NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Simeprevir is an oral, direct acting hepatitis C virus (HCV) protease inhibitor that is no longer available but was previously used in combination with other antiviral agents in the treatment of chronic hepatitis C, genotypes 1 and 4, but subsequently was withdrawn. Simeprevir has been linked to isolated, rare instances of mild, acute liver injury during treatment and to occasional cases of hepatic decompensation in patients with preexisting cirrhosis.
Background
The hepatitis C virus is a small RNA virus that is a major cause of chronic hepatitis, cirrhosis and hepatocellular carcinoma in the United States as well as worldwide. Various approaches to antiviral therapy of chronic hepatitis C have been developed, starting in the 1980s with interferon alfa which was replaced in the 1990s by long acting forms of interferon (peginterferon), to which was added the oral nucleoside analogue, ribavirin. Between 2010 and 2015, several potent oral, direct acting anti-HCV agents were developed and combinations of these found to have marked activity against the virus, allowing for highly effective therapy without use of interferon with treatment courses of 12 to 24 weeks only. These direct acting agents included HCV protease (NS3/4) inhibitors, structural replication complex (NS5A) inhibitors and the HCV RNA polymerase (NS5B) inhibitors.
Simeprevir (sim e' pre vir) was the third HCV protease inhibitor to become clinically available for therapy of hepatitis C. Like other HCV protease inhibitors, simeprevir blocks the activity of the viral encoded protease (HCV nonstructural [NS] region 3/4) that is essential in the posttranslational modification of the viral polypeptide that is cleaved into a series of structural and nonstructural (enzyme) regions. When used by itself, simeprevir results in rapid inhibition of HCV RNA levels, but resistance develops rapidly in a high proportion of patients. When combined with peginterferon and ribavirin, it was shown to provide a sustained inhibition of HCV RNA with a low rate of antiviral resistance. When given for 24 to 48 weeks, triple therapy using simeprevir increased the sustained virological response (SVR) rate from 40% to 50% (peginterferon and ribavirin alone) to 65% to 75% in patients with genotype 1. Even higher rates of response were found when simeprevir was combined with the HCV polymerase inhibitor sofosbuvir in an all-oral, interferon free regimen for 12 weeks. Simeprevir was approved for use in the United States in 2013 for patients with chronic hepatitis C, genotypes 1 and 4, in combination with peginterferon and ribavirin or as an all-oral regimen with sofosbuvir. It was voluntarily withdrawn by the sponsor in 2018 because of availability of other highly effective regimens for hepatitis C consisting of fixed doses in single tablet formulations. Interestingly, simeprevir has been shown to have antiviral activity against SARS-CoV-2 but has not been assessed in clinical cases of COVID-19. Simeprevir was previously available in 150 mg capsules under the brand name Olysio (formerly TMC435), and the recommended dose was 150 mg once daily for 12 weeks. Common side effects included rash, photosensitivity, pruritus, nausea, headache, muscle aches and abdominal discomfort.
Hepatotoxicity
In large randomized controlled trials, simeprevir was not linked to an increased rate of serum enzyme elevations during treatment or with instances of clinically apparent liver injury. Simeprevir can cause a mild increase in indirect bilirubin and some patients become visibly jaundiced; but the bilirubin elevations were generally mild, transient and not associated with changes in serum aminotransferase or alkaline phosphatase levels. After its approval and more wide scale use, however, simeprevir was implicated in at least one case of an acute hepatitis (Case 1). The latency to onset was 7 weeks and pattern of injury was hepatocellular without immunoallergic or autoimmune features. Recovery was rapid once therapy was stopped.
In addition, simeprevir, in combination with other agents, has been linked to instances of acute, seemingly spontaneous decompensation of HCV related cirrhosis. The role of simeprevir as opposed to the other HCV antivirals used in combination was often unclear. Rates of hepatic decompensation during simeprevir combination therapy of cirrhosis due to hepatitis C was approximately 2% to 3% when combined with peginterferon and ribavirin, and 0.5% to 1.0% when used with sofosbuvir. Because of the risk of decompensation, patients with cirrhosis who are treated with antiviral regimens (both all-oral and interferon based) should be monitored for evidence of worsening liver disease, particularly during the first 4 weeks of treatment. This complication is probably more common in patients with more advanced liver disease, Child’s Class B cirrhosis and those with a previous history of liver decompensation.
In addition, direct acting antiviral therapy of hepatitis C including regimens using simeprevir have been linked to instances of reactivation of hepatitis B with rise or denovo appearance of HBV DNA in serum followed by a flare of hepatitis that can be severe. The rate of reactivation is appreciable in patients with HBsAg and HBV DNA in serum, but also can occur rarely in persons without HBsAg but with anti-HBc in serum. For these reasons, screening for hepatitis B is recommended in patients who are to receive antiviral therapy of chronic hepatitis C.
Likelihood score: B (likely cause of clinically apparent liver injury usually manifesting as hepatic decompensation in patients with preexisting cirrhosis and probable rare cause of reactivation of hepatitis B in susceptible patients).
Mechanism of Injury
The mechanism by which simeprevir might cause liver injury is not known. It is metabolized in the liver largely via the cytochrome P450 system, predominantly CYP 3A and it is an inhibitor of the drug transporters P-glycoprotein and OATP1Ba/3 and the efflux transporters MDR1, MRP2 and BSEP, perhaps accounting for the indirect hyperbilirubinemia that occurs in some patients. Simeprevir is associated with drug-drug interactions and it can raise levels of some statins. The decompensation that occurs with simeprevir combination therapy may be due to a direct effect of the agent, or else represent a usual complication of the rapid eradication of HCV infection. Finally, the episodes of decompensation may be incidental and unrelated to the antiviral therapy.
Outcome and Management
Combination antiviral regimens with peginterferon and ribavirin are now rarely used, largely because of the superior efficacy and better tolerance of all-oral regimens for hepatitis C. The oral regimen of sofosbuvir and simeprevir, while highly effective, is more expensive than other equally effective oral regimens and simeprevir is no longer available in the United States. Simeprevir in combination with other antiviral agents has been associated with cases of decompensation in patients with preexisting cirrhosis and with reactivation of hepatitis B in patients with ongoing or previous HBV infection.
Because of the risk of hepatic decompensation in patients with preexisting cirrhosis, particularly those with a prior history of hepatic decompensation, regular monitoring is recommended for patients with hepatitis C related cirrhosis undergoing antiviral therapy, particularly during the first 4 weeks. Therapy should be suspended if signs or symptoms of hepatic failure arise such as marked rises in serum bilirubin (both direct and indirect) and any worsening of ascites, hepatic encephalopathy or prolongation of the prothrombin time. Antiviral therapy of hepatitis C is also a risk factor for reactivation of hepatitis B, and screening for HBsAg and anti-HBc before starting antiviral therapy is recommended. Those with evidence of ongoing or previous HBV infection should be treated prophylactically with antiviral agents with potent activity against HBV or monitored with tests for HBV DNA and started on anti-HBV therapy if levels rise.
Drug Class: Antiviral Agents, Hepatitis C Agents, HCV Protease Inhibitors
CASE REPORTS
Case 1. Acute hepatitis during therapy of chronic hepatitis C with simeprevir, peginterferon and ribavirin.(1)
A 65 year old Japanese man with chronic hepatitis C, genotype 1b, developed fatigue and abdominal bloating and was found to have worsening of serum aminotransferase levels 49 days after starting triple antiviral therapy with simeprevir [100 mg orally, once daily], peginterferon [100 μg subcutaneously, each week] and ribavirin [400 mg orally, twice daily]. Serum ALT which had fallen on antiviral therapy rose to 768 U/L with a serum bilirubin of 3.3 mg/dL, alkaline phosphatase 171 U/L, INR 1.2 and albumin 3.8 g/dL (Table). He had no previous history of liver disease other than hepatitis C which was attributed to a blood transfusion 45 years previously and for which he had not been previously treated. He also had a history of heavy alcohol use, but had stopped five years previously. His other medical conditions included diabetes for which he was taking voglibose (an alpha glucosidase inhibitor) and glimepiride (a sulfonylurea), and hyperuricemia for which he was taking allopurinol. All of his medications he had taken for more than a year and he denied use of other over-the-counter medications or nutritional or herbal supplements. Physical examination showed no evidence of fever or rash, and an eosinophil count was normal (1.2%). Tests for hepatitis A, B, C and E were negative as were tests for acute EBV and CMV infection. Both ANA and SMA were negative and serum globulins were not elevated. Imaging of the liver by ultrasound showed no evidence of biliary obstruction or masses. A liver biopsy showed evidence of acute hepatocellular injury superimposed upon on chronic hepatitis, but did not suggest autoimmune hepatitis. Antiviral therapy was suspended, and he improved rapidly. One month later, his symptoms had resolved and all liver tests, including ALT levels, were normal. He remained HCV RNA negative and was ultimately considered to have a sustained virologic response despite the early discontinuation of therapy.
Key Points
| Medication: | Simeprevir, peginterferon and ribavirin |
|---|---|
| Pattern: | Hepatocellular (R ratio=18) |
| Severity: | Moderate (jaundice and hospitalization) |
| Latency: | 7 weeks |
| Recovery: | 1 month |
| Other medications: | Ursodiol, voglibose, glimepiride and allopurinol chronically |
Laboratory Values
Comment
A man with known chronic hepatitis C for many years developed an asymptomatic worsening of disease with ALT rising to 301 U/L, for which reason he was started on combination therapy of simeprevir with peginterferon and ribavirin, a regimen that was available and approved for use in Japan. He did well for the first six weeks of treatment and was HCV RNA negative when tested at 4 weeks. After 7 weeks, however, he developed nonspecific symptoms of fatigue and was found to have marked elevations in ALT and mild jaundice. Thorough evaluation found no evidence of other forms of liver disease and he was, indeed, still negative for HCV RNA. He improved rapidly once antiviral therapy was stopped and all liver tests had returned to normal one month later. Fortunately, his HCV infection did not relapse and he appeared to have achieved an SVR with other 7 weeks of treatment. This was a very convincing case of liver injury due to the antiviral therapy. Because he was receiving three agents, one cannot say for sure which one was responsible, but attributing it to simeprevir appears appropriate.
Case 2. Acute hepatic decompensation in a patient with chronic hepatitis C and cirrhosis during therapy with simeprevir and sofosbuvir.(2)
A 56 year old man with chronic hepatitis C, genotype 1a, and Child’s Class B cirrhosis developed worsening fatigue, anorexia and jaundice 4 weeks after starting oral therapy with simeprevir and sofosbuvir. He had a history of excessive alcohol use, but had stopped drinking years before. He had been treated with peginterferon and ribavirin for hepatitis C on two occasions in the past, without a significant response and had undergone transjugular intrahepatic portosystemic stent shunting (TIPSS) two years previously because of refractory ascites and esophageal varices. He was evaluated for liver transplantation, having a MELD score of 17 with serum bilirubin 5.3 mg/dL, ALT 29 U/L, INR not given. He received approval for pretransplant, compassionate use therapy with the oral antiviral regimen of sofosbuvir and simeprevir (doses not provided). He had a rapid virological response becoming HCV RNA negative by week 4. However, the total serum bilirubin level rose concurrently to 6.6 by week 2 and 28.0 mg/dL by week 4, at which point the antiviral drugs were stopped (Table). His MELD score subsequently rose to 36, the INR to 4.4 and he underwent successful emergency liver transplantation 4 weeks after stopping therapy. In follow up, he remained HCV RNA negative, suggesting a SVR despite therapy for only 4 weeks.
Key Points
| Medication: | Simeprevir and sofosbuvir |
|---|---|
| Pattern: | Unknown (alkaline phosphatase not given) |
| Severity: | Fatal (liver transplantation) |
| Latency: | 4 weeks |
| Recovery: | None |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | AST (U/L) | Bilirubin (mg/dL) | HCV RNA (IU/mL) | Other |
|---|---|---|---|---|---|---|
| Day 0 | 0 | 29 | 52 | 5.3 | Present | Therapy started |
| 2 Weeks | 0 | 6.6 | ||||
| 4 Weeks | 0 | 90 | 229 | 28.0 | None | |
| 8 Weeks | 4 weeks | Normal | Normal | 14.2 | None | Liver transplantation |
| Normal Values | Not Provided | <1.2 | None | |||
Comment
A man with chronic hepatitis C and cirrhosis and a past history of alcoholism presented for a liver transplant evaluation and was found to have advanced liver disease with moderate decompensation (Child’s Class B). He had HCV genotype 1a infection and a history of “null” responses to courses of peginterferon and ribavirin. He was started on an oral regimen of simeprevir and sofosbuvir, which in clinical trials had been shown to yield sustained virological response rates of 73% in patients with Child’s B cirrhosis. Indeed, he rapidly became HCV RNA negative, but had worsening symptoms and jaundice with rises in serum ALT and INR, prompting emergent liver transplantation. Fortunately, the antiviral therapy was successful in eradicating HCV infection and he remained HCV RNA negative thereafter. While somewhat short on details, this case report was reasonably convincing as demonstrating liver injury due to antiviral therapy. The authors attributed the injury to simeprevir, but sofosbuvir was given concurrently making it difficult to attribute the episode to one versus the other agent. Another possibility was that the sudden decompensation was triggered by the rapid clearance of HCV infection, as occurs in some cases of chronic hepatitis B during initiation of antiviral therapy. Finally, the decompensation may have been coincidental and not related to the drugs or antiviral response at all. Several such instances of “spontaneous” hepatic decompensation arising during potent antiviral therapy have been described which argues for careful monitoring of patients with cirrhosis, particularly during the first few weeks of therapy. This case also shows that antiviral therapy of patients with advanced cirrhosis is best left to centers with expertise in managing end stage liver disease and where liver transplantation is available.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Simeprevir – Olysio®
DRUG CLASS
Hepatitis C Agents
Product labeling at DailyMed, National Library of Medicine, NIH
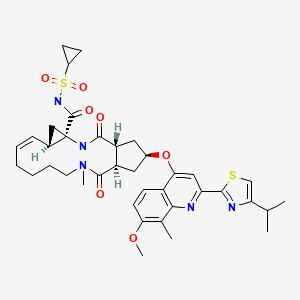
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Simeprevir | 923604-59-5 | C38-H47-N5-O7-S2 |
|
CITED REFERENCES
- 1.
- Igawa T, Fushimi S, Matsuo R, Ikeda F, Nouso K, Yoshino T, Nakatsukasa H. Severe liver injury associated with simeprevir plus pegylated interferon/ribavirin therapy in a patient with treatment-naïve genotype 1b hepatitis C virus: a case report. Clin J Gastroenterol. 2014;7:465–70. [PubMed: 26184030]
- 2.
- Stine JG, Intagliata N, Shah NL, Argo CK, Caldwell SH, Lewis JH, Northup PG. Hepatic decompensation likely attributable to simeprevir in patients with advanced cirrhosis. Dig Dis Sci. 2015;60:1031–5. [PMC free article: PMC6600814] [PubMed: 25373453]
ANNOTATED BIBLIOGRAPHY
References updated: 07 February 2022
[Abbreviation used: HBV, hepatitis B virus; HCV, hepatitis C virus; DDA, direct acting antiviral agent; SVR, sustained virological response.
- Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013.(Multi-authored textbook of hepatotoxicity published in 2013 does not discuss oral, direct acting antiviral agents used to treat hepatitis C).
- Kim JL, Morgenstern KA, Lin C, Fox T, Dwyer MD, Landro JA, Chambers SP, et al. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell. 1996;87:343–55. [PubMed: 8861917](Report of the crystal structure of the NS3/4 region of HCV with detailed description of the active serine protease catalytic site, the target for subsequent development of specific inhibitors of the HCV protease).
- Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, Marcellin P, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918–29. [PMC free article: PMC4112500] [PubMed: 23907700](Among 388 patients with chronic hepatitis C, genotype 1, randomized to 1 of 5 different regimens of simeprevir vs placebo combined with peginterferon and ribavirin for 24 or 48 weeks, SVR rates higher with simeprevir vs placebo [76% to 86% vs 66%], while side effects were similar in all groups and there were no concurrent increases in serum bilirubin and aminotransferase levels).
- Hayashi N, Seto C, Kato M, Komada Y, Goto S. Once-daily simeprevir (TMC435) with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1-infected patients in Japan: the DRAGON study. J Gastroenterol. 2014;49:138–47. [PMC free article: PMC3895197] [PubMed: 24005956](Among 92 Japanese patients with chronic hepatitis C, genotype 1, treated with simeprevir [50 or 100 mg] vs placebo for 12 or 24 weeks combined with peginterferon and ribavirin for 24 or 48 weeks, the SVR rates were higher with simeprevir [77% to 92% vs 46%], but adverse event rates were similar except for higher rates of mild bilirubin elevations and rash with simeprevir).
- Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naïve patients: the COSMOS randomised study. Lancet. 2014;384(9956):1756–65. [PubMed: 25078309](Among 167 patients with chronic hepatitis C, genotype 1, treated with sofosbuvir and simeprevir [with or without ribavirin] for 12 or 24 weeks, SVR rates were 90% to 94%; no mention of ALT elevations or hepatotoxicity).
- Simeprevir (Olysio) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1433):1–3. [PubMed: 24419295](Concise review of the efficacy, safety and costs of simeprevir shortly after its approval in the US as a part of combination therapy of chronic hepatitis C, genotype 1, mentions side effects of rash, photosensitivity, pruritus, nausea, fatigue and dyspnea; simeprevir can cause serum bilirubin elevations, but these are generally mild, transient and in the indirect(unconjugated) fraction and are not associated with ALT elevations or other evidence of liver injury).
- Schinazi R, Halfon P, Marcellin P, Asselah T. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int. 2014;34 Suppl 1:69–78. [PMC free article: PMC7737539] [PubMed: 24373081](Summary of safety and efficacy of various all-oral regimens for therapy of hepatitis C does not discuss hepatic decompensation, hepatotoxicity or ALT elevations during therapy).
- Rosenquist Å, Samuelsson B, Johansson PO, Cummings MD, Lenz O, Raboisson P, Simmen K, Vendeville S, et al. Discovery and development of simeprevir(TMC435), a HCV NS3/4A protease inhibitor. J Med Chem. 2014;57:1673–93. [PubMed: 24446688](Summary of the development of protease inhibitors for treatment of chronic hepatitis C including simeprevir [TMC435], a novel cyclopentane macrocyclic inhibitor identified by screening in HCV NS3 protease assays, application of structure based design and validation in HCV replicon systems).
- Forns X, Lawitz E, Zeuzem S, Gane E, Bronowicki JP, Andreone P, Horban A, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology. 2014;146:1669–79.e3. [PubMed: 24602923](Among 393 patients with previously treated chronic hepatitis C, genotype 1, who were treated with simeprevir vs placebo [12 weeks] combined with peginterferon and ribavirin [24 or 48 weeks], SVR rates were higher with simeprevir [79% vs 36%] and, except for hyperbilirubinemia and photosensitivity [3.5% vs 0], adverse event rates were similar).
- Izumi N, Hayashi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, et al. Once-daily simeprevir with peginterferon and ribavirin for treatment-experienced HCV genotype 1-infected patients in Japan: the CONCERTO-2 and CONCERTO-3 studies. J Gastroenterol. 2014;49:941–53. [PubMed: 24626851](Among 155 patients with previously treated chronic hepatitis C, genotype 1, treated with simeprevir [12 or 24 weeks] and peginterferon with ribavirin [24 or 48 weeks], SVR rates ranged from 36 to 96%; one patient discontinued therapy because of “abnormal hepatic function” and one for erythema multiforme, but few details provided).
- Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection(QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414–26. [PubMed: 24907224](Among 324 patients with previously untreated chronic hepatitis C, genotype 1, treated with simeprevir vs placebo [12 weeks] combined with peginterferon and ribavirin [24 or 48 weeks], SVR rates were 81% with simeprevir vs 50% with placebo, while adverse event rates were similar except that simeprevir treated patients had higher rate of rash [24% vs 11%] and photosensitivity [4% vs <1%]).
- Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403–13. [PubMed: 24907225](Among 394 previously untreated patients with chronic hepatitis C, genotype 1, treated with simeprevir or placebo [12 or 24 weeks] combined with peginterferon and ribavirin [24 to 48 weeks], SVR rates were higher with simeprevir [80% vs 50%], adverse event rates were similar and increases in ALT “were infrequent and similar between the treatment groups” and not associated with bilirubin elevations).
- Kumada H, Hayashi N, Izumi N, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, et al. Simeprevir (TMC435) once daily with peginterferon-α-2b and ribavirin in patients with genotype 1 hepatitis C virus infection: The CONCERTO-4 study. Hepatol Res. 2015;45:501–13. [PubMed: 24961662](Among 79 patients with chronic hepatitis C, genotype 1, treated with simeprevir [12 weeks] and peginterferon and ribavirin [24 or 48 weeks], SVR rates were higher in treatment naïve [92%] and relapse patients [100%] than in previous nonresponders [39%] and no serious liver related adverse events were recorded).
- Dieterich D, Rockstroh JK, Orkin C, Gutiérrez F, Klein MB, Reynes J, Shukla U, et al. Simeprevir (TMC435) with pegylated interferon/ribavirin in patients coinfected with HCV genotype 1 and HIV-1: a phase 3 study. Clin Infect Dis. 2014;59:1579–87. [PubMed: 25192745](Among 106 patients with chronic hepatitis C, genotype 1, and HIV coinfection treated with simeprevir [12 weeks] combined with peginterferon and ribavirin [24 or 48 weeks], the overall SVR rate was 74%, rash occurred in 16%, photosensitivity 2%, moderate bilirubin elevations 2%, but no patient had significant ALT elevations or clinically apparent liver injury).
- Manns MP, Fried MW, Zeuzem S, Jacobson IM, Forns X, Poordad F, Peeters M, et al. Simeprevir with peginterferon/ribavirin for treatment of chronic hepatitis C virus genotype 1 infection: pooled safety analysis from Phase IIb and III studies. J Viral Hepat. 2015;22:366–75. [PubMed: 25363449](In a pooled analysis of 5 clinical trials of simeprevir vs placebo combined with peginterferon and ribavirin for 24 to 48 weeks in 1464 patients with chronic hepatitis C, genotype 1 [11% with cirrhosis], bilirubin elevations occurred in 8.4% on simeprevir vs 2.8% on placebo, but none were accompanied by serum ALT elevations or evidence of hepatic injury; no liver related severe adverse events or deaths occurred nor any cases of DRESS or Stevens Johnson syndrome).
- Stine JG, Intagliata N, Shah NL, Argo CK, Caldwell SH, Lewis JH, Northup PG. Hepatic decompensation likely attributable to simeprevir in patients with advanced cirrhosis. Dig Dis Sci. 2015;60:1031–5. [PMC free article: PMC6600814] [PubMed: 25373453](Two men, ages 43 and 56 years, with chronic hepatitis C, genotype 1, and advanced cirrhosis developed progressive liver failure 2 and 4 weeks after starting sofosbuvir and simeprevir [bilirubin rising from 5.3 to 14.2 mg/dL and 9.5 to 25 mg/dL, ALT 90 and 53 U/L, Alk P not provided], one patient undergoing successful liver transplantation and the other dying, both remaining HCV RNA negative despite stopping therapy promptly).
- Pearlman BL, Ehleben C, Perrys M. The combination of simeprevir and sofosbuvir is more effective than that of peginterferon, ribavirin, and sofosbuvir for patients with hepatitis C-related Child's class A cirrhosis. Gastroenterology. 2015;148:762–70.e2. [PubMed: 25557952](Among 82 patients with chronic hepatitis C and compensated cirrhosis, genotype 1a, who were treated with sofosbuvir and either simeprevir vs peginterferon with ribavirin for 12 weeks, SVR rates were higher with simeprevir [93% vs 75%]; one patient on peginterferon developed hepatic decompensation, but there were no other liver related serious adverse events in either group).
- Pungpapong S, Aqel B, Leise M, Werner KT, Murphy JL, Henry TM, Ryland K, et al. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 after liver transplant. Hepatology. 2015;61:1880–6. [PubMed: 25722203](Among 123 patients with recurrent chronic hepatitis C, genotype 1, after liver transplant who were treated with simeprevir and sofosbuvir with or without ribavirin for 12 weeks, the overall SVR rate was 90% and severe side effects included acute pancreatitis, allergic lung injury and acute urinary obstruction; there were no ALT elevations above 5 times ULN or clinically apparent liver injury).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 12 were attributed to antiviral agents, but all were antiretroviral agents and no case was attributed to the oral direct acting agents used to treat hepatitis C).
- Gutierrez JA, Carrion AF, Avalos D, O'Brien C, Martin P, Bhamidimarri KR, Peyton A. Sofosbuvir and simeprevir for treatment of hepatitis C virus infection in liver transplant recipients. Liver Transpl. 2015;21:823–30. [PMC free article: PMC6658191] [PubMed: 25825070](Among 61 patients with recurrent hepatitis C after liver transplantation who were treated with simeprevir and sofosbuvir for 12 weeks, the overall SVR rate was 93%; two patients with cirrhosis developed hepatic decompensation, but no patient died or had hepatic rejection).
- Saxena V, Nyberg L, Pauly M, Dasgupta A, Nyberg A, Piasecki B, Winston B, et al. Safety and Efficacy of simeprevir/sofosbuvir in hepatitis C-infected patients with compensated and decompensated cirrhosis. Hepatology. 2015;62:715–25. [PMC free article: PMC4549204] [PubMed: 26033798](Among 156 patients with chronic hepatitis C, genotype 1, and cirrhosis treated with simeprevir and sofosbuvir with or without ribavirin for 12 weeks, overall SVR rates were 91% in compensated vs 73% in decompensated cases, and hepatic decompensation in 20% vs 3%, one patient dying of liver failure, but rates of adverse events were said to be similar to those of matched, untreated subjects).
- Collins JM, Raphael KL, Terry C, Cartwright EJ, Pillai A, Anania FA, Farley MM, Hepatitis B. Virus Reactivation During Successful Treatment of Hepatitis C Virus With Sofosbuvir and Simeprevir. Clin Infect Dis. 2015;61(8):1304–6. [PubMed: 26082511](2 cases: 55 year old man with chronic hepatitis C, genotype 1, and HBsAg with low levels of HBV DNA [2300 IU/mL] developed jaundice 8 weeks after starting sofosbuvir and simeprevir [bilirubin 12.2 mg/dL, ALT 1495 U/L, INR 1.96, HBV DNA 22 million IU/mL], with resolution within 6 weeks of stopping HCV agents and starting tenofovir and emtricitabine [Case 2]; 57 year old man with chronic hepatitis C, genotype 1a, and anti-HBc without HBsAg developed rising levels of HBV DNA during therapy with sofosbuvir and simeprevir [Pre <20, 2 weeks 353 and 4 weeks 11,255 IU/mL], which fell to undetectable levels within 8 weeks of starting tenofovir with emtricitabine [Truvada], ALT values remaining normal during the episode).
- Aqel BA, Pungpapong S, Leise M, Werner KT, Chervenak AE, Watt KD, Murphy JL, et al. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 in patients with cirrhosis. Hepatology. 2015;62:1004–12. [PubMed: 26096332](Retrospective review of course of 119 patients with chronic hepatitis C, genotype 1, and cirrhosis treated with sofosbuvir and simeprevir with or without ribavirin found overall SVR rate of 78%; bilirubin levels rose above 3 mg/dL in 4 patients, but none developed hepatic decompensation on therapy or had to discontinue treatment early because of liver related adverse events; one patient died of liver failure 6 weeks after completing treatment despite having cleared HCV RNA).
- Bailly F, Pradat P, Virlogeux V, Zoulim F. Antiviral therapy in patients with hepatitis C virus-induced cirrhosis. Dig Dis. 2015;33:613–23. [PubMed: 26159282](Review of the status of antiviral therapy of chronic hepatitis C with cirrhosis summarizing the high rate of adverse events including hepatic decompensation and death with peginterferon based regimens combined with boceprevir or telaprevir, and the more effective and better tolerated all oral regimens).
- Ferenci P, Kozbial K, Mandorfer M, Hofer H. HCV targeting of patients with cirrhosis. J Hepatol. 2015;63:1015–22. [PubMed: 26100497](Review of the status of antiviral therapy of chronic hepatitis C with cirrhosis, suggests that genotype 1 infected patients should receive an all-oral regimen such as the dual regimen of sofosbuvir with ledipasvir, daclatasvir or simeprevir or the triple combination of dasabuvir with ombitasvir and paritaprevir, the major issues being duration of therapy and the role of ribavirin).
- Igawa T, Fushimi S, Matsuo R, Ikeda F, Nouso K, Yoshino T, Nakatsukasa H. Severe liver injury associated with simeprevir plus pegylated interferon/ribavirin therapy in a patient with treatment-naïve genotype 1b hepatitis C virus: a case report. Clin J Gastroenterol. 2014;7:465–70. [PubMed: 26184030](65 year old man with chronic hepatitis C developed fatigue and serum ALT elevations 50 days after starting simeprevir, peginterferon and ribavirin [bilirubin 3.3 mg/dL, ALT 728 U/L, Alk P 171 U/L, INR 1.2], with rapid improvement on stopping therapy and persistent loss of HCV RNA despite early discontinuation).
- Shiffman ML, James AM, Long AG, Alexander PC. Treatment of chronic HCV with sofosbuvir and simeprevir in patients with cirrhosis and contraindications to interferon and/or ribavirin. Am J Gastroenterol. 2015;110:1179–85. [PubMed: 26215530](Among 120 cirrhotic patients with chronic hepatitis C, genotype 1, treated with sofosbuvir and simeprevir for 12 weeks, the SVR rate was 83%, but 14 patients developed complications of cirrhosis including hyperbilirubinemia in 8, sepsis in 2 [1 dying of multiorgan failure], hepatocellular carcinoma in 2 and variceal hemorrhage in 2).
- Ende AR, Kim NH, Yeh MM, Harper J, Landis CS. Fulminant hepatitis B reactivation leading to liver transplantation in a patient with chronic hepatitis C treated with simeprevir and sofosbuvir: a case report. J Med Case Rep. 2015;9:164. [PMC free article: PMC4535371] [PubMed: 26215390](59 year old woman with chronic hepatitis C, genotype 1, and anti-HBc without HBsAg or HBV DNA in serum, developed jaundice 11 weeks after starting sofosbuvir and simeprevir [bilirubin 9.1 mg/dL, ALT 2263 U/L, INR 1.9, HBV DNA 29 million IU/mL]; she was started on tenofovir, but developed progressive liver failure and underwent emergency liver transplantation 10 days after presentation).
- Lawitz E, Jacobson IM, Nelson DR, Zeuzem S, Sulkowski MS, Esteban R, Brainard D, et al. Development of sofosbuvir for the treatment of hepatitis C virus infection. Ann N Y Acad Sci. 2015;1358:56–67. [PubMed: 26235748](Review of the development of therapy of hepatitis C, focusing upon sofosbuvir and the prelicensure studies that lead to its approval).
- Modi AA, Nazario H, Trotter JF, Gautam M, Weinstein J, Mantry P, Barnes M, et al. Safety and efficacy of simeprevir plus sofosbuvir with or without ribavirin in patients with decompensated genotype 1 hepatitis C cirrhosis. Liver Transpl. 2016;22:281–6. [PubMed: 26335142](Among 42 patients with chronic hepatitis C, genotype 1, with decompensated cirrhosis treated with simeprevir and sofosbuvir with vs without ribavirin for 12 weeks, the overall SVR rate was 74% and no patient developed worsening hepatic decompensation or required hospitalization).
- European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199–236. [PubMed: 25911336](Guidelines for the antiviral therapy of chronic hepatitis C from the European liver disease research and academic society).
- AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–54. [PubMed: 26111063](Guidelines for the antiviral therapy of chronic hepatitis C from the US liver and infectious diseases research and academic societies).
- Lawitz E, Matusow G, DeJesus E, Yoshida EM, Felizarta F, Ghalib R, Godofsky E, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A Phase 3 study (OPTIMIST-2). Hepatology. 2016;64:360–9. [PMC free article: PMC5297873] [PubMed: 26704148](Among 103 patients with chronic hepatitis C, genotype 1, and cirrhosis treated with sofosbuvir and simeprevir for 12 weeks 103 [83%] had an SVR, and no patient developed liver failure or a treatment related serious adverse event).
- Kamar N, Marion O, Rostaing L, Cointault O, Ribes D, Lavayssière L, Esposito L, et al. Efficacy and safety of sofosbuvir-based antiviral therapy to treat hepatitis C virus infection after kidney transplantation. Am J Transplant. 2016;16:1474–9. [PubMed: 26587971](Among 25 kidney transplant recipients with chronic hepatitis C, genotype 1, treated with sofosbuvir in combination with other antiviral agents, 22 [88%] had an SVR, and "no adverse event was observed").
- Crittenden NE, Buchanan LA, Pinkston CM, Cave B, Barve A, Marsano L, McClain CJ, et al. Simeprevir and sofosbuvir with or without ribavirin to treat recurrent genotype 1 hepatitis C virus infection after orthotopic liver transplantation. Liver Transpl. 2016;22:635–43. [PubMed: 26915588](Among 56 patients with recurrent hepatitis C after liver transplantation who were treated with sofosbuvir and simeprevir for 12 weeks, 49 [88%] had an SVR, and two died of liver failure).
- Issa D, Eghtesad B, Zein NN, Yerian L, Cruise M, Alkhouri N, Adams R, et al. Sofosbuvir and simeprevir for the treatment of recurrent hepatitis C with fibrosing cholestatic hepatitis after liver transplantation. Int J Organ Transplant Med. 2016;7:38–45. [PMC free article: PMC4756263] [PubMed: 26889372](Among 5 patients with recurrent hepatitis C and fibrosing cholestatic hepatitis after liver transplant who were treated with sofosbuvir and simeprevir for 24 weeks, 4 had an SVR with resolution of jaundice, and one developed hepatic decompensation and died).
- Pillai AA, Wedd J, Norvell JP, Parekh S, Cheng N, Young N, Spivey JR, et al. Simeprevir and sofosbuvir (SMV-SOF) for 12 weeks for the treatment of chronic hepatitis C genotype 1 infection: a real world (transplant) hepatology practice experience. Am J Gastroenterol. 2016;111:250–60. [PubMed: 26832650](Among 170 patients with chronic hepatitis C, genotype 1, treated with sofosbuvir and simeprevir with or without ribavirin for 12 weeks in routine clinical practice, 133 [78%] had an SVR; adverse events were not discussed).
- Brown RS Jr, O'Leary JG, Reddy KR, Kuo A, Morelli GJ, Burton JR, Stravitz RT, et al. Hepatitis C Therapeutic Registry Research Network Study Group. Interferon-free therapy for genotype 1 hepatitis C in liver transplant recipients: Real-world experience from the hepatitis C therapeutic registry and research network. Liver Transpl. 2016;22:24–33. [PMC free article: PMC5208040] [PubMed: 26519873](Among 151 patients with recurrent hepatitis C after liver transplantation treated with sofosbuvir and simeprevir with or without ribavirin for 12 or 24 weeks, 133 [88%] had an SVR, and 8 [5%] developed hepatic decompensation and 3 patients died, all of whom had cirrhosis).
- Sulkowski MS, Vargas HE, Di Bisceglie AM, Kuo A, Reddy KR, Lim JK, Morelli G, et al. HCV-TARGET Study Group. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real-world patients with HCV genotype 1 infection. Gastroenterology. 2016;150:419–29. [PMC free article: PMC4727992] [PubMed: 26497081](Among 802 patients with chronic hepatitis C, genotype 1, treated with sofosbuvir and simeprevir with or without ribavirin for 12 weeks in clinical practices ["the real world"], 675 [84%] had an SVR, 44 [5.3%] had a serious adverse event, 10 [1.2%] hepatic decompensation and 2 [0.3%] died of liver failure).
- Modi AA, Nazario H, Trotter JF, Gautam M, Weinstein J, Mantry P, Barnes M, et al. Safety and efficacy of simeprevir plus sofosbuvir with or without ribavirin in patients with decompensated genotype 1 hepatitis C cirrhosis. Liver Transpl. 2016;22:281–6. [PubMed: 26335142](Among 42 patients with chronic hepatitis C, genotype 1, and decompensated cirrhosis treated with sofosbuvir, simeprevir and [n=35] ribavirin for 12 weeks, 31 [74%] had an SVR, and none developed decompensation requiring hospitalization).
- Fontana RJ, Brown RS, Moreno-Zamora A, Prieto M, Joshi S, Londoño MC, et al. Daclatasvir combined with sofosbuvir or simeprevir in liver transplant recipients with severe recurrent hepatitis C infection. Liver Transpl. 2016;22:446–58. [PubMed: 26890629](Among 97 liver transplant recipients treated with daclatasvir and either sofosbuvir or simeprevir with or without ribavirin for up to 24 weeks, 84 [87%] had an SVR, and 8 [8%] patients died, but none of the deaths were considered due to the antiviral therapy).
- Dyson JK, Hutchinson J, Harrison L, Rotimi O, Tiniakos D, Foster GR, Aldersley MA, et al. Liver toxicity associated with sofosbuvir, an NS5A inhibitor and ribavirin use. J Hepatol. 2016;64:234–238. [PubMed: 26325535](74 year old man and 36 year old woman with HCV related cirrhosis developed worsening hepatic decompensation within a few weeks of starting sofosbuvir, an NS5A inhibitor and ribavirin [peak bilirubin 23.4 and 30.5 mg/dL, ALT 65 and 96 U/L, Alk P 202 and 398 U/L], resulting in death in one and emergency liver transplant in the other [Case 1]).
- Marchan-Lopez A, Dominguez-Dominguez L, Kessler-Saiz P, Jarrin-Estupiñan ME. Liver failure in human immunodeficiency virus - hepatitis C virus coinfection treated with sofosbuvir, ledipasvir and antiretroviral therapy. J Hepatol. 2016;64:752–3. [PubMed: 26682727](Letter in response to Dyson [2016]: 49 year old man with chronic hepatitis C, cirrhosis [Child-Pugh class B] and HIV coinfection developed worsening hepatic decompensation 1 to 2 months after starting sofosbuvir and ledipasvir that worsened for two weeks after stopping [peak bilirubin 46.9 mg/dL, INR 3.17], and then resolved; he later tolerated reinitiation of antiretroviral drugs).
- Dyson JK, McPherson S. Reply to "Liver failure in human immunodeficiency virus - Hepatitis C virus coinfection treated with sofosbuvir, ledipasvir and antiretroviral therapy". J Hepatol. 2016;64:753–4. [PubMed: 26682725](Letter in reply to March-Lopez [2016] reporting another case of hepatic decompensation during sofosbuvir, ledipasvir and ribavirin therapy of a patient hepatitis C, cirrhosis and HIV coinfection, arising within 6 weeks of starting treatment [bilirubin 12.6 mg/dL, protime 17 sec], and leading to successful, emergency liver transplantation).
- Welker MW, Luhne S, Lange CM, Vermehren J, Farnik H, Herrmann E, Welzel T, et al. Lactic acidosis in patients with hepatitis C virus cirrhosis and combined ribavirin/ sofosbuvir treatment. J Hepatol. 2016;64:790–9. [PubMed: 26658684](Among 35 patients with chronic hepatitis C and advanced fibrosis or cirrhosis treated with sofosbuvir based regimens, 12 [34%] had a serious adverse event and 5 [14%] developed lactic acidosis, largely in those with Child-Pugh class B or C cirrhosis and in the context of hepatic decompensation, 2 of whom died).
- In brief: Hepatitis B reactivation with direct-acting antiviral drugs for hepatitis C. Med Lett Drugs Ther. 2016;58(1506):140. [PubMed: 27755511](Brief summary of the FDA warning letter concerning HBV reactivation caused by DAA therapies of hepatitis C).
- Hoofnagle JH. Hepatic decompensation during direct-acting antiviral therapy of chronic hepatitis C. J Hepatol. 2016;64:763–5. [PubMed: 26795828](Editorial in response to Welker [2016] discussing the occurrence of unexplained hepatic decompensation during antiviral therapy of hepatitis C and whether these episodes are coincidental, caused by hepatoxicity of the antiviral drugs, or are the paradoxical result of sudden eradication of the chronic viral infection).
- Bersoff-Matcha SJ, Cao K, Jason M, Ajao A, Jones SC, Meyer T, Brinker A. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus: a review of cases reported to the U.S. Food and Drug Administration adverse event reporting system. Ann Intern Med. 2017;166:792–798. [PubMed: 28437794](FDA summary of 29 reports of HBV reactivation among patients with chronic hepatitis C being treated with DAAs, including, presenting 14 to 196 days after starting therapy, with various DAA regimens, 3 resulting in death, 19 from Japan, 5 from the U.S., many treated after a delay in identifying the cause of sudden worsening of liver disease).
- Mahale P, Glenn JS, O'Brien TR. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus. Ann Intern Med. 2017;167:759–760. [PMC free article: PMC7325517] [PubMed: 29159388](Letter in response to Bersoff-Matcha [2017] suggesting that some cases of sudden worsening of liver disease in HBsAg positive patients might be due to superinfections with hepatitis D [Delta] virus).
- Bersoff-Matcha SJ, Cao K, Jason M, Jones SC, Brinker A. Hepatitis B virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus. Ann Intern Med. 2017;167:760. [PubMed: 29159389](Reply to the letter to the editor by Mahale [2017] by the authors).
- Serper M, Forde KA, Kaplan DE. Rare clinically significant hepatic events and hepatitis B reactivation occur more frequently following rather than during direct-acting antiviral therapy for chronic hepatitis C: Data from a national US cohort. J Viral Hepat. 2018;25(2):187–197. [PMC free article: PMC5969991] [PubMed: 28845882](Analysis of the large VA Medical system database of 17,400 veterans with chronic hepatitis C as well as anti-HBc who received oral DAA therapy, identified only 2 cases of HBV reactivation with serum ALT elevations above 300 U/L among 97 HBsAg positive patients).
- Aggeletopoulou I, Konstantakis C, Manolakopoulos S, Triantos C. Risk of hepatitis B reactivation in patients treated with direct-acting antivirals for hepatitis C. World J Gastroenterol. 2017;23:4317–4323. [PMC free article: PMC5487495] [PubMed: 28706414](Review of the literature on reactivation of HBV during antiviral therapy of chronic hepatitis C summarizing 7 cases in the literature).
- Calvaruso V, Ferraro D, Licata A, Bavetta MG, Petta S, Bronte F, Colomba G, et al. HBV reactivation in patients with HCV/HBV cirrhosis on treatment with direct-acting antivirals. J Viral Hepat. 2018;25:72–79. [PubMed: 28703895](Among 104 patients with cirrhosis and chronic hepatitis C who were treated with DAAs, reactivation of HBV occurred in 3 of 4 HBsAg positive patients not on anti-HBV therapy, but none developed ALT elevations or clinically apparent acute liver injury).
- Loggi E, Gitto S, Galli S, Minichiello M, Conti F, Grandini E, Scuteri A, et al. Hepatitis B virus reactivation among hepatitis C patients treated with direct-acting antiviral therapies in routine clinical practice. J Clin Virol. 2017;93:66–70. [PubMed: 28654775](Among 137 Italian adults with chronic hepatitis C treated with DAAs during a one-year period, reactivation [without ALT elevations] occurred in 1 of 2 subjects with HBsAg, but in none of 42 with anti-HBc without HBsAg).
- Kawagishi N, Suda G, Onozawa M, Kimura M, Maehara O, Ito J, Nakai M, et al. Hepatitis B virus reactivation during hepatitis C direct-acting antiviral therapy in patients with previous HBV infection. J Hepatol. 2017;67:1106–1108. [PubMed: 28438688](Letter describing course of 5 of 84 patients with chronic hepatitis C and serologic evidence of previous HBV infection [anti-HBc without HBsAg] developed evidence of HBV reactivation during various DAA regimens that was not accompanied by ALT elevations, but was persistent after DAA treatment in two patients).
- Belperio PS, Shahoumian TA, Mole LA, Backus LI. Evaluation of hepatitis B reactivation among 62,920 veterans treated with oral hepatitis C antivirals. Hepatology. 2017;66:27–36. [PubMed: 28240789](Analysis of VA Medical Database of 62,920 veterans with chronic hepatitis C treated with oral DAAs of whom 9 had evidence of HBV reactivation, 8 in patients with HBsAg and 1 with anti-HBc without HBsAg in serum, but only 3 patients had ALT elevations above twice ULN).
- Yeh ML, Huang CF, Hsieh MH, Ko YM, Chen KY, Liu TW, Lin YH, et al. Reactivation of hepatitis B in patients of chronic hepatitis C with hepatitis B virus infection treated with direct acting antivirals. J Gastroenterol Hepatol. 2017;32:1754–1762. [PubMed: 28230928](Among 64 patients with chronic hepatitis C and serologic evidence of ongoing or previous HBV infection who were treated with various DAA regimens, 4 of 7 HBsAg positive [57%] but none of 57 anti-HBc positive, HBsAg negative subjects developed HBV reactivation but all cases were subclinical, ALT elevations occurring in only one).
- Londoño MC, Lens S, Mariño Z, Bonacci M, Ariza X, Broquetas T, Pla A, et al. Hepatitis B reactivation in patients with chronic hepatitis C undergoing anti-viral therapy with an interferon-free regimen. Aliment Pharmacol Ther. 2017;45:1156–1161. [PubMed: 28206681](Among 352 patients with chronic hepatitis C receiving DAA treatment, 5 of 10 [50%] with concurrent HBsAg and 1 of 64 with anti-HBc without HBsAg developed a 1 log IU/mL increase in HBV DNA levels, but elevations were modest and none developed ALT elevations or clinically apparent liver injury).
- Kawagishi N, Suda G, Onozawa M, Kimura M, Maehara O, Ohara M, Izumi T, et al. Comparing the risk of hepatitis B virus reactivation between direct-acting antiviral therapies and interferon-based therapies for hepatitis C. J Viral Hepat. 2017;24:1098–1106. [PubMed: 28632923](Among 151 patients with chronic hepatitis C undergoing antiviral therapy, reactivation of HBV occurred in 1 of 4 patients with HBsAg in serum, and 5 of 151 with anti-HBc without HBsAg, but peak HBV DNA levels were low and all cases were without ALT elevations or symptoms and all 6 occurred in 79 patients receiving oral DAAs [8%] and none in the 72 who received interferon-based therapies [0%]).
- Mücke VT, Mücke MM, Peiffer KH, Weiler N, Welzel TM, Sarrazin C, Zeuzem S, et al. No evidence of hepatitis B virus reactivation in patients with resolved infection treated with direct-acting antivirals for hepatitis C in a large real-world cohort. Aliment Pharmacol Ther. 2017;46:432–439. [PubMed: 28627791](Among 848 patients with chronic hepatitis C treated in the "real world" with DAAs, 5 of 9 patients with HBsAg developed HBV reactivation [3 ultimately requiring anti-HBV therapy] and 8 of 263 patients with anti-HBc without HBsAg had low, but detectable levels of HBV DNA during therapy but without ALT elevations).
- Lawitz E, Poordad F, Gutierrez JA, Kakuda TN, Picchio G, Beets G, Vandevoorde A, et al. Simeprevir, daclatasvir and sofosbuvir for hepatitis C virus-infected patients with decompensated liver disease. J Viral Hepat. 2017;24:287–294. [PubMed: 27878906](Among 40 patients with chronic hepatitis C, genotype 1 or 4, and cirrhosis treated with sofosbuvir, simeprevir and daclatasvir for 12 weeks, all patients achieved an SVR and while 2 patients had an episode of decompensation on treatment and Child’s Pugh scores worsened in 7 patients, there were no deaths and no early discontinuations).
- Hézode C, Almasio PL, Bourgeois S, Buggisch P, Brown A, Diago M, Horsmans Y, et al. Simeprevir and daclatasvir for 12 or 24 weeks in treatment-naïve patients with hepatitis C virus genotype 1b and advanced liver disease. Liver Int. 2017;37:1304–1313. [PubMed: 28135777](Among 106 adults with chronic hepatitis C, genotype 1, and advanced liver disease treated with simeprevir and daclatasvir for 12 or 24 weeks, the overall SVR rate was 92% with both 12 and 24 weeks of treatment, 70% had adverse events, 6% serious adverse events but there was only one severe liver related event, a transient and asymptomatic rise in ALT and AST levels above 5 times ULN).
- Gaeta GB, Aghemo A, Menzaghi B, D'Offizi G, Giorgini A, Hasson H, Brancaccio G, et al. STIly Study Group. Effectiveness and safety of simeprevir-based regimens for hepatitis C in Italy: The STIly observational study. Medicine (Baltimore). 2018;97:e11307. [PMC free article: PMC6076166] [PubMed: 29979400](Among 342 Italian patients with chronic hepatitis C [genotypes 1 and 4] treated with 12 weeks of sofosbuvir and simeprevir with or without ribavirin, the overall SVR rate was 95% while serious adverse events arose in 1.2%, but all resolved without need for drug discontinuations; no mention of liver injury, hepatitis or ALT elevations).
- Miotto N, Mendes LC, Zanaga LP, Lazarini MSK, Goncales ESL, Pedro MN, Goncales FL Jr, Stucchi RSB, et al. All-oral direct antiviral treatment for hepatitis C chronic infection in a real-life cohort: The role of cirrhosis and comorbidities in treatment response. PLoS One. 2018;13:e0199941. [PMC free article: PMC6038991] [PubMed: 29990371](Among 527 Brazilian patients with chronic hepatitis C treated with interferon free regimens [including sofosbuvir, daclatasvir, ribavirin and simeprevir] between 2015 and 2018, the overall SVR rate was 91% and 22 of 272 patients [8%] with cirrhosis had decompensation during therapy).
- Sulkowski MS, Feld JJ, Lawitz E, Felizarta F, Corregidor AM, Khalid O, Ghalibv R, et al. Efficacy and safety of 6 or 8 weeks of simeprevir, daclatasvir, sofosbuvir for HCV genotype 1 infection. J Viral Hepat. 2018;25:631–639. [PubMed: 29274193](Among 68 adults with chronic hepatitis C, genotype 1, treated with simeprevir, daclatasvir and sofosbuvir for either 6 weeks [mild fibrosis] or 8 weeks [cirrhosis], the SVR rate was 86% in the 6 week and 100% in the 8 week arm, but relapse was frequent [12%]; there were no therapy related serious adverse events or elevations of serum ALT above 5 times ULN).
- Pariente A, Arpurt JP, Rémy AJ, Rosa-Hézode I, Causse X, Heluwaert F, Macaigne G, et al. Association nationale des gastroentérologues des hôpitaux (ANGH). Hepatitis C treatment with all-oral direct-acting antivirals: Effectiveness and tolerance in a multicenter, prospective, observational study from French general hospitals (APROVVIE, ANGH). Presse Med. 2019;48(3 Pt 1):e101–e110. [PubMed: 30853287](Among 1123 patients with chronic hepatitis C treated at 24 French general hospitals with various interferon-free antiviral regimens for 12 or 24 weeks the overall response rate was 91%, with lower responses among 553 patients with cirrhosis, two of whom developed hepatic decompensation and died).
- Laurain A, Metivier S, Haour G, Larrey D, Dorival C, Hezode C, Zoulim F, et al. ANRS/AFEF HEPATHER study group. Safety and efficacy of the combination simeprevir-sofosbuvir in HCV genotype 1- and 4-mono-infected patients from the French ANRS CO22 hepather cohort. BMC Infect Dis. 2019;19(1):300. [PMC free article: PMC6446259] [PubMed: 30940090](Among 599 French patients with chronic hepatitis C [genotypes 1 and 4] treated with 12 or 24 weeks of sofosbuvir and simeprevir with or without ribavirin, the overall SVR rate was 93%, while serious adverse events arose in 37 [9%] participants, one of which was drug induced liver injury; 4 patients died, but none from liver disease).
- Gammeltoft KA, Zhou Y, Duarte Hernandez CR, Galli A, Offersgaard A, Costa R, Pham LV, et al. Hepatitis C virus protease inhibitors show differential efficacy and interactions with remdesivir for treatment of SARS-CoV-2 in vitro. Antimicrob Agents Chemother. 2021;65:e0268020. [PMC free article: PMC8370243] [PubMed: 34097489](In a cell culture system, simeprevir and several other HCV protease inhibitors demonstrated antiviral activity against SARS-CoV-2, which was synergist with remdesivir).
- Recent References on Simeprevir: from PubMed.gov
- Trials on Simeprevir: from ClinicalTrials.gov
- [Jim: Check the links]
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Telaprevir.[LiverTox: Clinical and Researc...]Review Telaprevir.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Boceprevir.[LiverTox: Clinical and Researc...]Review Boceprevir.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Hepatitis C (HCV) Agents.[LiverTox: Clinical and Researc...]Review Hepatitis C (HCV) Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Hepatic decompensation likely attributable to simeprevir in patients with advanced cirrhosis.[Dig Dis Sci. 2015]Hepatic decompensation likely attributable to simeprevir in patients with advanced cirrhosis.Stine JG, Intagliata N, Shah NL, Argo CK, Caldwell SH, Lewis JH, Northup PG. Dig Dis Sci. 2015 Apr; 60(4):1031-5. Epub 2014 Nov 6.
- Do directly acting antiviral agents for HCV increase the risk of hepatic decompensation and decline in renal function? Results from ERCHIVES.[Aliment Pharmacol Ther. 2017]Do directly acting antiviral agents for HCV increase the risk of hepatic decompensation and decline in renal function? Results from ERCHIVES.Butt AA, Ren Y, Marks K, Shaikh OS, Sherman KE, ERCHIVES study. Aliment Pharmacol Ther. 2017 Jan; 45(1):150-159. Epub 2016 Nov 4.
- Simeprevir - LiverToxSimeprevir - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...