from before. She had no hepatomegaly or signs of chronic liver disease. Laboratory tests showed ALT 83 U/L, Alk P 371 U/L and total serum bilirubin 7.9 mg/dL. The total eosinophil count was 640/µL. Tests for hepatitis A, B and C were negative as were antinuclear and antismooth muscle antibodies. Abdominal ultrasound showed no evidence of biliary obstruction. A liver biopsy showed marked cholestasis, focal necrosis and portal inflammation with eosinophils, compatible with drug induced liver injury. Lymphocyte stimulation tests in response to propylthiouracil were negative on two occasions. Her thyroid disease was treated with radioactive iodine. Her jaundice deepened during the first two weeks of hospitalization with serum bilirubin peaking at 40.8 mg/dL. Thereafter she improved and, in follow up 6 weeks later, she was asymptomatic and anicteric. However, minor liver test abnormalities persisted for several years. A second liver biopsy was read as normal. Five years after exposure, all enzyme levels were normal.
Key Points
| Medication: | Propylthiouracil (150 mg daily for 3 days) |
|---|---|
| Pattern: | Cholestatic (R=0.8) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 3 days |
| Recovery: | 6 weeks for jaundice, >2 years for serum enzymes |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|
| Pre | 19 | 80 | <0.5 | |
| 5 days | 119 | 289 | ||
| 9 days | 83 | 371 | 7.9 | Admission |
| 15 days | 58 | 354 | 30.0 | |
| 22 days | 64 | 317 | 40.8 | |
| 26 days | 61 | 280 | 34.2 | Liver biopsy |
| 2 months | 64 | 353 | 2.1 | Asymptomatic |
| 2 years | 30 | 116 | <1.0 | |
| 3 years | 33 | 88 | <1.0 | Liver biopsy |
| 4 years | 38 | 131 | <1.0 | |
| 5 years | 28 | 123 | <1.0 | |
| 6 years | 24 | 94 | <1.0 | |
| Normal Values | <25 | <90 | <1.2 |
Comment
This case was unusual because of the short latency period and prolonged cholestatic course in response to only 3 days of propylthiouracil therapy. The patient denied taking propylthiouracil or similar medications previously. Other diagnoses were excluded and liver histology supported the diagnosis of propylthiouracil induced cholestatic liver injury. The persistent liver test abnormalities suggested chronic injury, but this was not documented by the liver biopsy. The residual minor enzyme elevations may have been unrelated to the drug induced liver injury.
Case 4. Acute liver failure due to propylthiouracil.(4)
A 14 year old girl with Graves disease, exophthalmos and goiter was treated with methimazole with gradual improvement, but relapsed when it was stopped after 3 months. One week after switching to propylthiouracil (450 mg daily), she developed fatigue, vomiting and diarrhea followed by dark urine and jaundice. She remained symptomatic of her thyroid disease with tachycardia and tremor. On examination, she was jaundiced, but did not have fever, rash or signs of chronic liver disease. Laboratory tests showed bilirubin of 13.2 mg/dL and marked elevations of serum ALT (1501 U/L) and AST (2800 U/L) with minimal increase in alkaline phosphatase (287 U/L). Propylthiouracil was stopped and she was maintained on propranolol. Because of worsening liver failure, she was transferred to a tertiary medical center. Tests for hepatitis A, B and C were negative as were antinuclear and smooth muscle antibodies. Thyroid studies showed a normal total and free thyroxine level. An open liver biopsy showed massive necrosis, and she underwent successful liver transplantation 10 days after onset of liver injury and 19 days after starting propylthiouracil.
Key Points
| Medication: | Propylthiouracil (450 mg daily) |
|---|---|
| Pattern: | Hepatocellular (R=56) |
| Severity: | 5+ (emergency liver transplant) |
| Latency: | 1 week |
| Recovery: | None |
| Other medications: | Propranolol |
Laboratory Values
Comment
More than two dozen cases of acute liver failure attributable to propylthiouracil therapy have been reported in the literature, with several cases occurring in children or adolescents. This severe outcome has led to the recommendation that propylthiouracil not be used in children and not be considered as a first line of therapy for Graves disease in adults. The injury is typically hepatocellular and associated with rapid development of acute liver failure and death. The latency to onset can be short as in this instance (one week) or prolonged to as long as a year. This patient recovered after liver transplantation and was later treated successfully with subtotal thyroidectomy.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Propylthiouracil – Generic
DRUG CLASS
Antithyroid Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Propylthiouracil | 51-52-5 | C7-H10-N2-O-S |
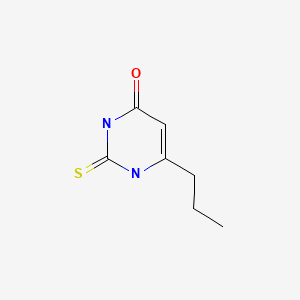
|
CITED REFERENCES
- 1.
- Kim HJ, Kim BH, Han YS, Yang I, Kim KJ, Dong SH, et al. The incidence and clinical characteristics of symptomatic propylthiouracil-induced hepatic injury in patients with hyperthyroidism: a single-center retrospective study. Am J Gastroenterol. 2001;96:165–9. [PubMed: 11197248]
- 2.
- Parker WA. Propylthiouracil-induced hepatotoxicity. Clin Pharm. 1982;1:471–4. [PubMed: 6897865]
- 3.
- Lock DR, Sthoeger ZM. Severe hepatotoxicity on beginning propylthiouracil therapy. J Clin Gastroenterol. 1997;24:267–9. [PubMed: 9252857]
- 4.
- Williams KV, Nayak S, Becker D, Reyes J, Burmeister LA. Fifty years of experience with propylthiouracil-associated hepatotoxicity: what have we learned? J Clin Endocrinol Metab. 1997;82:1727–33. [PubMed: 9177371]
ANNOTATED BIBLIOGRAPHY
References updated: 10 February 2020
- Zimmerman HJ. Antithyroid drugs. Hormonal derivatives and related drugs. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 579-81.(Expert review of hepatotoxicity of antithyroid medications published in 1999; mentions 35 recorded cases of jaundice attributed to propylthiouracil [usually hepatocellular] and 15 to methimazole [usually cholestatic]).
- Chitturi S, Farrell GC. Antithyroid drugs. Adverse effects of hormones and hormone antagonists on the liver. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 614-5.(Review of hepatotoxicity of antithyroid agents mentions that propylthiouracil can cause severe acute hepatitis and death from acute liver failure).
- Brent GA, Koenig RJ. Thyroid and anti-thyroid drugs. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 787-802.(Textbook of pharmacology and therapeutics).
- Livingston HJ, Livingston SF. Agranulocytosis and hepatocellular jaundice: toxic reactions following propylthiouracil therapy. JAMA. 1947;135:422–4. [PubMed: 20264947](68 year old man developed fever and malaise 6 weeks after starting propylthiouracil [200 mg/day] with leucopenia and jaundice, recovering with antibiotics and withdrawal of propylthiouracil, but details not provided).
- Juliar B, Harris TL. Fatal agranulocytosis occurring during propylthiouracil therapy: report of a case. JAMA. 1949;139:646–7. [PubMed: 18110874](25 year old woman developed fever, rash and agranulocytosis after several months of propylthiouracil, with progressive delirium and jaundice a few days before death).
- McCormick RV. Polyarteritis occurring during propylthiouracil therapy. JAMA. 1950;144:1453–4. [PubMed: 14794365](Elderly woman developed fever and diarrhea within 24 hours of restarting propylthiouracil, improving on withdrawal and recurring with a single rechallenge dose; later inadvertent reexposure led to fever, delirium, stroke and death; autopsy showed polyarteritis).
- Specht NW, Boehme EJ. Death due to agranulocytosis induced by methimazole therapy. JAMA. 1952;149:1010–1. [PubMed: 14938092](67 year old woman developed fever 4 weeks after starting methimazole with agranulocytosis and jaundice, dying within 1-2 days, liver at autopsy showing “central congestion”).
- Colwell AR Jr, Sando DE, Lang SJ. Propylthiouracil-induced agranulocytosis, toxic hepatitis, and death. JAMA. 1952;148:639–41. [PubMed: 14888549](60 year old woman with heart disease and arrhythmias developed fever and cough 1 month after starting propylthiouracil with pancytopenia followed by jaundice [bilirubin 10.9 mg/dL, normal Alk P] and death 3 days later, autopsy showing severe centrolobular necrosis).
- Eisen MJ. Fulminant hepatitis during treatment with propylthiouracil. N Engl J Med. 1953;249:814–6. [PubMed: 13111354](52 year old woman developed severe abdominal pain and nausea within 1 day of starting propylthiouracil and died 4 days later in shock but without jaundice; autopsy showed severe centrolobular hepatic necrosis interpreted as an acute hepatitis).
- Rosenbaum H, Reveno WS. Agranulocytosis and toxic hepatitis from methimazole. JAMA. 1953;152:27. [PubMed: 13034518](62 year old woman developed jaundice 7 weeks after starting methimazole followed by agranulocytosis, resolving rapidly with stopping methimazole; had tolerated propylthiouracil).
- Shipp JC. Jaundice during methimazole (‘Tapazole’) administration. Ann Intern Med. 1955;42:701–6. [PubMed: 14350490](63 year old woman developed pruritus followed by jaundice 2 weeks after starting methimazole [bilirubin 8.7 mg/dL, Alk P 3 times ULN], with subsequent worsening of jaundice and agranulocytosis responding to antibiotics; resolution of jaundice in 10 weeks).
- Tennenbaum JI, Dreskin OH. Toxic hepatitis during treatment with methimazole (Tapazole). Report of a case with apparent recovery. Ohio Med J. 1962;58:306–7. [PubMed: 13920238](38 year old woman developed rash, jaundice and pruritus ~4 weeks after starting methimazole [bilirubin 6.2 mg/dL, ALT 545 U/L, Alk P ~2 times ULN, 12% eosinophils], slowly resolving on stopping therapy).
- Martinez-Lopez JI, Greenberg SE, Kling RR. Drug-induced hepatic injury during methimazole therapy. Gastroenterology. 1962;43:84–7. [PubMed: 14470520](36 year old woman developed jaundice and pruritus 1 month after starting methimazole [bilirubin 18 m/dL, Alk P 3 times ULN, AST 400 U/L, protime 18 sec]; delay in stopping methimazole and jaundice persisted for 10 weeks).
- Greenberger NJ, Milligan FD, DeGroot LJ, Isselbacher KJ. Jaundice and thyrotoxicosis in the absence of congestive heart failure. A study of four cases. Am J Med. 1964;36:840–6. [PubMed: 14162890](4 patients, 2 men and 2 women, ages 20 to 51 years had jaundice and hyperthyroidism without congestive heart failure or other known causes of liver disease [bilirubin 1.3-6.4 mg/dL, Alk P 1-3 times ULN, AST 13-40 U/L], jaundice resolving with therapy of hyperthyroidism).
- Sambe K. Liver injury due to drugs. Acta Hepatol (Japan). 1965;6:69.(Review of pathology of 19 cases of drug induced liver disease; one due to methylthiouracil [similar to propylthiouracil, not used in US] and one to mercazol [carbimazole]; little clinical information given).
- Becker CE, Gorden P, Robbins J. Hepatitis from methimazole during adrenal steroid therapy for malignant exophthalmos. JAMA. 1968;26:1787–9. [PubMed: 4177058](54 year old woman on corticosteroids for severe exophthalmos developed jaundice 4 weeks after starting methimazole [bilirubin 5.1 mg/dL, ALT 414 U/L, Alk P 1.5 times ULN]; switched to propylthiouracil and recovered promptly).
- Amrhein JA, Kenny FM, Rose D. Granulocytopenia, lupus-like syndrome and other complications of propylthiouracil therapy. J Pediatr. 1970;76:54–63. [PubMed: 5410161](38 children treated with propylthiouracil were followed prospectively; 10 developed transient leucopenia, one a lupus-like syndrome, 4 urticaria, 2 rash and 2 drug fever; no clinically apparent liver disease).
- Fischer MG, Nayer HR, Miller A. Methimazole-induced jaundice. JAMA. 1973;223:1028–9. [PubMed: 4739295](74 year old woman developed jaundice 2 weeks after starting methimazole [bilirubin 13.6 mg/dL, Alk P 270 U/L, ALT 96 U/L]; jaundice lasted 2 months after stopping, even with prednisone therapy).
- Ishizuki Y. Horumon To Rinsho. 1974;22:1083–5. [2 cases of liver diseases caused by thyroid antagonists] Japanese. [PubMed: 4473289](Two cases, ages 62 and 75 years, with onset of jaundice 3 and 5 weeks after starting antithyroid therapy, with prolonged cholestasis in patient in whom methimazole was continued).
- Parker LN. Hepatitis and propylthiouracil. Ann Intern Med. 1975;82:228–9. [PubMed: 1172923](9 year old girl with Graves disease developed jaundice 2 months after starting propylthiouracil [bilirubin 48 mg/dL, AST 2524 U/L; protime 35 sec, ammonia elevated], resolving within 10 weeks of stopping; lymphocyte stimulation test negative).
- Fedotin MS, Lefer LG. Liver disease caused by propylthiouracil. Arch Intern Med. 1975;135:319–21. [PubMed: 1147734](56 year old man developed liver test abnormalities within a short but undefined time after starting propylthiouracil [bilirubin 2.1 mg/dL, ALT 238 U/L, Alk P 395 U/L], biopsy showing chronic hepatitis with portal inflammation and fibrosis; resolving rapidly upon stopping).
- Mihas AA, Holley P, Koff RS, Hirschowitz BI. Fulminant hepatitis and lymphocyte sensitization due to propylthiouracil. Gastroenterology. 1976;70:770–4. [PubMed: 1261772](34 year old woman developed jaundice 2-3 months after starting propylthiouracil which was continued until she became disoriented [bilirubin 20.7 mg/dL, ALT 1060 U/L, Alk P 274 U/L, protime 24.1 sec], resolving spontaneously; positive lymphocyte stimulation to propylthiouracil, biopsy showed bridging necrosis).
- Maddrey WC, Boitnott JK. Drug-induced chronic liver disease. Gastroenterology. 1977;72:1348–53. [PubMed: 323097](Review of drugs that cause chronic hepatitis; oxyphenisatin, isoniazid, methyldopa, nitrofurantoin, phenothiazines; mentioning 2 cases with bridging necrosis associated with propylthiouracil).
- Reddy CM. Propylthiouracil and hepatitis: a case report. J Natl Med Assoc. 1979;71:1185–6. [PMC free article: PMC2537460] [PubMed: 522183](10 year old girl with Hashimotos thyroiditis developed jaundice 23 days after starting propylthiouracil [bilirubin 10.5 mg/dL, AST 350 U/L, Alk P 400 U/L], resolving rapidly upon stopping therapy).
- Jacobsen BB. Ugeskr Laeger. 1979;141:3171–2. [Liver involvement in propylthiouracil treatment of juvenile thyrotoxicosis] Danish. [PubMed: 524495]
- Nielsen HK, Iversen TO. Ugeskr Laeger. 1980;142:3189–90. [Propylthiouracil-induced hepatitis and agranulocytosis] Danish. [PubMed: 7445231]
- Weiss M, Hassin D, Bank H. Propylthiouracil-induced hepatic damage. Arch Intern Med. 1980;140:1184–5. [PubMed: 6893265](Two cases; 28 year old woman developed jaundice 5 months after starting propylthiouracil [bilirubin 7.2 mg/dL, AST 250 U/L, Alk P normal], resolving in 2 months and recurring upon rechallenge, but then tolerating methimazole; 54 year old woman developed jaundice and pruritus 5 months after restarting propylthiouracil [bilirubin 2.4 mg/dL, AST 60 U/L, Alk P 220 U/L], with slow, incomplete recovery on stopping and biopsy later showing cirrhosis).
- Pacini F, Sridama V, Refetoff S. Multiple complications of propylthiouracil treatment: granulocytopenia, eosinophilia, skin reaction and hepatitis with lymphocyte sensitization. J Endocrinol Invest. 1982;5:403–7. [PubMed: 6221046](47 year old woman developed rash, fever and eosinophilia 4 weeks after starting propylthiouracil [bilirubin 3.5 mg/dL, AST 276 U/L, Alk P 588 U/L, neutropenia], with reversal on stopping propylthiouracil, positive lymphocyte stimulation test in immediate period of withdrawal).
- Parker WA. Propylthiouracil-induced hepatotoxicity. Clin Pharm. 1982;1:471–4. [PubMed: 6897865](22 year old woman developed jaundice and pruritus 5 months after starting propylthiouracil [bilirubin 10.8 mg/dL, AST 1385 U/L, Alk P 385 U/L], resolving within 3 weeks; patient switched to methimazole without recurrence: Case 2).
- Kimura T, Shindo T. Nippon Naika Gakkai Zasshi. 1982;71:685–91. [A case of insulin autoimmune syndrome with cholestatic hepatitis induced by methimazole and propylthiouracil] Japanese. [PubMed: 7130811](Randomized, placebo controlled trial of propylthiouracil [300 mg daily for 6 weeks] in 67 patients with acute alcoholic hepatitis; no difference in survival or time to improvement of any liver test abnormalities or symptoms; side effects of hypothyroidism, but no apparent hepatotoxicity).
- Safani MM, Tatro DS, Rudd P. Fatal propylthiouracil-induced hepatitis. Arch Intern Med. 1982;142:838–9. [PubMed: 7073426](20 year old woman developed fatigue and jaundice 2 weeks after starting propylthiouracil [bilirubin 22 mg/dL, AST 750 U/L, Alk P 248 U/L], with progressive hepatic failure, dying 4 months later).
- Cooper DS. Antithyroid drugs. N Engl J Med. 1984;311:1353–62. [PubMed: 6387489](Extensive review of mechanism of action, efficacy and safety of propylthiouracil and methimazole in treating hyperthyroidism; side effects occur in 1-5% of patients, including fever, rash, urticaria, transient leucopenia, and arthralgias particularly with higher doses; severe side effects include agranulocytosis, vasculitis, aplastic anemia, thrombocytopenia and nephritic syndrome).
- Hanson JS. Propylthiouracil and hepatitis. Two cases and a review of the literature. Arch Intern Med. 1984;144:994–6. [PubMed: 6608933](Two 20 year old women developed jaundice 3 months after starting propylthiouracil [bilirubin 11.4 and 24.6 mg/dL, AST 714 and 1260 U/L, Alk P 119 and 44 U/L], one progressing to liver failure and death, the other recovering within 6 weeks of stopping).
- Bloch CA, Jenski LJ, Balistreri WF, Dolan LM. Propylthiouracil-associated hepatitis. Arch Intern Med. 1985;145:2129–30. [PubMed: 4062471](12 year old boy developed jaundice 2 months after starting propylthiouracil [bilirubin 5.5 mg/dL, ALT 1716 U/L, Alk P 4 times ULN], resolving within 1 month of stopping; negative lymphocyte stimulation tests).
- Garty BZ, Kauli R, Ben-Ari J, Lubin E, Nitzan M, Laron Z. Hepatitis associated with propylthiouracil treatment. Drug Intell Clin Pharm. 1985;19:740–2. [PubMed: 4053978](12 year old girl developed urticarial rash 3 days after starting propylthiouracil and diarrhea and fever 2 days after restarting [bilirubin 4.2 mg/dL, AST 230 U/L, Alk P 350 U/L], tests improving within a week of stopping).
- Vitug AC, Goldman JM. Hepatotoxicity from antithyroid drugs. Horm Res. 1985;21:229–34. [PubMed: 4007783](Review of literature identified 29 cases of hepatic injury due to antithyroid drugs; propylthiouracil [n=17], methimazole [n=10] and carbimazole [n=3]; propylthiouracil cases were primarily hepatocellular with onset in 10 days to 5 months; the other drugs caused cholestatic injury, arising after 10 days to 8 weeks).
- Cofré C, Valdés E, Tapia A, Rodríguez J. Rev Med Chil. 1986;114:42–4. [Cholestatic hepatitis secondary to propylthiouracil] Spanish. [PubMed: 3764142](58 year old developed abdominal pain 18 days after starting methimazole [bilirubin 1.2 mg/dL, ALT 93 U/L, Alk P 572 U/L], with improvement on stopping and recurrent rise in Alk P with rechallenge).
- Seidman DS, Livni E, Ilie B, Blum I. Propylthiouracil-induced cholestatic jaundice. J Toxicol Clin Toxicol. 1986;24:353–60. [PubMed: 3528517](33 year old woman developed pruritus and jaundice 2 weeks after starting propylthiouracil [bilirubin 13.2 mg/dL, ALT 27 U/L, Alk P 339 U/L], resolving within 4 weeks of stopping; positive macrophage migration inhibition test to propylthiouracil).
- Limaye A, Ruffolo PR. Propylthiouracil-induced fatal hepatic necrosis. Am J Gastroenterol. 1987;82:152–4. [PubMed: 3812421](42 year old woman developed jaundice 1 year after starting propylthiouracil [bilirubin 22 mg/dL, AST 1755 U/L, Alk P 381 U/L, protime 21 sec], progressing to hepatic failure and death within 4 days; autopsy showed massive necrosis without fibrosis).
- Jonas MM, Eidson MS. Propylthiouracil hepatotoxicity: two pediatric cases and review of the literature. J Pediatr Gastroenterol Nutr. 1988;7:776–9. [PubMed: 3054039](Two cases; 6 year old girl developed jaundice 4 months after starting propylthiouracil 300 mg/day [bilirubin 11.9 mg/dL, ALT 999 U/L, ANA 1:320], improving rapidly upon stopping; 13 year old girl developed jaundice and pruritus 7 months after starting propylthiouracil 300 mg/day [bilirubin 14.7 mg/dL, ALT 1530 U/L, Alk P 369 U/L, ANA 1:160], progressing to liver failure before stopping drug and dying 9 days later, autopsy showing massive necrosis).
- Yao JD, Gross JB Jr, Ludwig J, Purnell DC. Cholestatic jaundice in hyperthyroidism. Am J Med. 1989;86:619–20. [PubMed: 2712072](42 year old man presented with jaundice and weight loss [bilirubin 16.7 rising to 36.0 mg/dL, ALT 76 U/L, Alk P 252 U/L], biopsy showed intrahepatic cholestasis, found to have hyperthyroidism, jaundice resolving with radioactive iodine therapy).
- Maggiore G, Larizza D, Lorini R, De Giacomo C, Scotta MS, Severi F. Propylthiouracil hepatotoxicity mimicking autoimmune chronic active hepatitis in a girl. J Pediatr Gastroenterol Nutr. 1989;8:547–8. [PubMed: 2723949](8 year old was found to have hepatomegaly 12 months after starting propylthiouracil [bilirubin normal, ALT 765 U/L, GGT 3 times ULN], biopsy showing chronic hepatitis; ANA negative but antithyroid antibodies present; rapid resolution on stopping drug and later tolerating methimazole).
- Baker B, Shapiro B, Fig LM, Woodbury D, Sisson JC, Beierwaltes WH. Unusual complications of antithyroid drug therapy: four case reports and review of literature. Thyroidology. 1989;1:17–26. [PubMed: 2484903](3 cases of hepatotoxicity; 34 year old woman developed jaundice 6 months after starting propylthiouracil [bilirubin 20.6 mg/dL, ALT 957 U/L, Alk P 176 U/L], delayed withdrawal and slow recovery; 9 year old girl developed jaundice 3 months after starting propylthiouracil [bilirubin 9.0 mg/dL, ALT 1407 U/L, Alk P 848 U/L], resolving rapidly upon stopping; 20 year old woman developed jaundice 8 months after starting methimazole [bilirubin 27 mg/dL, ALT 2040 U/L, Alk P 389 U/L], progressing to hepatic failure and death, autopsy showing massive necrosis).
- Werner MC, Romaldini JH, Bromberg N, Werner RS, Farah CS. Adverse effects related to thioamide drugs and their dose regimen. Am J Med Sci. 1989;297:716–9. [PubMed: 2523194](Among 389 patients with Graves disease, 5 had hepatotoxicity on antithyroid therapy, 4 of 131 [2%] on propylthiouracil vs 1 of 258 [0.5%] on methimazole, all recovered; liver injury typically arose during initial high dose therapy).
- Kirkland JL. Propylthiouracil-induced hepatic failure and encephalopathy in a child. DICP. 1990;24:470–1. [PubMed: 2343593](9 year old girl developed jaundice and delirium 4 months after starting propylthiouracil [bilirubin 14 mg/dL, AST 1215 U/L, protime 27.5 sec], but recovering ultimately after stopping medication).
- Kang H, Choi JD, Jung IG, Kim DW, Kim TB, Shin HK, Kim BT, Park CK, Yoo JY. A case of methimazole-induced acute hepatic failure in a patient with chronic hepatitis B carrier. Korean J Intern Med. 1990;5:69–73. [PMC free article: PMC4535001] [PubMed: 2271514](43 year old man with HBsAg carrier state developed jaundice 7 months after starting methimazole [bilirubin 5.0 mg/dL, ALT 180 U/L, Alk P 848 U/L, no detectable HBV DNA], developed worsening hepatic failure and died 30 days after admission, autopsy showing cirrhosis with severe cholestasis).
- Di Gregorio C, Ghini F, Rivasi F. Granulomatous hepatitis in a patient receiving methimazole. Ital J Gastroenterol. 1990;22:75–7. [PubMed: 2131935](56 year old woman developed abnormal liver tests 11 years after starting methimazole and not resolving when drug was stopped, biopsy showing active granulomas with giant cells suggestive of sarcoidosis [bilirubin normal, ALT 51-139 U/L, Alk P 484-652 U/L]).
- Findor J, Bruch Igartúa E, Sorda J, Jury R. Acta Gastroenterol Latinoam. 1991;21(2):115–9. [Jaundice caused by methimazole] Spanish. [PubMed: 1687933](Four patients with thyrotoxicosis and “cholestasis” [bilirubin 0.8-1.0 mg/dL, Alk P 300-548 U/L, GGT 17-166 U/L], biopsies showing intrahepatic cholestasis).
- Hayashida CY, Duarte AJ, Sato AE, Yamoshuro-Kanasbiro EH. Neonatal hepatitis and lymphocyte sensitization by placental transfer of propylthiouracil. J Endocrinol Invest. 1991;13:937–41. [PubMed: 2090674](Female newborn of a mother taking propylthiouracil had hyperthyroidism and liver injury [bilirubin 14 mg/dL, ALT 110 U/L, Alk P 2 times ULN], resolving spontaneously; positive lymphocyte stimulation test).
- Peter SA. Propylthiouracil-associated hepatitis. J Natl Med Assoc. 1991;83:75–7. [PMC free article: PMC2627005] [PubMed: 1994070](43 year old woman developed jaundice 10 weeks after starting propylthiouracil [bilirubin 6.4 mg/dL, AST 926 U/L, Alk P 292 U/L], worsening for 10 days and then resolving within 10 weeks of stopping).
- Fong TL, McHutchison JG, Reynolds TB. Hyperthyroidism and hepatic dysfunction. A case series analysis. J Clin Gastroenterol. 1992;14:240–4. [PubMed: 1564300](Among 18 patients with hyperthyroidism not on antithyroid therapy, 50% had bilirubin elevations [2 were deeply jaundiced] and 67% ALT elevations; highest rates present in those with heart failure due to hyperthyroidism).
- Frenkel J, Téllez R, Reyes C, González G, Michaud P. Rev Med Chil. 1993;121:1289–94. [Major adverse reactions to propylthiouracil in 586 cases of hyperthyroidism] Spanish. [PubMed: 8191137](60 patients with hyperthyroidism and normal baseline ALT levels were monitored on propylthiouracil, 28% had ALT elevations [40-231 U/L] all within 2 months and on initial dosage [300 mg/day], falling to normal with continuation [100 mg/day]; no symptoms, jaundice or Alk P elevations; liver biopsies in 3 patients showed spotty necrosis and ill-defined granulomas).
- Liaw YF, Huang MJ, Fan KD, Li KL, Wu SS, Chen TJ. Hepatic injury during propylthiouracil therapy in patients with hyperthyroidism. A cohort study. Ann Intern Med. 1993;118:424–8. [PubMed: 8439116](60 patients with hyperthyroidism and normal baseline ALT levels were monitored on propylthiouracil, 28% developed ALT elevations [40-231 U/L] all within 2 months of starting initial high dosage [300 mg/day], falling to normal with continuation [100 mg/day]; no symptoms, jaundice or Alk P elevations; liver biopsies in 3 patients showed spotty necrosis and ill defined granulomas).
- Sadoul JL, Canivet B, Freychet P. Toxic hepatitis induced by antithyroid drugs: four cases including one with cross-reactivity between carbimazole and benzylthiouracil. Eur J Med. 1993;2:473–7. [PubMed: 7504976](Retrospective analysis of 236 patients with hyperthyroidism treated at one center found 4 cases [1.7%] with hepatotoxicity due to carbimazole; only one with jaundice [bilirubin 3.5 mg/dL, ALT 162 U/L, Alk P 318 U/L], resolving within 4 weeks of stopping; other cases were anicteric and associated with drug-fever or rash, largely cholestatic enzyme patterns).
- Levy M. Propylthiouracil hepatotoxicity. A review and case presentation. Clin Pediatr (Phila). 1993;32:25–9. [PubMed: 8419095](14 year old girl developed jaundice 14 months after starting propylthiouracil with severe course, hepatic failure but ultimate slow recovery).
- Westphal SA. Hepatotoxicity from propylthiouracil. South Med J. 1994;87:943–7. [PubMed: 8091264](32 year old woman developed jaundice 2.5 months after switching from methimazole to propylthiouracil [bilirubin 9.5 mg/dL, ALT 2030 U/L, Alk P ~250 U/L], resolving within 4 months of stopping).
- Huang MJ, Li KL, Wei JS, Wu SS, Fan KD, Liaw YF. Sequential liver and bone biochemical changes in hyperthyroidism: prospective controlled follow-up study. Am J Gastroenterol. 1994;89:1071–6. [PubMed: 7912472](Prospective study of 95 patients with hyperthyroidism treated with propylthiouracil; 76% had at least one liver test abnormality before therapy, 37% in ALT [peak 169 U/L] and 64% in Alk P [peak 337 U/L]; ALT levels often decreased with treatment, but rose further in 38%, one developing jaundice [ALT 1490 U/L], resolving with stopping therapy).
- Hoffman DM, Burgess J, Hill P. Agranulocytosis and hepatic dysfunction following propylthiouracil treatment. Aust N Z J Med. 1994;24:409–10. [PubMed: 7980243](55 year old woman developed pruritus, jaundice and fever 6 weeks after starting propylthiouracil [bilirubin 17.4 mg/dL, ALT 165 U/L, Alk P 340 U/L, ANA positive], with agranulocytosis resolving within 3 months of stopping drug).
- Mamianetti A, Muñoz A, Ronchetti RD, Maccione E, Poggi U, Mugnolo R, et al. Medicina (B Aires). 1995;55:693–6. [Acquired sideroblastic anemia and cholestasis in a hyperthyroid patient treated with methimazole and atenolol] Spanish. [PubMed: 8731582](62 year old woman developed jaundice and pruritus within 10 days of starting methimazole [bilirubin 16 rising to 39 mg/dL, ALT 65 U/L, Alk P 670 U/L], with prolonged jaundice and sideroblastic anemia, resolving slowing with normal tests 14 months later).
- Arab DM, Malatjalian DA, Rittmaster RS. Severe cholestatic jaundice in uncomplicated hyperthyroidism treated with methimazole. J Clin Endocrinol Metab. 1995;80:1083–5. [PubMed: 7714072](48 year old man with hyperthyroidism and mild liver test abnormalities developed jaundice and worsening pruritus 1 month after starting methimazole [bilirubin 30.1 mg/dL, AST 40 U/L, Alk P 475 U/L], improving with stopping methimazole and achieving euthyroidism with radioactive iodide).
- Singh A, Thakur R. Scintigraphic study of propylthiouracil induced submassive hepatic necrosis. Clin Nucl Med. 1995;20:132–5. [PubMed: 7720304](44 year old woman developed nausea and vomiting 11 months after starting propylthiouracil [bilirubin 2.4 mg/dL, ALT 2610 U/L, Alk P 357 U/L, protime 13.7 sec, ANA 1: 1280], resolving on stopping; liver scans showing decreased uptake during episode).
- Deidiker R, deMello DE. Propylthiouracil-induced fulminant hepatitis: case report and review of the literature. Pediatr Pathol Lab Med. 1996;16:845–52. [PubMed: 9025882](13 year old girl developed jaundice 4 months after starting propylthiouracil [bilirubin 13.8 mg/dL, ALT 1716 U/L, Alk P not given, ANA 1:20], worsening and undergoing liver transplantation within 7 days, but dying postoperatively, explant showing massive necrosis and collapse).
- Schwab GP, Wetscher GJ, Vogl W, Redmond E. Methimazole-induced cholestatic liver injury, mimicking sclerosing cholangitis. Langenbecks Arch Chir. 1996;381:225–7. [PubMed: 8817448](68 year old man developed jaundice and pruritus 2 months after starting methimazole [bilirubin 3.1 rising to 12.2 mg/dL, ALT 61 U/L, Alk P 530 U/L], resolving within 3 months of stopping and with thyroidectomy).
- Hardee JT, Barnett AL, Thannoun A, Eghtesad B, Wheeler D, Jamal MM. Propylthiouracil-induced hepatotoxicity. West J Med. 1996;165:144–7. [PMC free article: PMC1303726] [PubMed: 8909171](37 year old woman developed jaundice 5 months after starting propylthiouracil [bilirubin 16.6 mg/dL, ALT 1135 U/L, Alk P 486 U/L, ANA 1:320, SMA 1:40, protime rising to 21.4 sec], with signs of hepatic failure, biopsy showing bridging necrosis, responding to support and prednisone, ultimately recovering).
- Ozenírler S, Tuncer C, Boztepe U, Akyol G, Alkim H, Cakir N, et al. Propylthiouracil-induced hepatic damage. Ann Pharmacother. 1996;30:960–3. [PubMed: 8876856](64 year old woman developed jaundice 1 year after starting propylthiouracil [bilirubin 7.3 mg/dL, ALT 990 U/L, Alk P 123 U/L, protime 20 sec, ANA negative], slow resolution and biopsy showing bridging necrosis; a year later, she had continued enzyme elevations and evidence of cirrhosis [ascites and splenomegaly]).
- Williams KV, Nayak S, Becker D, Reyes J, Burmeister LA. Fifty years of experience with propylthiouracil-associated hepatotoxicity: what have we learned? J Clin Endocrinol Metab. 1997;82:1727–33. [PubMed: 9177371](Two cases; 14 year old girl developed jaundice 3 weeks after restarting propylthiouracil [bilirubin 13.2 mg/dL, ALT 2501 U/L, Alk P 287 U/L], progressing to hepatic failure and successful liver transplant, explant showing massive necrosis [Case 4]; 54 year old man with thyrotoxicosis and liver test abnormalities developed jaundice 2 weeks after starting propylthiouracil [bilirubin 5.8 to 23.5 mg/dL, ALT 740 U/L, Alk P 258 U/L], with progressive hepatic failure and death 5 weeks later; review of literature found 28 cases of propylthiouracil hepatotoxicity, 25 in women, 7 fatal).
- Lock DR, Sthoeger ZM. Severe hepatotoxicity on beginning propylthiouracil therapy. J Clin Gastroenterol. 1997;24:267–9. [PubMed: 9252857](53 year old woman developed jaundice after only 3 days of propylthiouracil [bilirubin 7.9 rising to 40.8 mg/dL, ALT 83 U/L, Alk P 371 U/L], with clinical improvement within 6 weeks of stopping, but mild liver enzyme abnormalities persisting for more than 2 years: Case 3).
- Gürlek A, Cobankara V, Bayraktar M. Liver tests in hyperthyroidism: effect of antithyroid therapy. J Clin Gastroenterol. 1997;24:180–3. [PubMed: 9179740](At least one liver test abnormality found in 60% of patients with hyperthyroidism before therapy; Alk P in 44% and ALT in 23% and GGT, often improving on propylthiouracil therapy, but 15% developed de novo ALT elevations by 6 weeks, none were symptomatic, jaundiced or required dose modification).
- Khovidhunkit W, Farese RV Jr. Resolution of propylthiouracil-induced hepatic failure after treatment of thyrotoxicosis. West J Med. 1997;167:353–6. [PMC free article: PMC1304632] [PubMed: 9392993](21 year old woman with severe hyperthyroidism and liver test abnormalities developed jaundice 4 months after starting propylthiouracil [bilirubin 15.0 mg/dL, ALT 942 U/L, Alk P 205 U/L], not improving during first month after stopping, but then resolving with treatment of hyperthyroidism).
- Ichiki Y, Akahoshi M, Yamashita N, Morita C, Maruyama T, Horiuchi T, et al. Propylthiouracil-induced severe hepatitis: a case report and review of the literature. J Gastroenterol. 1998;33:747–50. [PubMed: 9773944](21 year old woman developed nausea 2 months after starting propylthiouracil [bilirubin 23.2 mg/L, ALT 502 U/L, Alk P 1442 U/L], resolving within 6 weeks on prednisolone; positive lymphocyte stimulation test; review of literature includes 25 cases with 5 fatalities).
- Waseem M, Seshadri KG, Kabadi UM. Successful outcome with methimazole and lithium combination therapy for propylthiouracil-induced hepatotoxicity. Endocr Pract. 1998;4:197–200. [PubMed: 15251733](49 year old man developed nausea 2 months and jaundice 4 months after starting propylthiouracil [bilirubin 20.4 mg/dL, ALT 1043 U/L, Alk P 186 U/L], values worsening for 1 month despite stopping and then slowly returning towards normal while on methimazole).
- Hung YT, Yu WK, Chow E. Delayed cholestatic hepatitis due to methimazole. Hong Kong Med J. 1999;5:200–1. [PubMed: 11821593](71 year old man developed jaundice a few weeks after stopping a 5 month course of methimazole with prolonged jaundice [bilirubin 40 mg/dL, ALT 40 U/L, Alk P 600 U/L], with slow recovery over more than 6 months).
- Babini G, Gurioli L, Rizzi R, Bertello P. Appearance of severe jaundice after radiometabolical treatment of thyrotoxicosis. J Endocrinol Invest. 1999;22:209–11. [PubMed: 10219889](63 year old man developed jaundice 2 weeks after receiving radioactive iodine for toxic goiter [bilirubin 6.8 mg/dL, ALT 86, Alk P 426 U/L], worsening for 2 weeks and then slowly improving and tolerating methimazole).
- Parolin MB, Lopes RW, Telles JE, Ioshii SO, Hajar N. Arq Gastroenterol. 2000;37:129–32. [Acute cholestatic hepatitis induced by propylthiouracil. Case report] Portuguese. [PubMed: 11144016]
- De Castro JJ, Nobre EL, Garcia e Costa J, Galvão-Teles A. Acta Med Port. 2001;14:523–7. [Asymptomatic hepatitis induced by propylthiouracil] Portuguese. [PubMed: 11878166]
- Kim HJ, Kim BH, Han YS, Yang I, Kim KJ, Dong SH, et al. The incidence and clinical characteristics of symptomatic propylthiouracil-induced hepatic injury in patients with hyperthyroidism: a single-center retrospective study. Am J Gastroenterol. 2001;96:165–9. [PubMed: 11197248](Retrospective analysis of 497 patients with hyperthyroidism treated with propylthiouracil; 6 [1.2%] developed clinically apparent hepatotoxicity 12 to 49 days after starting; 5 were jaundiced [peak bilirubin 0.5-42.7 mg/dL, ALT 33-296 U/L, Alk P 132-373 U/L], no autoantibodies, 2 with rash; 3 cholestatic, 2 mixed and 1 hepatocellular; resolution in 2-20 weeks, no fatalities; no predictive baseline features identified; another 16% of patients had transient, asymptomatic rises in serum aminotransferase levels during therapy; Case 1).
- Woeber KA. Methimazole-induced hepatotoxicity. Endocr Pract. 2002;8:222–4. [PubMed: 12467281](36 year old woman developed pruritus and jaundice 3 weeks after starting methimazole [bilirubin 12.1 rising to 25.8 mg/dL, ALT 127 U/L, Alk P 265 U/L], with slow recovery and Alk P abnormalities for ~12 months).
- Kontoleon P, Ilias I, Koutras DA, Kontogiannis D, Papapetrou PD. Successful treatment with carbimazole of a hyperthyroid pregnancy with hepatic impairment after propylthiouracil administration: a case report. Clin Exp Obstet Gynecol. 2002;29:304–5. [PubMed: 12635752](27 year old woman developed elevations in ALT [151 U/L], with normal bilirubin [0.5 mg/dL] after 12th week of pregnancy while on propylthiouracil, resolving within 10 days of switching to carbimazole).
- Ruiz JK, Rossi GV, Vallejos HA, Brenet RW, Lopez IB, Escribano AA. Fulminant hepatic failure associated with propylthiouracil. Ann Pharmacother. 2003;37:224–8. [PubMed: 12549953](Two cases: 30 year old woman presented with jaundice 4 months after starting propylthiouracil [bilirubin 29.5 mg/dL, ALT 207 U/L, Alk P 208 U/L, protime 17 sec], with progressive liver failure, sepsis and death within 1 month; 32 year old woman developed jaundice after 5 months of propylthiouracil in 5th month of pregnancy [bilirubin 32.8 mg/dL, ALT 686 U/L, Alk P 364 U/L, protime 19.9 sec], with progressive liver failure, loss of fetus and death of multiorgan failure in 2 months).
- Testa G, Trevino J, Bogetti D, Layden T, Wiley T, Sankary H, et al. Liver transplantation for propylthiouracil-induced acute hepatic failure. Dig Dis Sci. 2003;48:190–1. [PubMed: 12645809](17 year old girl developed jaundice 7 months after starting propylthiouracil [bilirubin 17.4 mg/dL, ALT 338 U/L, protime 40.8 sec], with progressive hepatic failure requiring liver transplantation).
- Russo MV, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among 2291 liver transplants for acute liver failure done in the US between 1990 and 2002, 357 were attributed to medications, 53% to acetaminophen; in remaining 137 cases, most common agents were isoniazid [24; 17.5%], propylthiouracil [13; 9.5%], phenytoin [10; 7.3%], valproate [10; 7.3%], nitrofurantoin [7; 5%], herbals [7; 5%], ketoconazole [6; 4%] and disulfiram [6; 4%]; none due to methimazole).
- Mikhail NE. Methimazole-induced cholestatic jaundice. South Med J. 2004;97:178–82. [PubMed: 14982270](43 year old woman developed jaundice and pruritus one month after starting methimazole [bilirubin 16.7 mg/dL, ALT 104 U/L, Alk P 289 U/L], resolving 4 months after discontinuation; review of literature found 20 cases, 18 in women, onset in 3 days to 3 months, largely cholestatic, no fatalities from liver disease, recurrence on rechallenge with methimazole or carbimazole).
- Piñero Madrona A, Pons Miñano JA, Madrid Conesa J, Parrilla Paricio P. Rev Clin Esp. 2004;204:388. [Methimazole hepatitis] Spanish. [PubMed: 15274789](46 year old woman with subclinical hyperthyroidism developed arthralgias and malaise 6 days after starting methimazole [bilirubin normal, ALT 1280 U/L, Alk P 520 U/L], resolving within 2 weeks of stopping).
- Brusco F, González G, Soto N, Arteaga E. Successful treatment of hyperthyroidism with amiodarone in a patient with propylthiouracil-induced acute hepatic failure. Thyroid. 2004;14:862–5. [PubMed: 15588385](20 year old woman developed jaundice 4 months after starting propylthiouracil [bilirubin 20.4 mg/dL, ALT 3827 U/L, Alk P 372 U/L, INR 4.4], with hepatic encephalopathy but ultimate recovery over the next several weeks; hyperthyroidism managed with amiodarone until recovery when radioactive iodine was used: Case 3).
- Lian XL, Bai Y, Dai WX, Jin ZM, Zeng ZP, Guo ZS. Zhonghua Nei Ke Za Zhi. 2004;43:442–6. [The clinical characteristics of symptomatic propylthiouracil-induced hepatic injury in patients with hyperthyroidism] Chinese. [PubMed: 15312442]
- Lian XL, Bai Y, Dai WX, Guo ZS, Li W, Lu L. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004;26:172–7. [Propylthiouracil-induced overt hepatic injury in patients with hyperthyroidism] Chinese. [PubMed: 15171556]
- Aydemir S, Ustundag Y, Bayraktaroglu T, Tekin IO, Peksoy I, Unal AY. Fulminant hepatic failure associated with propylthiouracil: a case report with treatment emphasis on the use of plasmapheresis. J Clin Apheresis. 2005;20:235–8. [PubMed: 16206173](22 year old woman developed jaundice 1.5 years after starting propylthiouracil and 3 weeks after raising the dose to 600 mg daily [bilirubin 26 mg/dL, ALT 1350 U/L, Alk P 266 U/L, protime 69 sec], with slow recovery over 4 months while undergoing multiple courses of plasmapheresis).
- Sipe WEB, Su M, Posselt A, Kim GE, Quiros JA, Rosenthal P. Propylthiouracil-associated liver failure presenting as probable autoimmune hepatitis in a child with Graves' disease. Pediatr Transplant. 2006;10:525–8. [PubMed: 16712616](7 year old girl developed jaundice 9 months after starting propylthiouracil for Graves disease [bilirubin 7.9 mg/dL, ALT 1634 U/L, Alk P 496 U/L, INR 2.4], with progressive hepatic failure requiring emergency liver transplant 12 days later; ANA positive, but little evidence of improvement with corticosteroids; explant showing massive necrosis).
- Casallo Blanco S, Valero MA, Marcos Sánchez F, de Matías Salces L, Blanco González JJ, Martín Barranco MJ. Gastroenterol Hepatol. 2007;30:268–70. [Methimazole and propylthiouracil induced acute toxic hepatitis] Spanish. [PubMed: 17493435](79 year old woman developed jaundice 1 month after starting methimazole [bilirubin 3.2 mg/dL, ALT 184 U/L, Alk P 574 U/L], with prompt improvement on stopping; then, 2 weeks after starting propylthiouracil, she redeveloped jaundice [bilirubin 5.5 mg/dL, ALT 448 U/L, Alk P 279 U/L], these normalizing within 2 months of stopping and with concurrent prednisone therapy).
- Benyounes M, Sempoux C, Daumerie C, Rahier J, Geubel AP. Propylthiouracyl-induced severe liver toxicity: an indication for alanine aminotransferase monitoring? World J Gastroenterol. 2006;12:6232–4. [PMC free article: PMC4088125] [PubMed: 17036403](2 cases; 35 year old woman developed jaundice 2-3 months after starting propylthiouracil [bilirubin 10 mg/dL, ALT 1575 U/L], with delayed withdrawal and progressive hepatic failure but ultimate recovery over next 4 months; 43 year old woman developed jaundice 5 months after starting propylthiouracil [bilirubin 10.9 mg/dL, ALT 5040 U/L], improving within 8 weeks of stopping; authors recommend ALT monitoring).
- Aycan Z, Arhan E, Cetinkaya E, Vidinlisan S, Menekşe N, Yücel H, et al. Propylthiouracil-induced hypersensitivity syndrome. Turk J Pediatr. 2006;48:162–5. [PubMed: 16848120](14 year old girl developed vasculitis 8 months after starting propylthiouracil which progressed on continuing therapy and improved with prednisone and switching to methimazole; no mention of liver involvement).
- Ramos-Bonner LS, Goldberg TH, Moyer S, Anastasopoulou C. Methimazole-induced cholestatic jaundice in an elderly hyperthyroid patient. Am J Geriatr Pharmacother. 2007;5:236–40. [PubMed: 17996663](76 year old woman developed jaundice and pruritus 6 weeks after starting methimazole [bilirubin 25.4 mg/dL, ALT 676 U/L, Alk P 620 U/L, INR 1.2], worsening for 5 days and then resolving slowly).
- Nakamura H, Noh JY, Itoh K, Fukata S, Miyauchi A, Hamada N. Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by Graves' disease. J Clin Endocrinol Metab. 2007;92:2157–62. [PubMed: 17389704](Controlled trial of 2 doses of methimazole vs propylthiouracil in 396 Japanese patients with Graves disease; hepatotoxicity occurred in 27% on propylthiouracil [n=104] vs 6.6% on methimazole [n=267]).
- Moral P, Rodríguez Soler M, Mancheño Franch N, Aguilera Sánchez-Tello V, Todolí Parra J, Ponce García J, et al. Gastroenterol Hepatol. 2008;31:474–5. [Acute cholestatic hepatitis due to propylthiouracil] Spanish. [PubMed: 18783701](51 year old man developed jaundice and pruritus [bilirubin 17.9 mg/dL, ALT 92 U/L, Alk P 228 U/L] a few days after restarting propylthiouracil, resolving 2 months after stopping).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Rochon J. Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to propylthiouracil or methimazole).
- Grzywa M, Orłowska-Florek R, Grzywa-Celińska A. Endokrynol Pol. 2009;60:396–400. [Two cases of serious hepatic injury caused by antithyroid drugs] Polish. [PubMed: 19885811](Two women, ages 49 and 43, with Graves disease developed jaundice after switching from methimazole to propylthiouracil [bilirubin 17.8 and 35.3 mg/dL, ALT 42 and 82 U/L, Alk P 397 and 232 U/L], both recovering within 2 months of stopping).
- Cooper DS, Rivkees SA. Putting propylthiouracil in perspective. J Clin Endocrinol Metab. 2009;94:1881–2. [PubMed: 19401361](Editorial summarizing issues of hepatotoxicity of propylthiouracil, 33 publications of hepatotoxicity in adults and 14 in children with UNOS reporting 16 liver transplants for acute liver failure due to propylthiouracil in adults and 7 in children between 1990 and 2007. In contrast, methimazole can cause liver injury, but fatalities are rare; these factors led to recommendations that methimazole be used instead of propylthiouracil except in first trimester of pregnancy [or for intolerance] and when surgery or radioiodine are not an option).
- Bahn RS, Burch HS, Cooper DS, Garber JF, Greenlee CM, Klein IL, et al. The role of propylthiouracil in the management of Graves’ disease in adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid. 2009;19:673–4. [PubMed: 19583480](The estimated fatality rate from acute liver failure due to propylthiouracil is 1:10,000 and perhaps as high as 1:2000 in children; UNOS has reported 16 deaths in adults and 7 in children since 1990; for these reasons propylthiouracil should not be considered the “first line” of treatment of Graves’ disease, methimazole being preferred except in first trimester of pregnancy).
- Rivkees SA, Mattison DR. Ending propylthiouracil-induced liver failure in children. N Engl J Med. 2009;360:1574–5. [PubMed: 19357418](Letter suggesting that propylthiouracil no longer be used as first line treatment of Graves disease in children, citing high rates of fatal hepatotoxicity from its use).
- Rivkees SA, Mattison DR. Propylthiouracil (PTU) Hepatoxicity in children and recommendations for discontinuation of use. Int J Pediatr Endocrinol. 2009;2009:132041. [PMC free article: PMC2777303] [PubMed: 19946400](Review of propylthiouracil induced liver injury; 29 cases reported in literature, 14 in children, 9 resulting in death [3 in children] and 3 in liver transplantation; in contrast, fatality or transplantation due to methimazole induced liver disease has not been reported; concluded that propylthiouracil should not be used as treatment of Graves disease in children).
- Gallelli L, Staltari O, Palleria C, De Sarro G, Ferraro M. Hepatotoxicity induced by methimazole in a previously health patient. Curr Drug Saf. 2009;4:204–6. [PubMed: 19534646](54 year old man developed fever, rash and then jaundice 14 days after starting methimazole [bilirubin 4.4 mg/dL, ALT 55 U/L, Alk P 374 U/L, GGT 627 U/L], resolving rapidly with stopping methimazole).
- Zhang M, Zhou H, He R, Di F, Yang L, Yang T. Steroids for the treatment of methimazole-induced severe cholestatic jaundice in a 74-year-old woman with type 2 diabetes. Endocrine. 2010;37:241–3. [PubMed: 20960257](74 year old woman developed jaundice and pruritus 1 month after starting methimazole [bilirubin 14.9 mg/dL, ALT 92 U/L, Alk P 301 U/L], resolving slowly and with 8 weeks of prednisone ).
- Livadas S, Xyrafis X, Economou F, Boutzios G, Christou M, Zerva A, Karachalios A, Palioura H, Palimeri S, Diamanti-Kandarakis E. Liver failure due to antithyroid drugs: report of a case and literature review. Endocrine. 2010;38:24–8. [PubMed: 20960098](34 year old woman developed pruritus 20 days after starting methimazole [bilirubin 2.4 rising to 3.6 mg/dL, ALT 141 to 1125 U/L, Alk P 189 to 396 U/L], resolving within 4 weeks of stopping).
- Shen C, Zhao CY, Liu F, Wang YD, Yu J. Acute-on-chronic liver failure due to thiamazole in a patient with hyperthyroidism and trilogy of Fallot: case report. BMC Gastroenterol. 2010;10:93. [PMC free article: PMC2928759] [PubMed: 20707932](24 year old man developed jaundice 1 year after starting methimazole [bilirubin 34.3 mg/dL, ALT 75 U/L, Alk P 133 U/L, INR 2.3], with progression to hepatic failure and death 27 days after presentation).
- Alvarez MP, Cano RL, Fernández CP, Méndez LF, García RG. Endocrinol Nutr. 2010;57:451–3. [Acute toxic hepatitis induced by methimazole: two cases] Spanish. [PubMed: 20675204](Two cases; 71 and 52 year old women developed enzyme elevations 6 weeks after starting methimazole [bilirubin 0.8 and 0.9 mg/dL, ALT 102 and 546 U/L, Alk P 889 and 999 U/L], resolving within 2 and 4 months of stopping).
- Rivkees SA, Szarfman A. Dissimilar hepatotoxicity profiles of propylthiouracil and methimazole in children. J Clin Endocrinol Metab. 2010;95:3260–7. [PubMed: 20427502](Analysis of FDA adverse event reports between 1969 and 2008 showed a higher rate of severe liver injury due to propylthiouracil than methimazole in children; “We are unaware of reports of death and liver failure in children and adolescents taking methimazole”).
- Malozowski S, Chiesa A. Propylthiouracil-induced hepatotoxicity and death. Hopefully, never more. J Clin Endocrinol Metab. 2010;95:3161–3. [PMC free article: PMC2928912] [PubMed: 20610609](Editorial in response to Rivkees [2010] summarizing the factors that led to the recommendation that propylthiouracil be avoided in children and be considered a second line drug for treating hyperthyroidism in adults).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, neither propylthiouracil or methimazole were listed in the top 41 causes).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which 5 were attributed to propylthiouracil, none to methimazole).
- Carrion AF, Czul F, Arosemena LR, Selvaggi G, Garcia MT, Tekin A, Tzakis AG, et al. Propylthiouracil-induced acute liver failure: role of liver transplantation. Int J Endocrinol. 2010;2010:910636. [PMC free article: PMC3014703] [PubMed: 21234410](Two women; one 29 year old developed ALT elevations 4 weeks and jaundice 10 weeks after starting propylthiouracil [bilirubin 19.3 mg/dL, ALT443 U/L, Alk P 509 U/L, INR 3.9], undergoing liver transplantation 8 days later; 34 year old developed jaundice 6 weeks after starting propylthiouracil [bilirubin 12.8 mg/dL, ALT 1227 U/L, Alk P 272 U/L, INR 4.3], undergoing transplant 5 days later).
- Rivkees SA. 63 years and 715 days to the "boxed warning": unmasking of the propylthiouracil problem. Int J Pediatr Endocrinol. 2010; 2010. doi:pii: 658267. [PMC free article: PMC2913555] [PubMed: 20706665](History of the debate on the safety of propylthiouracil that led to the FDA boxed warning and recommendation that methimazole be the first line drug for hyperthyroidism, particularly in children).
- Koch L. Therapy: Propylthiouracil use associated with severe hepatotoxicity in children. Nat Rev Endocrinol. 2010;6:416. [PubMed: 20681069](Editorial comment on Rivkees [2010]).
- Primeggia J, Lewis JH. Gone (from the Physicians' desk reference) but not forgotten: propylthiouracil-associated hepatic failure: a call for liver test monitoring. J Natl Med Assoc. 2010;102:531–4. [PubMed: 20575220](19 year old girl with Graves disease developed jaundice 3 months after starting propylthiouracil [bilirubin 6.5 mg/dL, ALT 1589 U/L, INR 3.0], with progressive liver failure and unsuccessful liver transplant 2 weeks after presentation).
- Regelmann MO, Miloh T, Arnon R, Morotti R, Kerkar N, Rapaport R. Graves disease presenting with severe cholestasis. Thyroid. 2012;22:437–9. [PubMed: 22458973](17 year old girl with acute hepatitis A and Graves disease developed marked cholestasis, which improved with therapy of hyperthyroidism).
- Sato H, Minagawa M, Sasaki N, Sugihara S, Kazukawa I, Minamitani K, Wataki K, et al. Comparison of methimazole and propylthiouracil in the management of children and adolescents with Graves' disease: efficacy and adverse reactions during initial treatment and long-term outcome. J Pediatr Endocrinol Metab. 2011;24:257–63. [PubMed: 21823520](Retrospective analysis of safety and efficacy of methimazole in 64 and propylthiouracil in 69 children with hyperthyroidism found similar response rates, but more frequent adverse events with initial high doses of propylthiouracil).
- Molleston JP, Fontana RJ, Lopez MJ, Kleiner DE, Gu J, Chalasani N., Drug-Induced Liver Injury Network. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN Prospective Study. J Pediatr Gastroenterol Nutr. 2011;53:182–9. [PMC free article: PMC3634369] [PubMed: 21788760](Among 30 children with drug induced liver injury enrolled in a prospective US database between 2004 and 2008, none were attributed to propylthiouracil or methimazole).
- Sequeira E, Wanyonyi S, Dodia R. Severe propylthiouracil-induced hepatotoxicity in pregnancy managed successfully by liver transplantation: A case report. J Med Case Rep. 2011;5:461. [PMC free article: PMC3183039] [PubMed: 21929775](36 year old pregnant woman developed Graves disease at 7 weeks gestation and jaundice at 17 weeks, 7 weeks after starting propylthiouracil [no liver test results provided], leading to liver transplantation and thyroidectomy, and Cesarean section at 37 weeks with delivery of a child with microcephaly).
- Sato I, Tsunekawa T, Shinohara Y, Nishio Y, Shimizu Y, Suzuki Y, Yoshioka S. A case of autoimmune hepatitis with Graves' disease treated by propylthiouracil. Nagoya J Med Sci. 2011;73:205–9. [PMC free article: PMC4831229] [PubMed: 21928702](58 year old woman developed ALT elevations 18 months after starting propylthiouracil that resolved on stopping, but recurred on restarting [bilirubin not provided, ALT 619 U/L, Alk P 445 U/L, ANA positive, IgG 2877 mg/dL], a biopsy suggested autoimmune hepatitis and she improved on prednisone therapy, but no information on outcome).
- Hackmon R, Blichowski M, Koren G. The safety of methimazole and propylthiouracil in pregnancy: a systematic review. J Obstet Gynaecol Can. 2012;34:1077–86. [PubMed: 23231846](Systematic review of the safety of propylthiouracil and methimazole during pregnancy concludes that propylthiouracil "has been associated with a rare, but serious form of hepatic failure").
- Karras S, Memi E, Kintiraki E, Krassas GE. Pathogenesis of propylthiouracil-related hepatotoxicity in children: present concepts. J Pediatr Endocrinol Metab. 2012;25:623–30. [PubMed: 23155684](Review of the mechanism of action of propylthiouracil and current concepts of the pathogenesis of its hepatotoxicity).
- Glinoer D, Cooper DS. The propylthiouracil dilemma. Curr Opin Endocrinol Diabetes Obes. 2012;19:402–7. [PubMed: 22820213](Review of FDA reports from 1982 to 2008 found 13 cases of hepatic failure due to propylthiouracil in children and 28 in adults; risk factors were longer duration of therapy, higher doses, pediatrics, female sex and potentially black race).
- Otsuka F, Noh JY, Chino T, Shimizu T, Mukasa K, Ito K, Ito K, Taniyama M. Hepatotoxicity and cutaneous reactions after antithyroid drug administration. Clin Endocrinol (Oxf). 2012;77:310–5. [PubMed: 22332800](Among 391 patients with hyperthyroidism, ALT levels >2 times ULN occurred in 9% on methimazole vs 26% on propylthiouracil arising after 12 to 60 days, but "...no serious liver failure was observed").
- Kwon H, Lee SH, Kim SE, Lee JH, Jee YK, Kang HR, Park BJ, Park JW, Hong CS. Spontaneously reported hepatic adverse drug events in Korea: multicenter study. J Korean Med Sci. 2012;27:268–73. [PMC free article: PMC3286773] [PubMed: 22379337](Summary of 2 years of adverse event reporting in Korea; of 9360 reports, 567 were liver related, including two attributed to propylthiouracil).
- de Campos Mazo DF, de Vasconcelos GB, Pereira MA, de Mello ES, Bacchella T, Carrilho FJ, et al. Clinical spectrum and therapeutic approach to hepatocellular injury in patients with hyperthyroidism. Clin Exp Gastroenterol. 2013;6:9–17. [PMC free article: PMC3579408] [PubMed: 23550044](Among 7 patients with hyperthyroidism found to have liver disease, 2 were attributed to propylthiouracil hepatotoxicity, 2 to autoimmune hepatitis and 3 to the underlying hyperthyroidism).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to antithyroid medications).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, five [including 2 deaths] were attributed to propylthiouracil but none to methimazole).
- Wang MT, Lee WJ, Huang TY, Chu CL, Hsieh CH. Antithyroid drug-related hepatotoxicity in hyperthyroidism patients: a population-based cohort study. Br J Clin Pharmacol. 2014;78:619–29. [PMC free article: PMC4243912] [PubMed: 25279406](Among 71,379 patients with hyperthyroidism initiating medical therapy between 2004 and 2009 identified in the Taiwanese National Health Insurance Research Database, methimazole/carbimazole had a higher rate of clinically apparent liver injury than propylthiouracil [3.2 vs 1.2] but a lower rate of acute liver failure [0.32 vs o.68: both per 1000-patient years]).
- Akmal A, Kung J. Propylthiouracil, and methimazole, and carbimazole-related hepatotoxicity. Expert Opin Drug Saf. 2014;13:1397–406. [PubMed: 25156887](Review of the hepatotoxicity of antithyroid medications concludes that methimazole and carbimazole are more likely to cause cholestatic liver injury, while propylthiouracil is more likely to cause acute liver failure for which reason its use should be limited to use in adults and in the first trimester of pregnancy and thyroid storm).
- Heidari R, Niknahad H, Jamshidzadeh A, Abdoli N. Factors affecting drug-induced liver injury: antithyroid drugs as instances. Clin Mol Hepatol. 2014;20:237–48. [PMC free article: PMC4197171] [PubMed: 25320726](Review of risk factors associated with liver injury from methimazole and propylthiouracil including age, gender, alcohol use and co-morbidities, none of which appear to play a very major role).
- Heidari R, Niknahad H, Jamshidzadeh A, Eghbal MA, Abdoli N. An overview on the proposed mechanisms of antithyroid drugs-induced liver injury. Adv Pharm Bull. 2015;5:1–11. [PMC free article: PMC4352210] [PubMed: 25789213](Review of the possible mechanisms of liver injury due to methimazole and propylthiouracil).
- Papachristos DA, Huynh J, Grossman M, MacIsaac RJ. Liver dysfunction and anti-thyroid therapy. SAGE Open Med Case Rep 2015; 3: 2050313X14568335. [PMC free article: PMC4857332] [PubMed: 27489677](49 year old man with Graves disease developed jaundice 2 weeks after starting carbimazole [bilirubin 34 mg/dL, ALT 104 U/L, Alk P 147 U/L], which resolved within 3 months of stopping and after radioactive iodine treatment making it difficult to assess whether the injury was due to the medication versus hyperthyroidism).
- Yang J, Li LF, Xu Q, Zhang J, Weng WW, Zhu YJ, Dong MJ. Analysis of 90 cases of antithyroid drug-induced severe hepatotoxicity over 13 years in China. Thyroid. 2015;25:278–83. [PubMed: 25384184](Among 90 patients with hyperthyroidism and drug induced liver injury due to antithyroid therapy seen at a single medical center over a 13 year period, 51 were attributed to methimazole [1 fatal] and 39 to propylthiouracil [none fatal]; both had similar latencies to onset [80% within 3 months, 60% within 1 month] and methimazole cases were more likely to be cholestatic [35% vs 18%] and less likely hepatocellular [43% vs 56%]).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 6 [0.7%] were attributed to antithyroid drugs including 3 to methimazole [thiamazole] all of which were cholestatic or mixed and self-limited in course, and 3 to propylthiouracil, one of which was fatal and one occurring in a woman treated during pregnancy).
- Čalkić L, Bajramović-Omeragić L. Relationship of recovered hepatitis B infection with appearance of toxic propylthiouracil hepatitis. Med Glas (Zenica). 2017;14:79–84. [PubMed: 28165437](45 year old woman with hyperthyroidism and anti-HBc without HBsAg in serum developed jaundice after switching from methimazole to propylthiouracil [bilirubin 8.8 rising to 16.2 mg/dL, ALT 570 U/L, Alk P 217 U/L], ultimately recovering, with no change in hepatitis B serology).
- Wu DB, Chen EQ, Bai L, Tang H. Propylthiouracil-induced liver failure and artificial liver support systems: a case report and review of the literature. Ther Clin Risk Manag. 2017;13:65–8. [PMC free article: PMC5238756] [PubMed: 28138249](16 year old Chinese girl developed jaundice 5 months after starting propylthiouracil for Graves disease [bilirubin 25.8 mg/dL, ALT 130 U/L, Alk P normal, INR 2.35] and was treated with 4 sessions of an artificial liver support system which was associated with a decrease in bilirubin, ALT, bile acids and ammonia levels each time and was followed by clinical recovery).
- Suzuki N, Noh JY, Hiruma M, Kawaguchi A, Morisaki M, Ohye H, Suzuki M, et al. Analysis of antithyroid drug-induced severe liver injury in 18,558 newly diagnosed patients with Graves' disease in Japan. Thyroid. 2019;29:1390–8. [PubMed: 31573408](Among 18,558 Japanese patients initiating medical therapy of Graves disease between 2005 and 2017, 416 [2.5%] developed evidence of liver injury [ALT above 8 times ULN or bilirubin above 3 times ULN], 1.4% on methimazole and 6.3% of propylthiouracil, 92% were women, onset usually within 30 days, rates being age-dependent and all patients recovered).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Methimazole.[LiverTox: Clinical and Researc...]Review Methimazole.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Antithyroid Agents.[LiverTox: Clinical and Researc...]Review Antithyroid Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Sustained control of Graves' hyperthyroidism during long-term low-dose antithyroid drug therapy of patients with severe Graves' orbitopathy.[Thyroid. 2011]Sustained control of Graves' hyperthyroidism during long-term low-dose antithyroid drug therapy of patients with severe Graves' orbitopathy.Laurberg P, Berman DC, Andersen S, Bülow Pedersen I. Thyroid. 2011 Sep; 21(9):951-6. Epub 2011 Aug 11.
- Comparison of standardized initial doses of two antithyroid drugs in the treatment of Graves' disease.[J Intern Med. 1996]Comparison of standardized initial doses of two antithyroid drugs in the treatment of Graves' disease.Kallner G, Vitols S, Ljunggren JG. J Intern Med. 1996 Jun; 239(6):525-9.
- Liver tests in hyperthyroidism: effect of antithyroid therapy.[J Clin Gastroenterol. 1997]Liver tests in hyperthyroidism: effect of antithyroid therapy.Gürlek A, Cobankara V, Bayraktar M. J Clin Gastroenterol. 1997 Apr; 24(3):180-3.
- Propylthiouracil - LiverToxPropylthiouracil - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...