NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Temozolomide is an orally administered alkylating agent used largely in the therapy of malignant brain tumors including glioblastoma and astrocytoma. Temozolomide has been associated with a low rate of serum enzyme elevations during treatment and with rare instances of clinically apparent cholestatic liver injury.
Background
Temozolomide (tem" oh zol' oh mide) is an imidazotetrazine derivative similar to dacarbazine (DTIC), which acts as an alkylating agent disrupting DNA replication, causing modification and cross linking of DNA, thus inhibiting DNA, RNA and protein synthesis and causing programmed cell death (apoptosis) in rapidly dividing cells. Temozolomide rapidly crosses the blood-brain barrier and has been evaluated largely in the therapy of malignant brain tumors. Temozolomide has been shown to induce tumor regression and remissions in patients with malignant astrocytoma and glioblastoma multiforme. It may also have activity in melanoma. Temozolomide was approved for use in the United States in 1999 and is now commonly used in treating patients with malignant brain tumors. Temozolomide is available in capsules of 5, 20, 100, 140, 180 and 250 mg and as a solution for injection generically and under the brand name of Temodar. The recommended dose regimen is calculated based on phase (initial cycles, maintenance), body weight, bone marrow toxicity and tolerance. It is typically given in a dose of 150 mg/m2 once a day during concurrent radiation therapy for 42 days followed by 75 mg/m2 on days 1 to 5 of each 28 day cycle. Temozolomide is considered somewhat less toxic and better tolerated than many other alkylating agents, but does have the common side effects of fatigue, nausea and vomiting, gastrointestinal upset, alopecia and bone marrow suppression. Less common but potentially severe adverse events included severe myelosuppression, myelodysplastic syndromes, pneumocystis pneumonia, hepatotoxicity and embryo-fetal toxicity.
Hepatotoxicity
Serum aminotransferase elevations occur during temozolomide therapy in up to 12% of patients, but these elevations are usually mild and self-limited, not requiring dose adjustment or drug discontinuation. An instance of serum aminotransferase elevation with jaundice was reported in the registration trials of temozolomide and subsequent to its approval. More strikingly, multiple single case reports and several case series of temozolomide hepatotoxicity have been reported in the literature. The onset of injury was typically within 2 to 8 weeks of starting temozolomide but several patients had received multiple courses before the onset of liver injury. The pattern of serum enzyme elevations was usually mixed initially, but the disease tended to be cholestatic. In several instances, jaundice was deep and prolonged. Features of hypersensitivity (rash, fever, eosinophilia) and autoantibody formation were not present. Liver histology demonstrated cholestasis and bile duct injury and a striking decrease in bile ducts (bile duct loss or paucity). Jaundice and pruritus tended to be prolonged and some patients developed vanishing bile duct syndrome, while others recovered clinically but had persistent serum alkaline phosphatase elevations during follow up and to the time of death from the brain tumor. Rechallenge was not done, but several patients subsequently received other antineoplastic agents, some of which were alkylating agents without recurrence of liver injury.
In addition, temozolomide has been associated with several cases of reactivation of chronic hepatitis B in patients who were hepatitis B surface antigen (HBsAg) positive at the start of chemotherapy. Clinical symptoms and signs of a flare of hepatitis B arose 6 to 12 weeks after starting temozolomide frequently in a cyclic pattern. Most patients had not received corticosteroids or other immunosuppressive agents that are more traditionally associated with reactivation. The episodes are marked by rises in HBV DNA levels and mild jaundice and responded to prompt antiviral therapy for hepatitis B which allowed for restarting of temozolomide in some cases. Fatal cases of reactivation have not been reported, but in general hepatitis B reactivation with jaundice has a mortality rate in excess of 10%.
Likelihood score: B (highly likely but uncommon cause of clinically apparent liver injury and reactivation of hepatitis B).
Mechanism of Injury
Temozolomide is hydrolyzed to the active intermediate at physiological pH and does not require hepatic metabolism or affect the cytochrome P450 (CYP) system to a major degree, perhaps accounting for its relative lack of direct hepatotoxicity. The cases of acute cholestatic liver injury have resembled idiosyncratic drug induced liver injury.
Outcome and Management
The severity of liver injury caused by temozolomide ranges from minor transient elevations in serum enzymes to severe cholestatic hepatitis that can be prolonged. Temozolomide has not been reported to cause acute liver failure but has been linked to instances of chronic liver injury and paucity of bile ducts on liver biopsy suggestive of mild vanishing bile duct syndrome. Because temozolomide is used as therapy of highly malignant brain tumors, long term follow up of liver injury from its use is rarely available. Most patients recover clinically, but may persist in having mild and asymptomatic alkaline phosphatase elevations. There is no evidence for cross sensitivity to hepatic injury between temozolomide and other alkylating agents. Because temozolomide has been linked to instances of reactivation of hepatitis B, it is appropriate to screen all patients scheduled to receive temozolomide for HBsAg and anti-HBc. Patients with serologic evidence of ongoing or previous hepatitis B should be monitored for evidence of reactivation (rise in HBV DNA). If evidence of reactivation arises, prompt therapy with an antiviral with potent activity against HBV is appropriate (such as entecavir or tenofovir). An alternative approach is to use the antiviral agent prophylactically in such patients. The antiviral therapy (either as prophylaxis or treatment) should be continued as long as the chemotherapy is planned and for 3 to 6 months thereafter.
Drug Class: Antineoplastic Agents, Alkylating Agents
CASE REPORT
Case 1. Severe mixed hepatocellular-cholestatic hepatitis due to temozolomide.(1)
A 67 year old woman developed jaundice 40 days after starting temozolomide therapy of a malignant glioblastoma. She had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. She had undergone resection of a left temporal lobe brain tumor 3 months earlier at which time she received local radiation and dexamethasone. Shortly thereafter she was started on temozolomide and levetiracetam along with dexamethasone (6 mg daily). She was also prescribed pantoprazole (40 mg daily) and ondansetron (4 mg orally as needed). Her past medical history was otherwise unremarkable, and she denied previous drug allergies. For many years, she had used a daily transdermal estradiol (0.0375 mg) patch and taken calcium, vitamin D and a multivitamin. When the jaundice was detected, she admitted to mild fatigue, but specifically denied fever, rash, itching, abdominal pain, anorexia or nausea. On examination, she was jaundiced but had no fever, rash, hepatic enlargement or tenderness. Laboratory tests showed a total bilirubin of 7.7 (6.6 mg/dL direct), ALT 896 U/L, AST 262 U/L, alkaline phosphatase 427 U/L and serum albumin 3.3 g/dL. Her serum ALT levels had been slightly abnormal at the time of her craniotomy, before temozolomide therapy (Table). Levetiracetam was stopped but temozolomide was continued. She developed deepening jaundice and serum enzyme elevations continued to rise, whereupon temozolomide was also stopped. Tests for hepatitis A, B and C (including HCV RNA and HBV DNA) were negative as were autoantibodies. Abdominal ultrasound showed no evidence of biliary obstruction. A liver biopsy showed marked cholestatic hepatitis with bile duct damage suggestive of drug induced liver injury. There was no reduction in the number of bile ducts. Serum bilirubin levels peaked at 46.8 mg/dL, ALT at 2783 U/L and alkaline phosphatase at 1219 U/L approximately 14 days after temozolomide was discontinued. Thereafter, serum bilirubin and enzymes slowly improved. Despite the height of the serum bilirubin and ALT, the prothrombin time remained normal and she had no signs of hepatic encephalopathy and continued to deny symptoms of abdominal pain, nausea or itching. Jaundice resolved after 3 months and liver tests were minimally abnormal 12 months later. Because of reappearance of seizures, levetiracetam was restarted without recurrence of jaundice or worsening of liver tests. Temozolomide was not restarted.
Key Points
| Medication: | Temozolomide |
|---|---|
| Pattern: | Hepatocellular-mixed (R=5.8, falling to 2.1 at time of peak bilirubin) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 5-6 weeks |
| Recovery: | 6 months |
| Other medications: | Levetiracetam |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | Pre | 67 | 68 | 0.4 | |
| 2 weeks | 0 | 74 | 63 | 0.4 | |
| 6 weeks | 0 | 896 | 427 | 7.7 | Protime 12.9 seconds |
| 7 weeks | 1 week | 2444 | 1142 | 24.3 | Liver biopsy |
| 8 weeks | 2 weeks | 2351 | 975 | 37.8 | Ursodiol started |
| 9 weeks | 3 weeks | 1218 | 1557 | 44.8 | |
| 10 weeks | 4 weeks | 627 | 1245 | 29.9 | |
| 3 months | 6 weeks | 366 | 565 | 6.8 | |
| 4 months | 2 months | 212 | 334 | 2.6 | |
| 4 months | 243 | 263 | 0.8 | ||
| 6 months | 134 | 256 | 0.6 | Levetiracetam restarted | |
| 8 months | 117 | 195 | 0.6 | ||
| 14 months | 12 months | 37 | 141 | 0.5 | |
| Normal Values | <42 | <115 | <1.2 | ||
Comment
Cholestatic hepatitis arose after 6 weeks of therapy with temozolomide and levetiracetam. Both drugs were stopped, but recovery was delayed. A liver biopsy showed cholestasis and bile duct injury, but importantly did not show massive or submassive necrosis. The patient had few symptoms but biochemical recovery was delayed. Levetiracetam (which rarely causes liver injury) was restarted without worsening of the liver condition, making the diagnosis of temozolomide induced liver injury more likely. While the liver injury ultimately resolved, it did cause an interruption of therapy and significantly disturbed the quality of life in an already compromised patient.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Temozolomide – Generic, Temodar®
DRUG CLASS
Antineoplastic Agents, Alkylating Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Temozolomide | 85622-93-1 | C6-H6-N6-O2 |
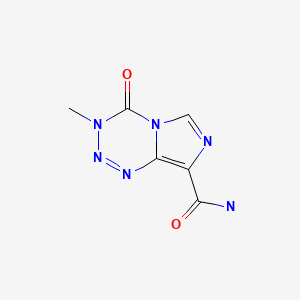
|
CITED REFERENCE
- 1.
- Clinical Center, National Institutes of Health.
ANNOTATED BIBLIOGRAPHY
References updated: 02 September 2020
- Zimmerman HJ. Alkylating agents: hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 678-80.(Expert review of hepatotoxicity of alkylating agents published in 1999 before the general availability of temozolomide).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 549-68.(Review of hepatotoxicity of cancer chemotherapeutic agents; does not discuss temozolomide specifically).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Cytotoxic agents. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1167-1201.(Textbook of pharmacology and therapeutics: "Temozolomide is the standard agent in combination with radiation therapy for patients with malignant glioma and for astrocytoma").
- Temozolomide for refractory anaplastic astrocytoma. Med Lett Drugs Ther. 1999;41:123–4. [PubMed: 10987014](Concise summary of efficacy of temozolomide in therapy of astrocytoma, the basis of its accelerated approval from the FDA in 1999; no mention of hepatotoxicity).
- Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–71. [PubMed: 10561351](Open label multicenter trial of temozolomide in 162 patients with astrocytoma; most common side effects were nausea, headache, fatigue, thrombocytopenia and neutropenia; no mention of ALT elevations or hepatotoxicity).
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. [PubMed: 15758009](Controlled trial of temozolomide and radiation vs radiation alone in 573 patients with glioblastoma showing increase in 2 year survival [26% vs 10%]; severe side effects included bone marrow suppression, fatigue, thromboembolism and infections; no mention of hepatotoxicity, jaundice or ALT elevations).
- Yang SH, Kim MK, Lee TK, Lee KS, Jeun SS, Park CK, Kang JK, et al. Temozolomide chemotherapy in patients with recurrent malignant gliomas. J Korean Med Sci. 2006;21:739–44. [PMC free article: PMC2729901] [PubMed: 16891823](Open label study of temozolomide in 25 Korean patients with recurrent gliomas; ALT elevations occurred in 12% of patients and one patient with preexisting liver disease developed "grade 2" toxicity).
- Dario A, Tomei G. The safety of the temozolomide in patients with malignant glioma. Curr Drug Saf. 2006;1:205–22. [PubMed: 18690931](Systematic review of the literature on the safety and adverse effects of temozolomide; the major toxicity is myelosuppression; common minor side effects are nausea, vomiting and fatigue; infections occur in 1 to 6% of treated patients; no discussion of hepatotoxicity).
- Grewal J, Dellinger CA, Yung WK. Fatal reactivation of hepatitis B with temozolomide. N Engl J Med. 2007;356:1591–2. [PubMed: 17429098](65 year old woman with glioblastoma developed jaundice 3 months after starting temozolomide and radiation therapy [bilirubin 2.8 rising to 25.9 mg/dL, ALT 1338 U/L, Alk P 107 U/L, HBV DNA above 500 million copies/mL], progressing to hepatic failure and death despite entecavir therapy; status of HBsAg before therapy was not reported).
- Chheda MG, Drappatz J, Greenberger NJ, Kesari S, Weiss SE, Gigas DC, Doherty LM, et al. Hepatitis B reactivation during glioblastoma treatment with temozolomide: a cautionary note. Neurology. 2007;68:955–6. [PubMed: 17372135](50 year old man with glioblastoma developed jaundice 6-8 weeks after starting radiation and chemotherapy with temozolomide and dexamethasone [bilirubin 2.9 mg/dL, ALT 1728 U/L, Alk P 109 U/L, HBV DNA 724,000 copies/mL], improving with lamivudine therapy and later tolerating temozolomide without recurrence of reactivation).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, antineoplastic agents were rarely implicated: 3 were considered due to mercaptopurine and 1 each due to bortezomib, cyclophosphamide, docetaxel, and temozolomide).
- Neyns B, Hoorens A, Stupp R. Valproic acid related idiosyncratic drug induced hepatotoxicity in a glioblastoma patient treated with temozolomide. Acta Neurol Belg. 2008;108:131–4. [PubMed: 19239041](48 year old patient developed cholestatic hepatitis during therapy with temozolomide, cilengitide and valproate which improved when all drugs were stopped and did not recur with restarting temozolomide).
- Trinh VA, Patel SP, Hwu WJ. The safety of temozolomide in the treatment of malignancies. Expert Opin Drug Saf. 2009;8:493–9. [PubMed: 19435405](Extensive review of pharmacology, clinical efficacy, and safety of temozolomide; dose limiting toxicity is bone marrow suppression; no mention of hepatotoxicity or ALT elevations).
- Clarke JL, Iwamoto FM, Sul J, Panageas K, Lassman AB, DeAngelis LM, Hormigo A, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27:3861–7. [PMC free article: PMC2727290] [PubMed: 19506159](Among 85 patients with glioblastoma treated with temozolomide, ALT or AST elevations occurred in 6% of patients overall, more commonly with continuous daily dosing [5 of 28: 18%] than cyclic dosing [1 of 31: 3%]).
- Perry JR, Bélanger K, Mason WP, Fulton D, Kavan P, Easaw J, Shields C, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28:2051–7. [PubMed: 20308655](Among 120 patients with recurrent glioblastoma treated with temozolomide for 0.3-24 months, nonhematologic toxicities were mild; no mention of ALT elevations or hepatotoxicity).
- Seiz M, Krafft U, Freyschlag CF, Weiss C, Schmieder K, Lohr F, Wenz F, et al. Long-term adjuvant administration of temozolomide in patients with glioblastoma multiforme: experience of a single institution. J Cancer Res Clin Oncol. 2010;136:1691–5. [PubMed: 20177703](Among 114 patients with glioblastoma treated with long term temozolomide, treatment was stopped in 34% because of hematologic side effects, but in none for liver related toxicities; no mention of ALT levels).
- Goldbecker A, Tryc AB, Raab P, Worthmann H, Herrmann J, Weissenborn K. Hepatic encephalopathy after treatment with temozolomide. J Neurooncol. 2011;103:163–6. [PubMed: 20730617](66 year old woman with glioblastoma developed jaundice 2 months after starting temozolomide [bilirubin 20.8 mg/dL, ALT 410 U/L, Alk P 2009 U/L], liver biopsy showing cholestasis and steatosis with slow and incomplete recovery).
- Gállego Pérez-Larraya J, Ducray F, Chinot O, Catry-Thomas I, Taillandier L, Guillamo JS, Campello C, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29:3050–5. [PubMed: 21709196](Among 77 elderly patients with glioblastoma treated with temozolomide, one developed ALT elevations >5 times ULN, but no specific details given).
- Niewald M, Berdel C, Fleckenstein J, Licht N, Ketter R, Rübe C. Toxicity after radiochemotherapy for glioblastoma using temozolomide--a retrospective evaluation. Radiat Oncol. 2011;6:141. [PMC free article: PMC3213071] [PubMed: 22017800](Retrospective analysis of 46 patients with glioblastoma treated with radiation and temozolomide found ALT elevations [>5 times ULN] in 5 patients [11%], leading to discontinuation in 2).
- Ohno M, Narita Y, Miyakita Y, Ueno H, Kayama T, Shibui S. Reactivation of hepatitis B virus after glioblastoma treatment with temozolomide--case report. Neurol Med Chir (Tokyo). 2011;51:728–31. [PubMed: 22027252](51 year old HBsAg positive man with glioblastoma developed reactivation of hepatitis B 10 weeks after starting chemotherapy [bilirubin not given, ALT rising from 28 to 744 U/L, HBV DNA from 3.8 to 5.8 log copies/mL], responding to entecavir therapy).
- Dixit S, Hingorani M, Afzal P, Campbell AP. Temozolomide induced liver injury. Acta Neurol Belg. 2011;111:249–51. [PubMed: 22141295](62 year old woman with glioblastoma developed jaundice 6 weeks after starting radiation therapy and temozolomide [bilirubin 1.9 mg/dL, ALT 2610 U/L, Alk P 1240 U/L], with prolonged cholestasis).
- Sarganas G, Orzechowski HD, Klimpel A, Thomae M, Kauffmann W, Herbst H, Bronder E, Garbe E. Severe sustained cholestatic hepatitis following temozolomide in a patient with glioblastoma multiforme: case study and review of data from the FDA adverse event reporting system. Neuro Oncol. 2012;14:541–6. [PMC free article: PMC3337307] [PubMed: 22394496](51 year old man with glioblastoma developed jaundice 6 weeks after starting temozolomide [bilirubin ~13.0 mg/dL, ALT 639 U/L, Alk P 261 U/L], with persistent jaundice until death from cancer 1 year later).
- Zamani N, Mohammad Alizadeh A. Drug-induced cholestatic hepatitis: how late can it occur even after the cessation of the culpable drug? Neuro Oncol. 2012;14:830. [PMC free article: PMC3379798] [PubMed: 22544734](Letter in response to Sarganas [2012] suggesting acetaminophen as the cause of the liver injury).
- Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, et al. NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707–15. [PubMed: 22578793](Controlled trial of temozolomide alone vs radiotherapy alone in 373 elderly patients with astrocytoma found similar efficacy; grade 3 enzyme elevations occurred in 30% of temozolomide vs 16% of radiation treated subjects).
- Fujimoto Y, Hashimoto N, Kinoshita M, Miyazaki Y, Tanaka S, Yakushijin T, Takehara T, et al. Hepatitis B virus reactivation associated with temozolomide for malignant glioma: a case report and recommendation for prophylaxis. Int J Clin Oncol. 2012;17:290–3. [PubMed: 21809177](49 year old HBsAg positive woman with glioma developed reactivation of hepatitis B 2 months after starting chemotherapy with temozolomide and radiation without corticosteroids [bilirubin 1.4 mg/dL, ALT 1098 U/L, Alk P 515 U/L, HBV DNA 1 billion copies/mL], responding to entecavir; temozolomide not restarted).
- Miller RJ, Gui X, Easaw JC, Tsang RY. Chemotherapy-associated steatohepatitis with temozolomide and dexamethasone. Can J Neurol Sci. 2012;39:547–9. [PubMed: 22896875](46 year old man with brain tumor developed elevations in serum enzymes 3-4 months after starting dexamethasone and temozolomide [peak bilirubin 0.8 mg/dL, ALT 355 U/L, Alk P 83 U/L], liver biopsy showing steatosis and liver tests improving after reduction in corticosteroid dose).
- Dixit S, Baker L, Walmsley V, Hingorani M. Temozolomide-related idiosyncratic and other uncommon toxicities: a systematic review. Anticancer Drugs. 2012;23:1099–106. [PubMed: 22850321](Systematic review of rare complications of temozolomide therapy mentions 5 published cases of idiosyncratic liver injury, usually cholestatic, 3 with prolonged jaundice and one dying of liver failure).
- Grant LM, Kleiner DE, Conjeevaram HS, Vuppalanchi R, Lee WM. Clinical and histological features of idiosyncratic acute liver injury caused by temozolomide. Dig Dis Sci. 2013;58:1415–21. [PMC free article: PMC3826911] [PubMed: 23212393](Among 1000 cases of drug induced liver injury enrolled in a prospective US database between 2004 and 2011, 4 [0.5%] were attributed to temozolomide, 3 men and 1 woman, ages 47 to 70 years, with onset of injury after 25-168 days, 3 with jaundice [peak bilirubin 0.5-32 mg/dL, ALT 530-1754 U/L, Alk P 151-780 U/L], all resolving, but often with prolonged jaundice and Alk P elevations).
- Temozolomide: fatal hepatic failure. Prescrire Int. 2014;23:214. [PubMed: 25325123](Report of fatal case of hepatotoxicity linked to temozolomide).
- Becker F, Hecht M, Schmidtner J, Semrau S, Fietkau R. Temozolomide-induced liver damage. A case report. Strahlenther Onkol. 2014;190:408–10. [PubMed: 24452817](57 year old woman developed liver injury 5 weeks after starting temozolomide for glioblastoma multiforme [ALT 1997 U/L, GGT 422 U/L, bilirubin 0.5 rising to 9.5 mg/dL], resolving within 2 months, but with persistence of mild GGT elevations).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to temozolomide).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attribute to temozolomide).
- Melchardt T, Magnes T, Weiss L, Grundbichler M, Strasser M, Hufnagl C, Moik M, et al. Liver toxicity during temozolomide chemotherapy caused by Chinese herbs. BMC Complement Altern Med. 2014;14:115. [PMC free article: PMC3994275] [PubMed: 24679099](56 year old woman with glioblastoma was treated with radiation therapy and temozolomide after surgery and developed jaundice one month later [bilirubin 16.7 mg/dL, ALT 585 U/L, Alk P not given], and was found to also be taking a mixture of Chinese herbs including atractylodes, astragali, mandarin orange, black cohosh, celery, kudzu, eucommia and skullcap, several of which can also cause liver injury, and patient later tolerated reintroduction of temozolomide without recurrence).
- Mason M, Adeyi O, Fung S, Millar BA. Vanishing bile duct syndrome in the context of concurrent temozolomide for glioblastoma. BMJ Case Rep. 2014;2014:bcr2014208117. [PMC free article: PMC4248108] [PubMed: 25432915](62 year old woman with glioblastoma underwent surgery and radiation therapy and developed pruritus and jaundice 15 days after starting temozolomide [bilirubin 16.9 mg/dL, ALT 747 U/L, Alk P 1402 U/L], liver biopsy showing bile duct loss [only 40% of portal areas with bile ducts], jaundice and pruritus lasting 2 months despite prompt discontinuation of temozolomide, but ultimately resolving).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 [5%] were attributed to antineoplastic agents including 4 due to temozolomide [Grant 2013]).
- Grieco A, Tafuri MA, Biolato M, Diletto B, Di Napoli N, Balducci N, Vecchio FM, Miele L. Severe cholestatic hepatitis due to temozolomide: an adverse drug effect to keep in mind. Case report and review of literature. Medicine (Baltimore). 2015;94:e476. [PMC free article: PMC4554001] [PubMed: 25816026](67 year old man with glioblastoma developed jaundice 1 month after starting temozolomide [bilirubin 7.96 rising to 25.1 mg/dL, ALT 1128 U/L, Alk P 458 U/L], with death from sepsis while still jaundiced one month later).
- Balakrishnan A, Ledford R, Jaglal M. Temozolomide-induced biliary ductopenia: a case report. J Med Case Rep. 2016;10:33. [PMC free article: PMC4743416] [PubMed: 26846183](58 year old woman with glioblastoma developed jaundice 6 weeks after starting temozolomide [bilirubin 7.9 rising to 36.8 mg/dL, ALT 1028 U/L, Alk P 291 U/L], biopsy showing bile duct loss).
- Aygun C, Altınok AY, Çakır A, Agan AF, Balaban Y. Acute temozolomide induced liver injury: mixed type hepatocellular and cholestatic toxicity. Acta Gastroenterol Belg. 2016;79:363–5. [PubMed: 27821033](53 year old man with glioblastoma developed jaundice after 9 weeks of temozolomide therapy [bilirubin 15.1 mg/dL, ALT 632 U/L, Alk P 1143 U/L, INR normal], biopsy showing cholestatic hepatitis, resolving 8 weeks after stopping drug).
- Efferth T, Schöttler U, Krishna S, Schmiedek P, Wenz F, Giordano FA. Hepatotoxicity by combination treatment of temozolomide, artesunate and Chinese herbs in a glioblastoma multiforme patient: case report review of the literature. Arch Toxicol. 2017;91:1833–46. [PubMed: 27519711](65 year old patient with glioblastoma developed fatigue, weight loss and serum enzyme elevations approximately 3 months after starting temozolomide and one month after starting several Chinese herbs including artesunate and Coptis-Kush [bilirubin not provided, ALT 157 rising to 238 U/L, GGT 29 rising to 347 U/L], with improvement upon stopping the Chinese herbs; no mention of whether temozolomide was stopped).
- Khoury T, Chen S, Abu Rmeileh A, Daher S, Yaari S, Benson AA, Cohen J, et al. Acute liver injury induced by levetiracetam and temozolomide co-treatment. Dig Liver Dis. 2017;49:297–300. [PubMed: 28034663](Among 94 patients with brain tumors treated with temozolomide or levetiracetam or both, serum enzyme elevations were more common among those receiving both agents than those receiving either alone and one subject on the combination developed acute liver failure and died, compared to none receiving temozolomide alone).
- Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, Fontana RJ, et al. U.S. Drug Induced Liver Injury Network Investigators. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65:1267–77. [PMC free article: PMC5360519] [PubMed: 27981596](Among 363 patients with acute drug induced liver injury who underwent liver biopsy, 26 had bile duct loss including 3 receiving temozolomide, all of whom had cholestatic hepatitis and a prolonged course with persistent Alk P elevations in follow up at 6-12 months).
- Desai VCA, Quinlan SC, Deitz AC, He J, Holick CN, Lanes S. Risk of severe acute liver injury among patients with brain cancer treated with temozolomide: a nested case-control study using the healthcore integrated research database. J Neurooncol. 2017;134:89–95. [PubMed: 28717885](A case controlled study based upon a US health insurance research database of adults with brain tumors seen between 2006 and 2014 found no increased rates of exposure to temozolomide among 61 patients with severe acute liver injury [18%] compared to 305 matched controls [22%]).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Temozolomide and treatment of malignant glioma.[Clin Cancer Res. 2000]Review Temozolomide and treatment of malignant glioma.Friedman HS, Kerby T, Calvert H. Clin Cancer Res. 2000 Jul; 6(7):2585-97.
- Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial.[Lancet Oncol. 2012]Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial.Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M, et al. Lancet Oncol. 2012 Jul; 13(7):707-15. Epub 2012 May 10.
- Review 65 YEARS OF THE DOUBLE HELIX: Treatment of pituitary tumors with temozolomide: an update.[Endocr Relat Cancer. 2018]Review 65 YEARS OF THE DOUBLE HELIX: Treatment of pituitary tumors with temozolomide: an update.Syro LV, Rotondo F, Ortiz LD, Kovacs K. Endocr Relat Cancer. 2018 Aug; 25(8):T159-T169. Epub 2018 Mar 13.
- Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide.[J Clin Oncol. 2002]Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide.Stupp R, Dietrich PY, Ostermann Kraljevic S, Pica A, Maillard I, Maeder P, Meuli R, Janzer R, Pizzolato G, Miralbell R, et al. J Clin Oncol. 2002 Mar 1; 20(5):1375-82.
- Temozolomide-induced biliary ductopenia: a case report.[J Med Case Rep. 2016]Temozolomide-induced biliary ductopenia: a case report.Balakrishnan A, Ledford R, Jaglal M. J Med Case Rep. 2016 Feb 5; 10:33. Epub 2016 Feb 5.
- Temozolomide - LiverToxTemozolomide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...