NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
8-methoxsalen and 5-methoxsalen are furocoumarins referred to collectively as psoralens that have photosensitizing activity and are used orally and topically in conjunction with ultraviolet irradiation for the therapy of psoriasis and vitiligo. Psoralens have been linked to a low rate of transient serum enzyme elevations during therapy and to rare instances of clinically apparent acute liver injury.
Background
Psoralen (sor’ a len) is a natural furocoumarin found in the seeds of Psoralea corylifolia and other botanicals and used for their photosensitizing activity in the therapy of psoriasis and vitiligo. Psoralen is actively taken up by epidermal cells and intercalates into DNA. Upon exposure to ultraviolet (UV) light, psoralen forms cross links between DNA causing cell injury and death. The most commonly used form of psoralen, known as 8-methoxsalen (meth ox’ a len) or 8-methoxypsoralen, has been available in the United States since the 1950s and was typically administered orally or topically as an ointment with ultraviolet light treatment. 8-methoxsalen with ultraviolet radiation (PUVA) was approved for use in refractory psoriasis in the United States in 1982. Its current indications are limited to severe, recalcitrant and disabling psoriasis. It also has been used to treat vitiligo and cutaneous T cell lymphoma. Psoralen is now not commonly used, largely because of concerns over the long term safety of ultraviolet light therapy and the availability of newer, more effective and better tolerated agents for psoriasis. 8-methoxsalen is available in 10 mg capsules for oral use and as lotions of 1% methoxsalen for topical administration generically and under the brand name Oxsoralen. The typical oral dose is adjusted by weight and ranges from 10 to 70 mg, 2 to 4 times weekly taken 1 to 2 hours before a controlled dose of ultraviolet irradiation. Common side effects include nausea, headache, dizziness, fatigue, depression and erythematous and pruritic skin reactions to UV light. Rare, but potentially severe adverse reactions include increased risk of basal cell carcinoma and melanoma.
Hepatotoxicity
In open label trials, serum ALT or AST elevations occurred in 2% to 12% of subjects treated with methoxsalen and UV light. The elevations were usually mild-to-moderate in severity, asymptomatic and self-limited in course. Clinically apparent acute liver injury has also been reported with oral methoxsalen therapy, but only in isolated case reports including one instance attributed to topical methoxsalen therapy. The time to onset has ranged from 1 to 5 months, the typical latency being 6 to 8 weeks. The onset is generally insidious, with appearance of nausea and abdominal pain followed by jaundice. Fever occurs in some cases, but rash and eosinophilia are not common. The typical pattern of injury is hepatocellular. Most published cases of psoralen hepatotoxicity have been mild-to-moderate in severity, but severe jaundice and death from hepatic failure has been described in patients with preexisting cirrhosis who developed further acute liver injury attributed to methoxsalen. Most cases resolve within 6 to 8 weeks.
Psoralen is also present in many herbal products used to treat various conditions including psoriasis and vitiligo. Case reports of acute liver injury have been reported with the use of seeds, powder and teas prepared from Psoralea corylifolia under various Chinese names such as Boh Gol Zhee, Xin Cu Hei Su and Qu Bai Ba Bu Gi Pian. Chemical analyses have shown the presence of psoralen in these products. The clinical features of these cases have resembled those attributed to methoxsalen with a latency of 1 to 2 months, a hepatocellular pattern of injury, absence of immunoallergic or autoimmune features, and self-limited course with recovery within 6 to 8 weeks.
Likelihood score: C (probable rare cause of clinically apparent liver injury)
Mechanism of Injury
The mechanism of possible liver injury due to psoralen is unknown. Psoralen is metabolized in the liver, and the liver injury may be due to a rare intermediate of its metabolism. In some cases, hypersensitivity may play a role.
Outcome and Management
While most cases of acute liver injury due to psoralen have been mild-to-moderate in severity and self-limited in course, fatal instances have been reported in patients with preexisting liver disease. Several instances of rapid recurrence of injury with rechallenge have been reported and rechallenge should be avoided.
Drug Class: Dermatologic Agents, Psoriasis Agents
CASE REPORTS
Case 1. Acute hepatitis due to methoxypsoralen.(1)
A 55 year old woman with widespread psoriasis who had failed to respond to acitretin, ultraviolet (UV) light and triamcinolone cream developed nausea, abdominal pain and fatigue after 40 treatments with 5-methoxypsoralen. Methoxypsoralen was started in a dose of 20 mg and raised to 40 mg given orally three times weekly just before each UV light treatment. She had a history of a previous episode of jaundice 6 years previously that was attributed to flucloxacillin. She had no other major medical illnesses, had no risk factors for viral hepatitis and was taking no other medications or herbal preparations. Acitretin had been stopped 10 weeks before presentation and her liver tests had been normal before starting antipsoriasis therapy. Physical examination showed an improvement in her psoriatic lesions and mild jaundice. She had no fever, new onset rash or lymphadenopathy. Laboratory tests showed a total serum bilirubin of 3.2 mg/dL, ALT 2727 U/L, AST 1444 U/L and GGT 857 U/L. The white blood cell eosinophil count was normal. Tests for hepatitis A, B and C were negative as were routine autoantibodies (ANA, AMA). The methoxypsoralen and UV light treatment were discontinued. She worsened for a few days, serum bilirubin rising to 8.8 mg/dL. Thereafter, the abnormalities began to resolve and all tests were near normal 7 weeks after presentation (Table).
Key Points
| Medication: | 5-methoxypsoralen (40 mg three times weekly; 40 courses) |
|---|---|
| Pattern: | Hepatocellular-mixed (R=5.1) |
| Severity: | 3+(jaundice and hospitalization) |
| Latency: | ~3 months |
| Recovery: | ~2 months |
| Other medications: | Triamcinolone cream |
Laboratory Values
Comment
This woman with refractory psoriasis had a good clinical response to photochemical therapy using 5-methoxypsoralen (also known as methoxsalen) and narrow beam UV light, but developed constitutional symptoms followed by jaundice after approximately 3 months of therapy. The pattern of serum enzyme elevations was mixed, with prominent increases in serum aminotransferase as well as GGT levels (alkaline phosphatase was not reported, so that the calculation of the R value had to use GGT instead which yielded a value in the low "hepatocellular" range). Recovery was slow, but there was no evidence of hepatic failure. Most cases of psoralen induced liver injury have had a similar latency of 1 to 4 months and a hepatocellular pattern of injury, with no immunoallergic or autoimmune features.
Case 2. Acute hepatitis due to herbal agent containing psoralen.(2)
A 44 year old Korean woman developed nausea and fatigue 4 weeks after starting an herbal agent called "Boh-Gol-Zhee", followed by jaundice 3 weeks later. She was taking the preparation for menopausal symptoms and ingested it with a cup of black tea "every 1 hour for 7 weeks". Physical examination showed jaundice, but no fever, rash or enlargement of the liver or spleen. Laboratory tests showed a total serum bilirubin of 7.3 mg/dL (direct 4.2 mg/dL), ALT 398 U/L, AST 774 U/L, alkaline phosphatase 367 U/L and GGT 192 U/L. Tests for hepatitis A, B and C were negative as were routine autoantibodies (ANA, SMA, anti-LKM). Ultrasonography showed an abnormal liver texture, but no evidence of obstruction. Liver biopsy showed severe hepatitis with confluent necrosis. The herbal preparation was stopped and she was admitted for observation for ten days. She improved without specific therapy and in follow up 4 months later, all liver tests were normal.
Key Points
| Medication: | Boh Gol Zhee (dried Psoralea corylifolia seeds) |
|---|---|
| Pattern: | Mixed (R~3.6) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | ~1-2 months |
| Recovery: | Within 2 months |
| Other medications: | None mentioned |
Laboratory Values
Comment
Boh-Gol-Zhee is the Korean name for the herbal product prepared from dried mature seeds of Psoralea corylifolia, which is used predominantly to treat menopausal symptoms. The seeds have more than 35 identified chemical constituents including psoralen, psoralidin, backuchiol, bavachin, genistein (a phytoestorgen), various resins and fatty oils. Powdered extracts of P. corylifolia have been linked to several instances of jaundice, and psoralen has been considered the most likely culprit in causing liver injury. The cases of liver injury from herbal sources of psoralen have resembled those attributed to methoxsalen treatment of psoriasis and vitiligo.
Case 3. Acute liver failure attributed to methoxsalen therapy.(3)
A 54 year old man with cirrhosis due to long term methotrexate therapy of psoriasis developed jaundice and evidence of hepatic failure a few weeks after starting 8-methoxsalen with UV irradiation. He had progressive weakness, nausea, abdominal pain, confusion, ascites and peripheral edema. He was transferred to a liver transplant center where laboratory test results showed a total serum bilirubin of 39.6 mg/dL (direct 16.8 mg/dL), ALT 83 U/L, AST 144 U/L, alkaline phosphatase 114 U/L and GGT 20 U/L. The prothrombin time was 23 seconds and serum albumin 2.7 g/dL. Tests for hepatitis B, CMV, EBV and HSV were negative. Emergency liver transplantation was done 3 days after transfer and the explant showed cirrhosis, sinusoidal fibrosis, acute hepatocellular necrosis and collapse, and marked cholestasis. He recovered and was discharged 6 weeks later and was active and working when the report was published 6 years later.
Key Points
| Medication: | 8-methoxypsoralen (dose not given) |
|---|---|
| Pattern: | Acute on chronic liver failure |
| Severity: | 5+ (liver failure, transplantation) |
| Latency: | ~4 weeks (not clearly stated) |
| Recovery: | None |
| Other medications: | None mentioned, methotrexate previously for 15 years |
Comment
A 54 year old man with well compensated cirrhosis attributed to long term methotrexate therapy of psoriasis developed acute hepatic decompensation after being started on therapy with methoxsalen and ultraviolet light. The latency to onset of symptoms and jaundice was not clearly defined in the report and pretreatment laboratory values were not provided. Drug induced liver injury superimposed upon chronic liver disease or cirrhosis often has a different pattern of clinical presentation, usually defined as "acute-on-chronic". Laboratory values may also be unusual, the only change from baseline abnormalities being a sudden appearance of jaundice followed rapidly by signs and symptoms of hepatitis failure. It remains unclear whether drug induced liver injury is more severe in patients with preexisting liver disease or if the injury is just less well tolerated. The two published instances of hepatic failure due to psoralen hepatotoxicity occurred in patients with known, preexisting cirrhosis.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
8-Methoxsalen – Generic, Oxsoralen®
DRUG CLASS
Dermatologic Agents, Psoriasis Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| 8-Methoxsalen | 298-81-7 | C12-H8-O4 |
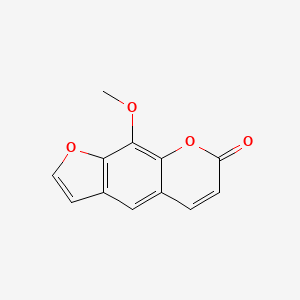
|
CITED REFRENCES
- 1.
- Stephens RB, Cooper A. Hepatitis from 5-methoxypsoralen occurring in a patient with previous flucloxacillin hepatitis. Australas J Dermatol 1999; 40: 217-9. 10570561. [PubMed: 10570561]
- 2.
- Nam SW, Baek JT, Lee DS, Kang SB, Ahn BM, Chung KW. A case of acute cholestatic hepatitis associated with the seeds of Psoralea corylifolia (Boh-Gol-Zhee). Clin Toxicol (Phila) 2005; 43: 589-91. 16255343. [PubMed: 16255343]
- 3.
- Markin RS, Donovan JP, Shaw BW Jr, Zetterman RK. Fulminant hepatic failure after methotrexate and PUVA therapy for psoriasis. J Clin Gastroenterol 1993; 17: 311-3. 8308218. [PubMed: 8308218]
ANNOTATED BIBLIOGRAPHY
References updated: 16 January 2020
Abbrevations used: UV, ultraviolet.
- Reuben A. Hepatotoxicity of immunosuppressive drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2011, pp. 569-91.(Review of hepatotoxicity published in 1999 mentions that methoxsalen has been associated with both hepatocellular and cholestatic injury, which can be fatal).
- Sewell MJ, Burkhart C, Morrell D, Goldsmith L. Dermatological pharmacology. In, Brunton LL, Chabner BA, Hillal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1271-96.(Textbook of pharmacology and therapeutics).
- Fitzpatrick TB, Imbrie JD, Labby D. Effect of methoxsalen on liver function. JAMA 1958; 167: 1586-9. 13563146. [PubMed: 13563146](Among 24 normal volunteers given methoxsalen or placebo for 3 months, serial determinations of liver function tests, including thymol and zinc turbidity, cephalin-flocculations and BSP excretion, remained normal in all).
- Tucker HA. Clinical and laboratory tolerance studies in volunteers given oral methoxsalen. J Invest Dermatol 1959; 32: 277-80. 13641797. [PubMed: 13641797](Among 100 healthy male volunteers given methoxsalen and 20 given placebo for 21 days, none developed hepatic function test abnormalities [bilirubin, thymol and zinc turbidity and cephalin-flocculation]).
- Parrish JA, Fitzpatrick TB, Tanenbaum L, Pathak MA. Photochemotherapy of psoriasis with oral methoxsalen and long-wave ultraviolet light. N Engl J Med 1974; 291: 1207-11. 4422691. [PubMed: 4422691](Among 21 patients with psoriasis treated with oral methoxsalen and long wave ultraviolet (UV) phototherapy for an unstated amount of time, all had improvement in skin lesions and serum bilirubin, and AST and Alk P remained normal in all).
- Swanbeck G, Thyresson-Hök, Bredberg A, Lambert B. Treatment of psoriasis with oral psoralens and longwave ultraviolet light. Therapeutic results and cytogenetic hazards. Acta Derm Venereol 1975; 55: 367-76. 52973. [PubMed: 52973](Among 40 patients with psoriasis treated with 8-methoxypsoralen and UV phototherapy, 6 had transient elevations in serum aminotransferase levels, but further specifics were not given).
- Dahl KB, Brodthagen H. [Psoriasis vulgaris. Treatment with carbon-arc light baths and 8-methoxypsoralen. A double-blind investigation]. Ugeskr Laeger 1976; 138: 2221-4. Danish. 788271. [PubMed: 788271](Among 31 patients with psoriasis treated with 8-methoxsalen and UV phototherapy, all except one had a clinical response, but 4 [13%] developed transient ALT elevations, 3 seemingly precipitated by alcohol ingestion).
- Weismann K, Howitz J, Bro-Jorgensen A. [Treatment of psoriasis with 8-methoxypsoralen and long-wave ultraviolet light]. Ugeskr Laeger 1976 138: 2225-8. Danish. 968972. [PubMed: 968972](Among 73 patients with psoriasis treated with 8-methoxsalen or placebo followed by UV phototherapy for an average of 6 weeks, 40% had side effects of nausea, dyspepsia and vertigo, and 2 [3%] had transient AST elevations that resolved with stopping).
- Melski JW, Tanenbaum L, Parrish JA, Fitzpatrick TB, Bleich HL. Oral methoxsalen photochemotherapy for the treatment of psoriasis: a cooperative clinical trial. J Invest Dermatol 1977; 68: 328-35. 864273. [PubMed: 864273](Among 1308 patients with psoriasis treated with oral methoxsalen and UV phototherapy for up to 18 months, psoriasis cleared in 88% and side effects were uncommon and generally mild; serum bilirubin, AST and Alk P levels remained normal or decreased).
- Bjellerup M, Bruze M, Hanssen A, Krook G, Ljunggren JB. Liver injury following administration of 8-methoxypsoralen during PUVA therapy. Acta Derm Venereol (Stockh) 1979; 59: 371-2. 92158. [PubMed: 92158](57 year old woman with psoriasis developed symptoms 7 weeks after starting 8-methoxsalen and UV phototherapy 4 times weekly [bilirubin normal, ALT 368 U/L, Alk P normal], resolving within 7 weeks with two positive rechallenges [ALT ~ 2 times ULN]).
- Pariser DM, Wyles RJ. Toxic hepatitis from oral methoxsalen phototherapy(PUVA). J Am Acad Dermatol 1980; 3: 248-50. 7451691. [PubMed: 7451691](59 year old woman with psoriasis resistant to methotrexate developed fatigue and fever 5 hours after starting a 14th course of 8-methoxsalen and UV phototherapy [bilirubin 1.4 mg/dL, AST 103 rising to 775 U/L, Alk P 100 U/L], resolving in 3 days and recurring with oral, but not topical reexposure).
- Chretien P, Galmiche JP, Payeeneville JM, Fouin-Fortunet H, Lauret P, Boismare F, Colin R. Effects of oral methoxy-psoralen photochemotherapy(PUVA) on liver function and antipyrin kinetics. Int J Clin Pharmacol Res 1983; 3: 343-7. 6678827. [PubMed: 6678827](Among 19 patients with psoriasis treated with methoxsalen and UV phototherapy for 28 days, liver tests remained normal, but antipyrine clearance decreased, likely because of CYP 450 interactions).
- Freeman K, Warin AP. Deterioration of liver function during PUVA therapy. Photodermatol Photoimmunol Photomed 1984; 1: 147-8. 6527958. [PubMed: 6527958](57 year old man with psoriasis and alcoholic liver disease developed jaundice 8 weeks after starting oral methoxsalen and UV phototherapy, with progressive liver failure and death; autopsy showing cirrhosis and alcoholic hepatitis).
- Nyfors A, Dahl-Nyfors B, Hopwood D. Liver biopsies from patients with psoriasis related to photochemotherapy(PUVA): findings before and after 1 year of therapy in twelve patients. A blind study and review of literature on hepatotoxicity of PUVA. J Am Acad Dermatol 1986; 14(1): 43-8. 3950113. [PubMed: 3950113](Among 12 patients with psoriasis treated with psoralen and UV phototherapy [average 79 treatments], liver biopsies done before and after a year of treatment showed no change in any light microscopic finding).
- Hann SK, Park YK, Im S, Koo SW, Haam IB. The effect on liver transaminases of photoxic drugs used in systemic photochemotherapy. J Am Acad Derm 1992; 26: 646-8. 1597556. [PubMed: 1597556](Among 162 patients with psoriasis or vitiligo treated with various forms of psoralen and UV phototherapy, only 3 [2%] developed ALT elevations [peak values 44, 52 and 275], all without symptoms or jaundice, resolving rapidly).
- Markin RS, Donovan JP, Shaw BW Jr, Zetterman RK. Fulminant hepatic failure after methotrexate and PUVA therapy for psoriasis. J Clin Gastroenterol 1993; 17: 311-3. 8308218. [PubMed: 8308218](54 year old man with psoriasis and cirrhosis after long term methotrexate developed jaundice within a few weeks of starting oral 8-methoxsalen and UV phototherapy, with progression to liver failure [bilirubin 39.6 mg/dL, ALT 83 U/L, Alk P 114 U/L] and undergoing urgent liver transplant, the explant showing cirrhosis and acute necrosis with collapse).
- Perharic L, Shaw D, Colbridge M, House I, Leon C, Murray V. Toxicological problems resulting from exposure to traditional remedies and food supplements. Drug Saf 1994; 11: 284-94. 7848547. [PubMed: 7848547](Retrospective and prospective survey identified 1070 enquiries following exposure to herbal products, including 4 cases of liver from Chinese herbs, one case of which was attributed to psoralea coryliflora, no details given).
- Park YM, Kim TY, Kim CW. Reproducible elevation of liver transaminases by topic 8-methoxypsoralen. Photodermatol Photoimmunol Photomed 1994; 10: 261-3. 7727284. [PubMed: 7727284](17 year old woman with vitiligo developed jaundice and pruritus 3 months after starting topical 8-methoxsalen and UV phototherapy [bilirubin 7.6 mg/dL, ALT 1150 U/L, Alk P 554 U/L], resolving within 4 months of stopping and recurring upon reexposure to psoralen alone without UV light UV phototherapy).
- Berg M, Ros AM. Treatment of psoriasis with psoralens and ultraviolet A. A double-blind comparison of 8-methoxypsoralen and 5-methoxypsoralen. Photodermatol Photoimmunol Photomed 1994; 10: 217-20. 7880762. [PubMed: 7880762](Among 38 patients with psoriasis treated with either 5- or 8-methoxsalen and UV phototherapy, efficacy was similar as were side effects; one patient developed liver injury 3 weeks after starting 5-methoxsalen [ALT 1500 U/L, bilirubin and Alk P not provided], which returned to normal between 6 months and one year after stopping).
- Choi HJ, Kim S, Hann SK, Park YK. The effect on liver transaminases of 5-methoxypsoralen used in systemic photochemotherapy. Ann Dermatol. 1995;7:51–3. Not in PubMed.(Among 80 patients with psoriasis or vitiligo treated with 5-methoxsalen and UV phototherapy, 4 had ALT elevations [59, 59, 235, 266 U/L] after 3.5-14 months of therapy, resolving within 2 months of stopping; no mention of jaundice or symptoms).
- McNeely W, Goa KL. 5-methoxypsoralen: a review of its effects in psoriasis and vitiligo. Drugs 1998 56: 667-90. 9806110. [PubMed: 9806110](Review of chemistry, mechanism of action, pharmacology, efficacy and safety of 5-methoxypsoralen states that side effects are lower with 5- compared to 8-methoxypasoralen, the most common being gastrointestinal upset, skin redness and pruritus; mild to moderate ALT elevations occurred in 5% of patients in one study [Choi 1995]).
- Stephens RB, Cooper A. Hepatitis from 5-methoxypsoralen occurring in a patient with previous flucloxacillin hepatitis. Australas J Dermatol 1999; 40: 217-9. 10570561. [PubMed: 10570561](55 year old woman with psoriasis developed nausea and abdominal pain 5 months after starting 5-methoxypsoralen [bilirubin 3.2 mg/dL, ALT 2727 U/L, GGT 857 U/L], resolving within 2 months of stopping).
- Nam SW, Baek JT, Lee DS, Kang SB, Ahn BM, Chung KW. A case of acute cholestatic hepatitis associated with the seeds of Psoralea corylifolia (Boh-Gol-Zhee). Clin Toxicol (Phila) 2005; 43: 589-91. 16255343. [PubMed: 16255343](44 year old woman developed jaundice 7 weeks after starting daily ingestion of Psoralea corylifolia tea "every hour" [bilirubin 7.3 mg/dL, ALT 398 U/L, Alk P 367 U/L], with rapid improvement on stopping).
- Cheung WI, Tse ML, Ngan T, Lin J, Lee WSK, Poon WT, Mak TWL, et al. Liver injury associated with the use of Fructus Psoraleae(Bol-gol-zhee or Bu-gu-zhi) and its related proprietary medicine. Clin Toxicol 2009; 47: 683-5. 19640237. [PubMed: 19640237](3 cases of liver injury after use of dried seeds of P. corylifolia to treat skin conditions, including 2 men and 1 woman, ages 20-39 years, taking product for 2-8 weeks, developing jaundice [bilirubin 5.1-5.5 mg/dL, ALT 720-2248 U/L, Alk P 115-177 U/L, INR 1.1-1.2], resolving within 4-9 weeks of stopping; analysis of the products [each with a different name] revealed psoralen, isopsoralen and bakuchiol).
- Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, Gottlieb A, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol 2010; 62: 114-35. 19811850. [PubMed: 19811850](Guidelines on use of phototherapy in psoriasis by the American Academy of Dermatology mentions acute common toxicities of erythema, pruritus, serosis, pigmentation and gastrointestinal symptoms and potential long term toxicities of squamous cell carcinoma and melanoma, but does not mention ALT elevations or hepatotoxicity).
- Walker D, Jacobe H. Phototherapy in the age of biologics. Semin Cutan Med Surg 2011; 30: 190-8. 22123416. [PubMed: 22123416](Discussion of role of psoralen and UV phototherapy in psoriasis and other skin conditions in view of availability of newer biologic agents, concluding that phototherapy is a valuable option; ordered October 12, 2014).
- Almutawa F, Alnomair N, Wang Y, Hamzavi I, Lim HW. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol 2013; 14: 87-109. 23572293. [PubMed: 23572293](Systematic review of safety and efficacy of UV based therapy of psoriasis discusses erythema and tolerability, but does not mention hepatotoxicity or ALT elevations).
- Carrascosa JM, Plana A, Ferrándiz C. Effectiveness and safety of psoralen-UVA (PUVA) topical therapy in palmoplantar psoriasis: a report on 48 patients. Actas Dermosifiliogr 2013; 104: 418-25. 23680012. [PubMed: 23680012](Among 48 patients with palmoplantar psoriasis treated with psoralen and UV A phototherapy, beneficial responses occurred in 63% and side effects in 25%; ALT and hepatotoxicity were not reported).
- Bansal S, Sahoo B, Garg V. Psoralen-narrowband UVB phototherapy for the treatment of vitiligo in comparison to narrowband UVB alone. Photodermatol Photoimmunol Photomed 2013; 29: 311-7. 24118425. [PubMed: 24118425](Among 45 Indian patients with vitiligo treated with narrowband UV B phototherapy with or without psoralen, response rates were better in those receiving psoralen, but adverse events were also more common, including nausea; no mention of ALT elevations or hepatotoxicity).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. 24552865. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to psoralen).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. 25754159. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were due to psoralen).
- El-Mofty M, Rasheed H, El-Eishy N, Hegazy RA, Hafez V, Shaker O, El-Samanoudy SI. A clinical and immunological study of phototoxic regimen of ultraviolet A for treatment of alopecia areata: a randomized controlled clinical trial. J Dermatolog Treat 2019; 30: 582-7. 30411986. [PubMed: 30411986](Among 40 patients with alopecia areata treated with topical psoralen and UV A phototherapy vs intralesional injections of corticosteroids, response rates [hair regrowth] were similar in both groups, whereas adverse events were more frequent with psoralen including itching and local erythemia; ALT elevations and hepatotoxicity were not mentioned).
- Drugs for psoriasis. Med Lett Drugs Ther 2019; 61(1574): 89-96. 31381544. [PubMed: 31381544](Concise review of current recommendations on drug therapy of psoriasis mentions that UV A phototherapy can be used for widespread disease and addition of topical or oral psoralen with UV phototherapy is also effective but may increase the risk of skin cancer; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Psoralens percutaneous permeation across the human whole skin and the epidermis in respect to their polarity (in vitro study).[J Dermatol Sci. 1997]Psoralens percutaneous permeation across the human whole skin and the epidermis in respect to their polarity (in vitro study).Saïd A, Makki S, Muret P, Humbert P, Millet J. J Dermatol Sci. 1997 Feb; 14(2):136-44.
- Review Clinical pharmacokinetics of methoxsalen and other psoralens.[Clin Pharmacokinet. 1986]Review Clinical pharmacokinetics of methoxsalen and other psoralens.de Wolff FA, Thomas TV. Clin Pharmacokinet. 1986 Jan-Feb; 11(1):62-75.
- Electrophoretic studies of serum proteins in vitiligenous patients treated with oral psoralens and ultra-violet irradiation.[J Invest Dermatol. 1963]Electrophoretic studies of serum proteins in vitiligenous patients treated with oral psoralens and ultra-violet irradiation.EL-HEFNAWI H, EL-HAWARY MF, RASHEED A. J Invest Dermatol. 1963 Feb; 40:111-9.
- An unusual cause of burn injury: unsupervised use of drugs that contain psoralens.[J Burn Care Rehabil. 1999]An unusual cause of burn injury: unsupervised use of drugs that contain psoralens.Türegün M, Oztürk S, Selmanpakoğlu N. J Burn Care Rehabil. 1999 Jan-Feb; 20(1 Pt 1):50-2.
- Review Psoralen-protein photochemistry--a forgotten field.[J Photochem Photobiol B. 1995]Review Psoralen-protein photochemistry--a forgotten field.Schmitt IM, Chimenti S, Gasparro FP. J Photochem Photobiol B. 1995 Feb; 27(2):101-7.
- Psoralen - LiverToxPsoralen - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...