NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Varenicline is a partial agonist of the nicotinic acetylcholine receptor and is used to help in smoking cessation. Varenicline has been associated with a low rate of serum enzyme elevations during therapy and, since approval and its widescale use, with rare instances of clinically apparent mild liver injury.
Background
Varenicline (var en' i kleen) is a partial agonist of the α4 β2 nicotinic acetylcholine receptor and appears to act by blocking the binding of nicotine to this receptor while providing partial agonist effect thus relieving nicotine craving. Use of varenicline in a program to stop smoking has been shown to increase the rate of smoking cessation and to decrease relapse. Varenicline was approved for use in the United States in 2006 and is widely used in smoking cessation programs. Varenicline is available in tablets of 0.5 and 1 mg under the brand name Chantix. The usually recommended regimen is to start with 0.5 mg once daily and increase to a maintenance dose of 1 mg twice daily, continuing therapy for at least 12 weeks after smoking cessation. Common side effects include nausea, vivid dreams, insomnia, anxiety, depression, dizziness, drowsiness, headache, dry mouth, and change in appetite, some of the symptoms being those of nicotine withdrawal. Varenicline has been reported to cause hypersensitivity reactions including Stevens Johnson syndrome.
Hepatotoxicity
Varenicline has not been associated with rates of serum enzyme elevations during therapy greater than occurs with placebo therapy, but information on these abnormalities is limited and occasional instances of asymptomatic ALT elevations leading to drug discontinuation have been reported. In prelicensure pivotal registration trials in several thousand patients, varenicline was not associated with cases of jaundice or hepatitis. Since licensure, rare case reports of serum enzyme elevations without jaundice arising within 4 weeks of starting varenicline have been published, but largely in patients with other causes of liver injury (alcoholic liver disease, hepatitis C). The injury was self-limited in course and not associated with immunoallergic or autoimmune features. In Iceland, a single case of varenicline hepatotoxicity has been reported (Case 1), there having been an estimated 20,000 persons treated with the drug in the country since its introduction.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which varenicline might cause liver injury is not known. Varenicline undergoes minimal hepatic metabolism and is excreted largely unchanged in the urine.
Outcome and Management
The rare reports of hepatotoxicity attributed to varenicline therapy have been mild and self-limiting. Varenicline has not been linked to cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome.
Agents in clinical use to aid in smoking cessation and to treat nicotine withdrawal symptoms include bupropion, nicotine, and varenicline.
Drug Class: Substance Abuse Treatment Agents
CASE REPORT
Case 1. Mild acute hepatitis arising 3 weeks after starting varenicline.(1)
A 50 year old woman developed nausea and fatigue 3 weeks after starting varenicline for smoking cessation. She stopped the medication promptly but during the next week developed itching, dark urine, and jaundice. She had no history of liver disease, alcohol abuse, risk factors for viral hepatitis or drug allergies. Her other medical conditions included hypertension and cerebral aneurysm for which she was taking atenolol, amlodipine and a thiazide diuretic. On examination, she was jaundiced but had no signs of chronic liver disease. Blood tests showed a total serum bilirubin of 5.6 mg/dL, ALT 466 U/L, AST 207 U/L, alkaline phosphatase 249 U/L and GGT 976 U/L. There was no fever, rash or peripheral eosinophilia. Tests for hepatitis A, B, C, E and Epstein Barr virus and cytomegalovirus were negative as well serum autoantibodies. An abdominal CT scan showed no liver or biliary abnormalities. She was not admitted to the hospital, did not undergo liver biopsy and received no specific therapy. Her routine medications were continued. Over the next few weeks she improved (Table) and liver tests were normal or near normal when she was seen 6 weeks after onset.
Key Points
| Medication: | Varenicline (3 weeks), initially 0.5 mg increasing to 2 mg daily |
|---|---|
| Pattern: | Mixed (R=4.3) |
| Severity: | 2+ moderate (jaundice, not hospitalized) |
| Latency: | 3 weeks to onset of symptoms |
| Recovery: | 5-6 weeks |
| Other medications: | Atenolol, amlodipine, bendroflumethiazide with potassium |
Laboratory Values
Comment
The patient developed a mild, self-limited acute hepatitis 3 weeks after starting varenicline. Other diagnoses were carefully ruled out. Very few cases of varenicline hepatotoxicity have been described, and most were not completely convincing because of the presence of other liver diseases. This case with a latency of 3 weeks and a self-limited mild course of a mixed hepatitis is reasonably convincing. Varenicline has little hepatic metabolism and it is given in low doses (2 mg daily), and thus does not have features usually associated with hepatotoxicity.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Varenicline – Chantix®
DRUG CLASS
Substance Abuse Treatment Agents
Product labeling at DailyMed, National Library of Medicine, NIH
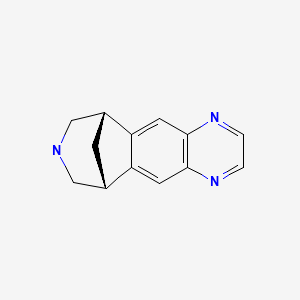
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Varenicline | 249296-44-4 | C13-H13-N3 |
|
CITED REFERENCE
- 1.
- Mogensen H, Björnsson ES. Varenicline-induced acute liver injury with jaundice. Hepatology. 2015;61:2110–1. [PubMed: 25820383]
ANNOTATED BIBLIOGRAPHY
References updated: 22 July 2020
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999 before the availability of varenicline).
- O'Brien CP. Nicotine. Drug us disorders and addiction. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 437-9.(Textbook of pharmacology and therapeutics mentions that varenicline partially stimulates nicotinic receptors, thereby reducing craving and preventing most withdrawal symptoms).
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, et al. the Varenicline Phase 3 study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. [PubMed: 16820546](Controlled trial of varenicline vs bupropion vs placebo for 12 weeks in 1025 smokers; no instances of hepatitis or clinically apparent liver injury were reported, but results of blood chemistry testing not discussed).
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, et al. Varenicline Phase 3 study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. [PubMed: 16820547](Controlled trial of varenicline vs bupropion vs placebo for 12 weeks in 1027 smokers; no instances of hepatitis or jaundice were reported; results of serial blood chemistry determinations not given).
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296:64–71. [PubMed: 16820548](Controlled trial of varenicline vs placebo continuation for 12 weeks after achieving abstinence with initial 12 weeks of therapy; no hepatic serious adverse events occurred, no discussion of ALT elevations).
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist. Arch Intern Med. 2006;166:1561–8. [PubMed: 16908788](Controlled trial of several doses of varenicline vs bupropion or placebo for 12 weeks in 626 smokers; "The frequency of clinically significant laboratory test abnormalities was low and similar across all treatment groups").
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, et al. Varenicline Study Group. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–7. [PubMed: 16908789](Controlled trial of 3 regimens of varenicline vs placebo for 12 weeks in 647 smokers; "results of clinical laboratory tests...demonstrated no clinically meaningful differences between varenicline and placebo").
- Williams KE, Reeves KR, Billing CB Jr, Pennington AM, Gong J. A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Curr Med Res Opin. 2007;23:793–801. [PubMed: 17407636](Among 377 patients enrolled in a placebo controlled trial of varenicline for smoking cessation, the most common side effects were nausea, gastrointestinal symptoms, weight gain, insomnia and abnormal dreams; 2 patients stopped therapy because of ALT or AST elevations, but values and follow up not provided).
- Hays JT, Ebbert JO, Sood A. Efficacy and safety of varenicline for smoking cessation. Am J Med. 2008;121(4) Suppl 1:S32–42. [PubMed: 18342165](Review of mechanism of action, clinical efficacy and safety of varenicline; in placebo controlled trials "the frequency of clinically significant laboratory test abnormalities was low and similar across the treatment groups").
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to varenicline).
- Franck AJ, Sliter LR. Acute hepatic injury associated with varenicline in a patient with underlying liver disease. Ann Pharmacother. 2009;43:1539–43. [PubMed: 19638471](53 year old man with end-stage alcoholic liver disease [initial ALT ~30 U/L, Alk P ~130 U/L] awaiting liver transplantation developed nausea and pruritus 1 week after starting a course of varenicline [bilirubin 1.0 mg/dL, ALT 657 U/L, Alk P 183 U/L], resolving within 1-2 months of stopping).
- Silva RR. Two recent updates for psychiatric conditions. J Child Adolesc Psychopharmacol. 2009;19:207. [PubMed: 19364299](Brief editorial review of the postmarketing product update on varenicline and bupropion warning of suicidal ideation and behaviors with their use).
- Jiménez-Ruiz C, Berlin I, Hering T. Varenicline: a novel pharmacotherapy for smoking cessation. Drugs. 2009;69:1319–38. [PubMed: 19583451](Review of the structure, pharmacology, metabolism, clinical efficacy and safety of varenicline; no discussion of hepatotoxicity).
- Safety of smoking cessation drugs. Med Lett Drugs Ther. 2009;51(1319):65. [PubMed: 19696706](Concise review of safety of medications used for smoking cessation; varenicline is mentioned as the most effective drug available, common side effects are nausea, sleep disturbances, vivid dreams, headache, constipation, vomiting, flatulence and dry mouth; no mention of hepatic effects).
- McNeil JJ, Piccenna L, Ioannides-Demos LL. Smoking cessation-recent advances. Cardiovasc Drugs Ther. 2010;24:359–67. [PubMed: 20602163](Review of mechanisms of action, efficacy and safety of smoking cessation therapies; hepatotoxicity is not discussed).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none to varenicline or other agents used to treated substance abuse).
- Cui Q, Robinson L, Elston D, Smaill F, Cohen J, Quan C, McFarland N, et al. Safety and tolerability of varenicline tartrate (Champix(®)/Chantix(®)) for smoking cessation in HIV-infected subjects: A pilot open-label study. AIDS Patient Care STDS. 2012;26:12–9. [PMC free article: PMC3242617] [PubMed: 22007690](Among 36 HIV-positive smokers treated with varenicline for 24 weeks, mild ALT elevations occurred in 19%, but none were above 2.5 times ULN or associated with symptoms or jaundice).
- Sprague D, Bambha K. Drug-induced liver injury due to varenicline: a case report. BMC Gastroenterol. 2012;12:65. [PMC free article: PMC3407017] [PubMed: 22681894](69 year old man developed nausea followed by jaundice within 1 week of starting varenicline and stopped it promptly, presenting several weeks later [bilirubin 12 mg/dL, ALT 1592 U/L, Alk P 254 U/L], resolving within two months; patient was also positive for anti-HCV and HCV RNA and liver biopsy during follow up showed chronic hepatitis).
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, et al. NCIG (National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group) Study Group. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7:277–86. [PMC free article: PMC3914416] [PubMed: 23728065](Among 200 adults with alcohol dependence treated with varenicline or placebo for 13 weeks, percent of heavy drinking days decreased more on varenicline, but abstinence rates were similar, and adverse events included nausea [37% vs 18%], abnormal dreams [28% vs 12%], constipation [9% vs 2%], and abnormalities of “liver function tests” [0% vs 1%]).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to varenicline).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attribute to varenicline).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to varenicline).
- Mogensen H, Björnsson ES. Varenicline-induced acute liver injury with jaundice. Hepatology. 2015;61:2110–1. [PubMed: 25820383](50 year old Icelandic woman developed fatigue 3 weeks after starting varenicline, which she promptly stopped but went on to develop jaundice and itching [bilirubin 5.6 mg/dL, ALT 466 U/L, Alk P 249 U/L], with rapid recovery: Case 1).
- Sakakibara M, Ohkawa K, Nawa T, Abe Y, Kusakabe A, Imai T, Katayama K. Two-step progression of varenicline-induced autoimmune hepatitis. Clin J Gastroenterol. 2018;11:184–7. [PubMed: 29383494](46 year old Asian woman developed elevations in serum enzymes while on varenicline, which improved upon stopping but rose again 8 weeks later with appearance of ANA positivity, ultimately requiring corticosteroid therapy).
- Drugs for smoking cessation. Med Lett Drugs Ther. 2019;61(1576):105–10. [PubMed: 31381546](Concise review of the mechanisms of action, clinical efficacy, safety and costs of drugs for smoking cessation mentions that side effects of varenicline include nausea and constipation, dizziness and headache and sleep disturbances with insomnia and vivid dreams; no mention of ALT elevations or hepatotoxicity).
- Desai RJ, Good MM, San-Juan-Rodriguez A, Henriksen A, Cunningham F, Hernandez I, Good CB. Varenicline and nicotine replacement use associated with US Food and Drug Administration drug safety communications. JAMA Netw Open. 2019;2:e1910626. [PMC free article: PMC6754175] [PubMed: 31483473](Varenicline has been labelled with warnings concerning serious neuropsychiatric and cardiovascular adverse events, but in postmarketing studies these complications have not been found more frequent with varenicline than placebo or no treatment, and analyses of Veterans Administration and Medicare/Medicaid prescribing has documented declines in the use of the drug after the FDA warnings which may have had the effect of an increase in smoking related morbidity and mortality).
- Carson-Chahhoud KV, Smith BJ, Peters MJ, Brinn MP, Ameer F, Singh K, Fitridge R, et al. Two-year efficacy of varenicline tartrate and counselling for inpatient smoking cessation (STOP study): A randomized controlled clinical trial. PLoS One. 2020;15:e0231095. [PMC free article: PMC7190140] [PubMed: 32348306](Among 1959 Australian adults admitted with smoking related illnesses to 3 hospitals between 2008 and 2011 who were treated with counseling with or without varenicline, smoking cessation was more frequent with varenicline which was well tolerated, common adverse events being nausea [16% vs 1.5%], abnormal dreams [6% vs 1%], headache [6% vs 1.5%], insomnia [5% vs 2%] and dizziness [2% vs 0.5%]; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- A randomized, placebo-controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers.[Clin Ther. 2007]A randomized, placebo-controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers.Tsai ST, Cho HJ, Cheng HS, Kim CH, Hsueh KC, Billing CB Jr, Williams KE. Clin Ther. 2007 Jun; 29(6):1027-39.
- Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers.[Clin Ther. 2007]Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers.Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Clin Ther. 2007 Jun; 29(6):1040-56.
- Procognitive effects of varenicline in the animal model of schizophrenia depend on α4β2- and α 7-nicotinic acetylcholine receptors.[J Psychopharmacol. 2018]Procognitive effects of varenicline in the animal model of schizophrenia depend on α4β2- and α 7-nicotinic acetylcholine receptors.Potasiewicz A, Golebiowska J, Popik P, Nikiforuk A. J Psychopharmacol. 2018 Dec 3; 33(1):269881118812097. Epub 2018 Dec 3.
- Review Varenicline: for smoking cessation.[Kathmandu Univ Med J (KUMJ). 2...]Review Varenicline: for smoking cessation.Rao J, Shankar PK. Kathmandu Univ Med J (KUMJ). 2009 Apr-Jun; 7(26):162-4.
- Review Nicotine receptor partial agonists for smoking cessation.[Cochrane Database Syst Rev. 2008]Review Nicotine receptor partial agonists for smoking cessation.Cahill K, Stead LF, Lancaster T. Cochrane Database Syst Rev. 2008 Jul 16; (3):CD006103. Epub 2008 Jul 16.
- Varenicline - LiverToxVarenicline - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...