NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Spironolactone is an aldosterone receptor antagonist and potassium-sparing diuretic widely used in the therapy of edema, particularly in patients with cirrhosis in which hyperaldosteronism appears to play a major role. Spironolactone has been linked to rare cases of clinically apparent drug induced liver disease.
Background
Spironolactone (spir on oh lak' tone) is a competitive inhibitor of the mineralocorticoid receptor in the late distal tubule and collecting duct of the kidneys, which causes a decrease in sodium reabsorption and potassium excretion in the distal tubule. As a result, spironolactone promotes a sodium diuresis, but maintains body potassium levels. Spironolactone is particularly helpful in edematous states caused or exacerbated by hyperaldosteronism, which is typical of the edema and ascites caused by cirrhosis. Because of its potassium-sparing actions, spironolactone is also used in combination with thiazide or loop diuretics in an attempt to prevent hypokalemia. Chronic low dose therapy with spironolactone has also been reported to improve survival in patients with heart failure after myocardial infarction. Spironolactone was approved for use in the United States in 1960 and continues to be widely used. Spironolactone is available in 25, 50, 75 and 100 mg tablets generically and under the brand name of Aldactone. Fixed combinations of spironolactone and hydrochlorothiazide are also available under the brand name Aldactizide. The typical dose of spironolactone is 25 mg one to three times daily initially, with modification of the dose based upon clinical efficacy and tolerance to maintenance doses of 75 to 450 mg daily. Spironolactone is usually well tolerated but can cause hyperkalemia and dehydration. In addition, spironolactone has antiandrogen-like effects and can have troublesome side effects of excess and abnormal hair growth and gynecomastia.
Hepatotoxicity
Clinically apparent liver injury from spironolactone is rare and only a few instances have been reported as isolated case reports. The liver injury typically arises after 4 to 8 weeks of therapy and the pattern of serum enzyme elevations is usually hepatocellular or mixed. Immunoallergic features (rash, fever, eosinophilia) are rare as is autoantibody formation. Recovery has occurred within 1 to 3 months of stopping and all cases have been mild and self-limited in course (Case 1).
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of spironolactone hepatic injury is unknown, but is most likely due to a metabolic idiosyncrasy.
Outcome and Management
Reported cases of liver injury due to spironolactone have been mild with either no or minimal jaundice, and recovery within a few months of stopping the medication. Recurrence on rechallenge has been reported, but there is no information or cross reactivity to the hepatic injury with other diuretics. Because eplerenone has a similar chemical structure, it is likely to cause a similar hepatic injury.
Drug Class: Diuretics, Potassium-Sparing Diuretics
Other Drugs in the Subclass: Amiloride, Eplerenone, Triamterene
CASE REPORT
Case 1. Spironolactone induced liver injury.(1)
A 53 year old woman with primary hyperaldosteronism due to an adrenal adenoma was found to have serum enzyme elevations (ALT 430 U/L, AST 130 U/L, Alk P 225 U/L) without symptoms 1 month after starting spironolactone (100 mg three times daily). Spironolactone was stopped and serum enzymes fell into the normal range within the next two months. One year later, spironolactone was restarted (100 mg twice daily) and serum enzymes were again found to be abnormal one month later. She was not taking other medications and tests for hepatitis B and for autoantibodies were negative. There was no rash, fever or eosinophilia. A liver biopsy showed mild spotty hepatitis. Upon withdrawal of spironolactone, serum enzymes fell to normal within 6 weeks.
Key Points
| Medication: | Spironolactone (100 mg 2-3 times daily) |
|---|---|
| Pattern: | Hepatocellular (R=6.7) |
| Severity: | Mild (enzyme elevations without symptoms or jaundice) |
| Latency: | 4 weeks |
| Recovery: | 6-8 weeks |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | GGT (U/L) | Other |
|---|---|---|---|---|---|
| Pre | Pre | 15 | 80 | 10 | |
| Spironolactone (100 mg three times daily) given for 1 month | |||||
| 4 weeks | 0 | 430 | 185 | 90 | |
| 6 weeks | 2 weeks | 255 | 225 | 140 | |
| 8 weeks | 4 weeks | 70 | 150 | 80 | |
| 12 weeks | 8 weeks | 35 | 105 | 20 | |
| 24 weeks | 20 weeks | 30 | 90 | ||
| 1 year | 1 year | 25 | 85 | 20 | |
| Spironolactone (100 mg two times daily) restarted for 1 month | |||||
| 4 weeks | 0 | 140 | 145 | 45 | |
| 6 weeks | 2 weeks | 125 | |||
| 10 weeks | 6 weeks | 20 | 80 | 30 | |
| Normal Values | <40 | <110 | < 40 | ||
Comment
This patient developed elevations in serum aminotransferases and alkaline phosphatase within a month of starting spironolactone. She was evidently asymptomatic and anicteric and the liver injury resolved rapidly upon withdrawal. The rare instances of hepatic injury reported with spironolactone use have been mild and self-limited. The recurrence of injury with the same latency and severity without immunoallergic features suggests metabolic idiosyncrasy as the cause of the hepatotoxicity.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Spironolactone – Generic, Aldactone®
DRUG CLASS
Diuretics
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Spironolactone | 52-01-7 | C24-H32-O4-S |
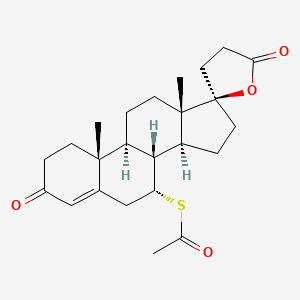
|
CITED REFERENCES
- 1.
- Shuck J, Shan S, Owensky L, Leftik M, Cucinell S. Spironolactone hepatitis in primary hyperaldosteronism. Ann Intern Med. 1981;95:708–10. [PubMed: 7305153]
ANNOTATED BIBLIOGRAPHY
References updated: 13 October 2021
- Zimmerman HJ. Diuretic drugs. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 662-4.(Expert review of hepatotoxicity of diuretics published in 1999 mentions that clinically apparent liver injury due to diuretics is rare; hepatocellular jaundice has been reported with triamterene; no mention of spironolactone).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 519-40.(Review of hepatotoxicity of cardiovascular agents, mentions that thiazide diuretics can rarely cause cholestatic hepatitis; no mention of potassium sparing diuretics).
- Jackson EK. Drugs affecting renal excretory function. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 445-70.(Textbook of pharmacology and therapeutics).
- Shuck J, Shan S, Owensky L, Leftik M, Cucinell S. Spironolactone hepatitis in primary hyperaldosteronism. Ann Intern Med. 1981;95:708–10. [PubMed: 7305153](53 year old woman found to have elevations in ALT [430 U/L] and Alk P [225 U/L] 1 month after starting spironolactone, falling to normal in 2 months and rising again [ALT 140 U/L] within 2 months of rechallenge: Case 1, spironolactone).
- Renkes P, Gaucher P, Tréchot P. Spironolactone and hepatic toxicity. JAMA. 1995;273:376–7. [PubMed: 7823379](74 year old man developed jaundice 7 weeks after starting spironolactone, biopsy showing intrahepatic cholestasis, resolving within 3 months of stopping; no details given).
- Thai KE, Sinclair RD. Spironolactone-induced hepatitis. Australas J Dermatol. 2001;42:180–2. [PubMed: 11488711](50 year old woman developed fatigue and pruritus 6 weeks after starting spironolactone [bilirubin 2.3 mg/dL, ALT 997 U/L, Alk P 742 U/L], resolving within 3 months of stopping).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 137 [0.5%] were done for idiosyncratic drug induced acute liver failure, none were attributed to a diuretic).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10-year period. Gastroenterology. 2005;129:512–21. [PubMed: 16083708](Reports of drug induced liver injury to a Spanish network found 570 cases; diuretics not mentioned as cause).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–8. [PubMed: 16054882](Survey of drug induced liver fatalities reported to WHO database between 1968-2003 revealed 4690 reports [89% from the US]; no diuretic found in the 20 most commonly implicated agents).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Population based survey of 126 cases of acute liver injury [24 with acute liver failure] due to drugs between 1993-1999 in Spain calculated relative risk of injury compared to the general population: hydrochlorothiazide was being taken by 7 and furosemide by 8 patients, but relative risk was not increased in comparison to a control group).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, no case was attributed to a diuretic).
- Drugs for hypertension. Treat Guidel Med Lett. 2009;7:1–10. [PubMed: 19107095](Brief overview of currently available drugs for hypertension with guidelines on their use and information on prices and toxicities: “thiazide diuretics are the first-line therapy for many patients with hypertension”).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](Among 313 cases of drug induced liver injury seen over a 12 year period at a large hospital in Bangalore, India, none were attributed to a diuretic).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Among 624,673 adverse event reports in children between 2000 and 2006 in the WHO VigiBase, no diuretic was mentioned among the 30 most common causes of liver injury).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which none were attributed to a diuretic).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to a diuretic).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to a diuretic).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to a diuretic).
- Drugs for hypertension. Med Lett Drugs Ther. 2020;62(1598):73–80. [PubMed: 32555118](Concise summary of efficacy, safety and costs of drug therapy of hypertension including the diuretics, focusing upon relative usefulness; no mention of hepatic adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- [PLACE OF ALDACTONE IN THE THERAPY OF EDEMA].[J Med Bord. 1964][PLACE OF ALDACTONE IN THE THERAPY OF EDEMA].APARICIO. J Med Bord. 1964 Feb; 141:245-54.
- IDIOPATHIC EDEMA ND HYPERALDOSTERONURIA: POSTURAL VENOUS PLASMA POOLING.[Pediatrics. 1965]IDIOPATHIC EDEMA ND HYPERALDOSTERONURIA: POSTURAL VENOUS PLASMA POOLING.FISHER DA, MORRIS MD. Pediatrics. 1965 Mar; 35:413-24.
- Inhibition of furosemide-induced kaliuresis in the rat by trilostane, an inhibitor of adrenal steroidogenesis.[Proc Soc Exp Biol Med. 1984]Inhibition of furosemide-induced kaliuresis in the rat by trilostane, an inhibitor of adrenal steroidogenesis.Harding HR, Creange JE, Potts GO, Schane HP. Proc Soc Exp Biol Med. 1984 Dec; 177(3):388-91.
- Review ALDOSTERONE IN CLINICAL MEDICINE.[Calif Med. 1964]Review ALDOSTERONE IN CLINICAL MEDICINE.BIGLIERI EG, SLATON PE Jr. Calif Med. 1964 Sep; 101(3):191-5.
- Review Aldosterone antagonists in hypertension and heart failure.[Ann Endocrinol (Paris). 2000]Review Aldosterone antagonists in hypertension and heart failure.Mantero F, Lucarelli G. Ann Endocrinol (Paris). 2000 Feb; 61(1):52-60.
- Spironolactone - LiverToxSpironolactone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...