NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Choline magnesium trisalicylate is a nonacetylated dimer of salicylic acid that is used in the therapy of chronic arthritis and for general management of pain and fever. Trisalicylate has been linked to rare instances of acute, clinically apparent liver injury.
Background
Trisalicylate (trye" sa lis' i late) is a nonacetylated salicylic acid that has antiinflammatory, analgesic and antipyretic actions similar to aspirin. The antiinflammatory and analgesic effects of trisalicylate are probably mediated by its inhibition prostaglandin synthesis. Choline magnesium trisalicylate is similar in efficacy to aspirin, but is reported to have fewer side effects. Although available for several decades, it is less commonly used than typical nonsteroidal antiinflammatory agents. Trisalicylate is indicated for the treatment of chronic arthritis due to osteoarthritis or rheumatoid arthritis and for minor-to-moderate pain. Trisalicylate is available by prescription only as tablets of 500, 750 and 1000 mg in generic forms and under the brand name of Trilisate. It is also available as an oral solution. The recommended regimen is 750 to 1500 mg twice daily, based upon response and tolerance. Common side effects are intestinal upset, nausea, headache, dizziness, somnolence, tinnitus, rash and hypersensitivity reactions.
Hepatotoxicity
Prospective studies show that a proportion of patients taking choline magnesium trisalicylate experience at least transient serum aminotransferase elevations. These may resolve even with drug continuation, particularly if the dose is reduced. Marked aminotransferase elevations (>3 fold elevated) can occur with high doses in patterns that resemble aspirin induced hepatotoxicity. At least one instance of clinically apparent liver injury with jaundice has been reported with onset within a week of starting trisalicylate and characterized by a mixed pattern of enzyme elevations and potentially severe course. Fever, rash and eosinophilia did not occur and autoantibodies were not present. Trisalicylate is probably capable of inducing Reye syndrome in a susceptible child or adolescent and, like aspirin, should be avoided in those age groups.
Likelihood score: A[HD]: (well established cause of clinically apparent liver injury when given in high doses).
Mechanism of Injury
The mechanism of trisalicylate hepatotoxicity is likely hypersensitivity in instances of jaundice associated with standard doses. In addition, trisalicylate in high doses probably has a direct hepatotoxic effect resembling that of aspirin.
Outcome and Management
A single case report of acute liver failure with ultimate recovery has been published. No instances of chronic liver disease or vanishing bile duct syndrome due to trisalicylate have been published. Patients with hypersensitivity to trisalicylate should probably avoid other nonacetylated salicylates.
Drug Class: Salicylates
Other Drugs in the Class: Aspirin, Diflunisal, Salsalate
CASE REPORT
Case 1. Serum enzyme elevations during choline magnesium trisalicylate therapy.(1)
A 21 year old woman had systemic lupus erythematosus, but was on no specific therapy. She developed a persistent cough that was then followed by fever, headache and fatigue. Evaluation found no specific cause and she was started on trisalicylate in a dose of 1500 mg twice daily to treat a suspected worsening of the underlying lupus erythematosus. Within four days, serum enzymes which had been mildly abnormal began to rise (Table). Trisalicylate was stopped and one day later serum ALT levels began to fall, although they were still elevated at the time of discharge and no follow up was available. Testing done on admission suggested that the fever and cough were due to cytomegalovirus infection and not to worsening of lupus erythematosus.
Key Points
| Medication: | Choline magnesium trisalicylate (3.0 g daily) |
|---|---|
| Pattern: | Mixed (R=4.5) |
| Severity: | 1+ (enzyme elevations without jaundice) |
| Latency: | 4 days |
| Recovery: | Rapid, but not documented to be complete |
| Other medications: | None mentioned |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | Pre | 79 | 164 | 0.8 | Admission for fever and headache |
| Pre | 84 | 290 | 0.4 | IgM anti-CMV positive | |
| Pre | 15 | 100 | 0.6 | ||
| Trisalicylate started (1.5 g twice daily) | |||||
| 3 days | 63 | 357 | 0.2 | ||
| 6 days | 0 | 555 | 371 | 0.3 | Trisalicylate stopped |
| 7 days | 1 day | 590 | 376 | 0.3 | |
| 8 days | 2 days | 383 | 326 | 0.5 | |
| 9 days | 3 days | 190 | 275 | 0.4 | |
| Patient discharged; no follow up available | |||||
| Normal Values | <41 | <110 | <1.2 | ||
Comment
A typical pattern of serum enzyme elevation in salicylate hepatotoxicity occurring within days of starting therapy with choline magnesium trisalicylate and improving within a few days of stopping. CMV hepatitis is also a possibility, although the timing in relationship to starting the medication favors drug induced liver injury. Salicylate hepatotoxicity is generally asymptomatic, anicteric and rapidly reversible.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Trisalicylate – Generic, Trilisate® (not available in U.S.), Generic
DRUG CLASS
Antiinflammatory Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
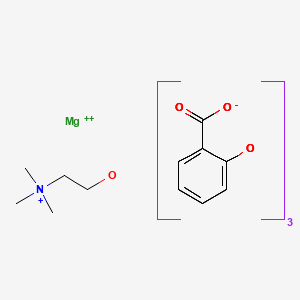
| Choline Magnesium Salicylate | 64425-90-7 | C7-H6-O3.C7-H5-O3. C5-H14-N-O.1/2Mg |
|
CITED REFERENCE
- 1.
- Cersosimo RJ, Matthews SJ. Hepatotoxicity associated with choline magnesium trisalicylate: case report and review of salicylate-induced hepatotoxicity. Drug Intell Clin Pharm. 1987;21:621–5. [PubMed: 3301251]
ANNOTATED BIBLIOGRAPHY
References updated: 15 August 2020
- Zimmerman HJ. Drugs used to treat rheumatic and musculospastic disease. Chapter 19: The NSAIDS. In Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott Williams & Williams, 1999, pp. 517-41.(Review of hepatotoxicity of salicylates published in 1999, discusses aspirin).
- Lewis JH, Stine JG. Nonsteroidal anti-inflammatory drugs and leukotriene receptor antagonists: pathology and clinical presentation of hepatotoxicity. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd Edition. Amsterdam: Elsevier, 2013. pp. 370-402.(Expert review of liver injury caused by NSAIDs mentions that choline magnesium trisalicylate can cause hypersensitivity reactions and accompanying liver injury).
- Burke A, Smyth E, FitzGerald GA. Analgesic-antipyretic and antiinflammatory agents: pharmacotherapy of gout. Chapter 26. In, Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill, 2006, pp 671-715.(Textbook of pharmacology and therapeutics).
- Blechman WJ, Lechner BL. Clinical comparative evaluation of choline magnesium trisalicylate and acetylsalicylic acid in rheumatoid arthritis. Rheumatol Rehabil. 1979;18:119–24. [PubMed: 377449](Comparison of trisalicylate with aspirin in rheumatoid arthritis; trisalicylate was as effective and had fewer side effects, and no abnormal laboratory results were found on testing after 7 weeks of therapy).
- Cersosimo RJ, Matthews SJ. Hepatotoxicity associated with choline magnesium trisalicylate: case report and review of salicylate-induced hepatotoxicity. Drug Intell Clin Pharm. 1987;21:621–5. [PubMed: 3301251](21 year old woman with lupus developed rising serum aminotransferase levels when placed on trisalicylate, resolving with discontinuation: no jaundice: Case 1).
- Le Gallez P, Bird HA, Wright V. A comparison of choline magnesium trisalicylate and acetylsalicylic acid in patients with rheumatoid arthritis. Curr Med Res Opin. 1990;12:71–5. [PubMed: 2202551](Crossover 3 week study in 19 patients with rheumatoid arthritis with ALT monitoring, "as far as one could tell, there was no significant differences in the frequency of side-effects").
- Nadkarni MM, Peller CA, Retig J. Eosinophilic hepatitis after ingestion of choline magnesium trisalicylate. Am J Gastroenterol. 1992;87:151–3. [PubMed: 1728115](66 year old woman developed jaundice 3 days after stopping 3 day course of trisalicylate for osteoarthritis [bilirubin 7.0 rising to 14.3 mg/dL, ALT 774 U/L, Alk P 542 U/L], with severe course, but ultimate recovery; biopsy showed hepatitis with eosinophils; no rash or fever, ANA negative).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, NSAIDs were implicated as a sole agent in 8 cases [4 diclofenac, 2 celecoxib, 1 meloxicam and 1 oxaprozin] and as one of several agents in 3 cases [1 diclofenac, 1 celecoxib, 1 ibuprofen]; trisalicylate not mentioned).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were due to choline magnesium trisalicylate or other salicylates).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were due to choline magnesium trisalicylate or other salicylates).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 28 were due to nonsteroid antiinflammatory agents, one to salsalate but none to choline magnesium trisalicylate).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Hepatotoxicity associated with choline magnesium trisalicylate: case report and review of salicylate-induced hepatotoxicity.[Drug Intell Clin Pharm. 1987]Review Hepatotoxicity associated with choline magnesium trisalicylate: case report and review of salicylate-induced hepatotoxicity.Cersosimo RJ, Matthews SJ. Drug Intell Clin Pharm. 1987 Jul-Aug; 21(7-8):621-5.
- Review Choline Magnesium Salicylate.[Drugs and Lactation Database (...]Review Choline Magnesium Salicylate.. Drugs and Lactation Database (LactMed®). 2006
- Treatment of acute rheumatic carditis with choline magnesium trisalicylate in a patient needing surgery.[J Pediatr Pharmacol Ther. 2003]Treatment of acute rheumatic carditis with choline magnesium trisalicylate in a patient needing surgery.Moffett BS, Arora G, Bricker JT. J Pediatr Pharmacol Ther. 2003 Oct; 8(4):284-6.
- A comparison of choline magnesium trisalicylate and acetylsalicylic acid in patients with rheumatoid arthritis.[Curr Med Res Opin. 1990]A comparison of choline magnesium trisalicylate and acetylsalicylic acid in patients with rheumatoid arthritis.Le Gallez P, Bird HA, Wright V. Curr Med Res Opin. 1990; 12(2):71-5.
- Eosinophilic hepatitis after ingestion of choline magnesium trisalicylate.[Am J Gastroenterol. 1992]Eosinophilic hepatitis after ingestion of choline magnesium trisalicylate.Nadkarni MM, Peller CA, Retig J. Am J Gastroenterol. 1992 Jan; 87(1):151-3.
- Trisalicylate - LiverToxTrisalicylate - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...