Reviewers
External Reviewers
This document was externally reviewed by content experts and the following individuals granted permission to be cited.
Brian Bressler MD, MS, FRCPC
Founder, The IBD Centre of BC
Director, Advanced IBD Training Program Clinical Associate Professor of Medicine, Division of Gastroenterology, University of British Columbia
Abbreviations
- ASA
Aminosalicylate
- AZA
Azathioprine
- CDAI
Crohn’s Disease Activity Index
- CDEIS
Crohn’s Disease Endoscopic Index of Severity
- CI
Confidence interval
- HBI
Harvey-Bradshaw Index
- HR
Hazard ratio
- RCT
Randomized controlled trial
- RR
Relative risk
- SD
Standard deviation
- SES-CD
Simple Endoscopic Score for Crohn’s Disease
- TNF
Tumor Necrosis Factor
Context and Policy Issues
Crohn’s disease is a chronic relapsing inflammatory condition, which primarily affects the gastrointestinal tract.1 The disease is characterized by both clinical symptoms and objective findings from endoscopy and pathology studies.1 Common symptoms include diarrhea, weight loss, abdominal pain, and fever.2 Endoscopic findings include ulcers, inflammation, and lesions.1 The onset is typically between age 20 and 30 years, though there is a second small peak in incidence around age 50; the disease may also present in childhood or adolescence.1,3 There are three phenotypes of disease: inflammatory, structuring, and penetrating, and patients can have one or more of these phenotypes.3
The incidence and prevalence of Crohn’s disease is increasing worldwide, and Canada has a prevalence among the highest in the world.4 The incidence in Canada was reported as 20.2 per 100,000 persons in a 2012 systematic review.5 A 2018 report suggested that the incidence varies by province, from 8.8 per 100,000 in British Columbia to 22.6 per 100,000 in Nova Scotia.4 The prevalence was estimated at 725 per 100,000 in Canada in 2018.4 A 2011 systematic review of international studies reported that 4 per 100,000 develop Crohn’s disease in childhood or adolescence.6
People with Crohn’s disease generally experience remissions and relapses over time.3 There may also be complications such as strictures or fistulas throughout the disease course.2 People with Crohn’s disease may require bowel resection surgery at some point to manage their disease.3 Further, those with Crohn’s disease often experience regular hospital admissions. The annual incidence in hospital admission is 20%, while around 50% of patients require surgery in the 10 years after diagnosis.1 Thus, health resource utilization related to Crohn’s disease may be high.
The main goals of medical treatment are to achieve both clinical and endoscopic remission (e.g., mucosal healing), with the aim of preventing complications and surgery.1,3 Medical management, in persons with early disease or a flare, aims to achieve rapid symptom relief and long-term disease control.2 Rapid symptom relief may be achieved using a corticosteroid or a tumor necrosis factor inhibitor (anti-TNF or “biologic”, e.g., infliximab).2 Maintenance of remission is often achieved with an immunomodulator (immunosuppressant) such as azathioprine (AZA) or methotrexate and/or an anti-TNF.2 The exact choice of agents and treatment approach depends on the phenotype, disease activity, severity, and individual patient characteristics.1–3 Two possible approaches are the conventional (or “step-up”) approach and early biologic (“top-down”) approach.
Traditionally, a “step-up” approach has been used, where treatment begins with a corticosteroid.7 If a person does not respond to this treatment, an immunomodulator could then be added.7 If the person is still refractory to treatment, an anti-TNF can then be added.7 Two reviews7,8 have suggested that the rationale for this approach is centered on using relatively safer (i.e. lower risk of adverse drug effects) but less efficacious options early in therapy and proceeding to more efficacious but less safe options if a patient is refractory to treatment. Lower cost has also been suggested as a rationale for the step-up approach.8 One possible challenge is that the step-up approach still results in high rates of surgical intervention.7 Corticosteroids may produce clinical remission but not affect mucosal healing.9 While using corticosteroids, inflammation may persist and result in tissue damage and disease progression.9 Mucosal healing has been noted to be particularly important in Crohn’s disease, as it can change the course of the disease.10 It is argued that step-up treatment can delay initiation of treatment that induces mucosal healing.9 The “top-down” approach involves early initiation of anti-TNF agents (with or without immunomodulators).7 When anti-TNF agents are given with immunomodulators, this may be called an “early combined” treatment approach. The theory behind top-down treatment is that it may induce mucosal healing early and subsequently may reduce the risk of downstream negative outcomes (e.g., surgery, relapse, hospitalization).9 It also appears to have positive effects on clinical outcomes. For example, the SONIC trial found that infliximab alone, or in combination with an immunosuppressant, improved rates of clinical remission at 26 weeks in treatment naïve adults.11
As mentioned, the appropriateness of these individual approaches likely depends on the severity and phenotype of disease, along with the clinical characteristics of the patient.7 Given the theorized benefits of a top-down approach (early use of biologics), there is growing interest in its clinical effectiveness, safety, and cost-effectiveness compared to a conventional treatment approach, both in children and adults.7,8 As such, this report sought to summarize the evidence regarding the clinical and cost-effectiveness of early biologic therapy compared to conventional treatment for Crohn’s disease in children and adults.
This is an upgrade of a previous CADTH rapid response titled “Early Treatment versus Conventional Treatment for the Management of Luminal Crohn’s Disease: A Review of Comparative Clinical Effectiveness and Cost-Effectiveness”.
Research Questions
What is the clinical effectiveness of early biologic treatment compared with conventional treatment for the management of Crohn’s disease?
What is the cost-effectiveness of early biologic treatment compared with conventional treatment for the management of Crohn’s disease?
Key Findings
The clinical effectiveness of early biologic therapy compared to conventional therapy for Crohn’s disease in adults is unclear based on available evidence, due to a limited number of studies and heterogeneity of existing studies. The evidence identified in this report did not suggest consistent benefits of early biologic therapy compared to conventional treatment but did point to possible benefits which require further study.
There were six non-randomized studies conducted in children, which were limited both in terms of the size and quality. No firm conclusions can be drawn about the clinical effectiveness of early biologic therapy for Crohn’s disease based on current evidence.
One cost-effectiveness analysis conducted in Italy suggested that early combined therapy may be both more effective and cost-saving compared to conventional treatment for Crohn’s disease in adults.
Methods
Literature Search Methods
This project was based on a literature search performed for a previous CADTH report. A literature search was conducted on key resources including PubMed, The Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases, Canadian and major international health technology agencies, as well as a focused Internet search. No methodological filters were applied to limit by study type. Where possible, retrieval was limited to the human population. The search was also limited to English language documents published between January 1, 2008 and November 22, 2018.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications or were published prior to January 1, 2008.
Critical Appraisal of Individual Studies
The included randomized controlled trials (RCTs) were appraised using Cochrane’s Risk of Bias 2.0 tool.12 Non-randomized studies were appraised using Downs and Black checklist13 and economic studies were appraised using the Drummond checklist14.
Summary of Evidence
Quantity of Research Available
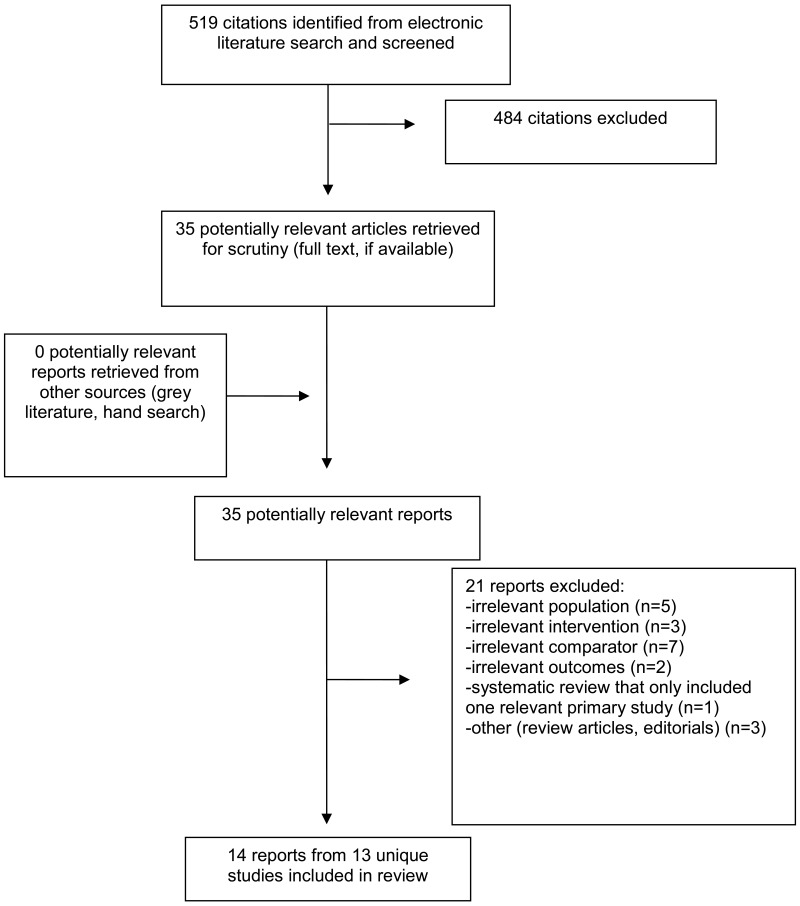
A total of 519 citations were identified in the literature search. Following screening of titles and abstracts, 484 citations were excluded and 35 potentially relevant reports from the electronic were retrieved for full-text review. No potentially relevant publications were retrieved from the grey literature search for full text review. Of the 35 potentially relevant articles, 21 publications were excluded for various reasons and 14 publications (from 13 unique studies) met the inclusion criteria and were included in this report. There were 2 RCTs (and 1 study reporting long-term follow-up from one of the RCTs) conducted in adults, 10 non-randomized studies (4 in adults and 6 in children), and 1 economic evaluation in adults. Appendix 1 presents the PRISMA15 flowchart.
Summary of Study Characteristics
Additional detail regarding the characteristics of included studies is provided in Appendix 2. A description of scales used in the eligible studies is included at the beginning of Appendix 2.
Study Design in Adults
There were two parallel group RCTs16,17 that included adults, and one long-term follow up study of one trial.18 One (n = 1,982) was an open-label cluster randomized trial17 and one (n = 133) was an open-label randomized trial16 with long-term follow up study (n = 119 of 133 in original trial).18
There were four non-randomized studies in adults.19–22 One (n=2,662) was a prospective registry-based study20, one (n = 77) was a controlled before-after study21, one (n=93) was a retrospective chart review19, and one (n = 3,750) was a retrospective cohort study.22
There was one economic evaluation, which was a cost-effectiveness study23 using a Markov model, with a time horizon of 5 years from the perspective of the Italian healthcare system. The clinical inputs were based on an RCT16 and costs based on the Italian healthcare system. The model assumed an average weight of 60 kg, a purchase cost for infliximab of €512 and that 20% of patients having surgery would have early complications.
Study Design in Children
There were six non-randomized studies that included children.24–29 Two (n = 76 and n = 552) were prospective observational studies,27,29 two (n = 28 and n = 36) retrospective chart reviews25,26 and two (n = 29 and n = 51) retrospective observational studies.28,30
Country of Origin for Studies in Adults
One RCT was conducted in Canada and Belgium17 and one was conducted in Belgium, Netherlands, and Germany.16 The long-term follow-up study was conducted in the RCT in Belgium, Netherlands, and Germany.18 One non-randomized study used data from patients in Austria, Belgium, France, Germany, Italy, Netherlands, Spain, Sweden, and the United Kingdom,20 two were conducted in the USA,19,22 and one was conducted in China.21
Country of Origin for Studies in Children
Five studies were conducted in South Korea24–28 and one study was conducted in Canada (using data from Canada and USA).29
Patient Population in Adults
Both RCTs recruited people 18 years or older. The mean age was 44 in one trial17 and approximately 30 in the other.16 In the trial by Khanna et al., persons were included regardless of disease activity, severity or duration, or treatment history – approximately 56% were in steroid-free remission at baseline, 7% had active fistula and the mean duration of disease was around 150 months.17 In D’Haens et al., included people that were newly diagnosed with Crohn’s disease (mean 2 weeks from diagnosis to treatment), had clinically active disease, and were treatment naïve.16 Approximately 60% of the participants in both trials were female.
In the non-randomized studies, the median age at baseline ranged from 2521 to 40 years22 in the three studies that reported it. The other study only reported age at diagnosis, which was 28 (mean duration of disease was 11 years).19 The proportion of female participants ranged from 53 to 60%. D’Haens et al. (2017) included patients regardless of disease severity or phenotype – approximately 30% of patients had fistulas and the mean time since diagnosis was 8.5 years.20 The study by Fan et al. involved newly diagnosed persons with moderate to severe luminal disease who were treatment naïve.21 Ghazi et al. included patients regardless of severity or phenotype (48% had “penetrating” disease) and the mean duration of disease was 11 years.19 Finally, Rubin et al. did not report criteria for severity or duration.22
The economic evaluation involved a population from one of the RCTs.16 These were newly diagnosed patients (mean age 30, median disease duration 10 weeks) with active disease, who were treatment naïve.
Patient Population in Children
Two studies reported median age at diagnosis, which was 14 and 15.27,30 The other four studies reported age at study baseline which ranged between 12 and 14.25,26,28,29 The proportion of female participants ranged from 14 to 48% in the five studies24–27,29 that reported it. Kang et al. included people with newly-diagnosed moderate to severe luminal Crohn’s disease, but people in the step-up group had previously failed conventional treatment (duration of disease was 0.7 months in top-down group versus 8 months in step-up).27 Lee et al. (2015) included newly-diagnosed patients with moderate to severe disease, regardless of disease type (around 60% had fistulas; duration of disease was 1 month in top-down versus 10 months in step-up).30 Walters et al. included participants newly diagnosed with luminal Crohn’s disease regardless of severity.29 Lee et al.(2012) and Kim et al.25,28 included persons with either moderate to severe Crohn’s disease or therapy resistant disease (duration and type not specified; although in one study 62% had fistulas). Finally, the Lee et al. (2010) study included newly diagnosed persons (type and severity not specified).26
Interventions and Comparators in Adults
Khanna et al. tested an early combined immunosuppression intervention (an algorithm involving combined anti-TNF and immunomodulator treatment if persons failed a steroid induction of 4- or 12-weeks) against conventional management (described as usual care).17 In this trial, 20% of people ended up on an early combined therapy in the algorithm group compared to 10% in the conventional treatment arm. The trial by Khanna et al. differs from the other studies in included this review in that early biologic therapy was part of a treatment algorithm in this study, and the trial tested the algorithm against conventional treatment. Therefore, not all participants in the in the “early biologic” group necessarily received a biologic. In the other studies included in this review, people receiving an intervention of early biologic (or top-down) therapy were assigned that treatment from baseline (or were deemed to have received early in treatment course in the case of observational studies).
D’Haens et al. (2008) tested early combined immunosuppression (infliximab plus azathioprine) against conventional treatment (the economic evaluation used the approach from this trial).16
D’Haens et al. (2017) identified three groups from a prospective register: (1) people who started infliximab with 30 days of enrollment, (2) conventional treatment (without ever receiving anti-TNF agents, (3) conventional treatment involving switch to infliximab.20 Fan et al. tested combined treatment initiation with infliximab and AZA against initiation with corticosteroid and AZA.21 Ghazi et al. tested early biologic treatment with or without an immunomodulator against step-up treatment (immunomodulator alone or immunomodulator before anti-TNF). Finally, Rubin et al. involved three groups: (1) 5-aminosalicylate (5-ASA) and/or corticosteroids and/or immunomodulator prior to anti-TNF, (2) immunomodulator only prior to anti-TNF, or (3) early initiation with anti-TNF.22
Interventions and Comparators in Children
Four of the studies tested top-down treatment approaches (early infliximab plus AZA) against step-up eventually requiring infliximab (e.g. oral corticosteroid plus AZA and/or 5-ASA, then infliximab).25,27,28,30 Walters et al. identified three groups: (1) early anti-TNF without an immunomodulator, (2) early immunomodulator, and (3) no early immunotherapy.29 Lee et al. (2010) identified three groups of persons receiving: (1) prednisolone then mesalamine, (2) prednisolone then AZA, (3) infliximab plus AZA.26
Outcomes in Adults
A description of scales used in the eligible studies is included at the beginning of Appendix 2.
The primary outcome in both RCTs16,17 was remission. The proportion of patients in remission was compared between two groups. In Khanna et al, remission was defined as having a Harvey-Bradshaw Index (HBI) less than 5 and being corticosteroid-free at 12 months.17 The proportions were compared using analysis of covariance adjusting for cluster size, country, practice size and baseline remission rate. The trial by D’Haens et al. defined remission as a Crohn’s disease activity index (CDAI) score of less than 150, the absence of corticosteroids and no intestinal resection at 26 and 52 weeks.16 The proportion remission in each group was compared using a χ2 test. In the long-term follow-up of the D’Haens et al. RCT, the primary outcome was also remission but this was not defined. Khanna et al. examined difference in mean HBI, time to adverse outcome (surgery, disease complication, hospital admission), time to corticosteroids, adverse events, mortality, and quality of life using 36-item short form survey (SF-36) and EuroQoL 5 dimension scale (EQ-5D).17 D’Haens et al. also evaluated the time to relapse (CDAI increase >50 to a score >200), mean CDAI and inflammatory bowel disease questionnaire (IBDQ), endoscopic score (4-point scale, from 0=no ulcers to 3=ulcerated stenosis, at five regions for a total score of 15) and proportion without ulcers at 24 months.16
One non-randomized study was designed to assess safety of infliximab and reported the frequency and incidence of adverse events over 5 years.20 This study reported the crude proportion of persons experiencing adverse effects and also risk of adverse events using Cox proportional hazards models. The primary outcome in Fan et al. was deep remission (CDAI < 150 plus complete absence of mucosal ulcerations).21 The proportion in deep remission was compared using a χ2 test. This study also evaluated clinical remission (not defined) and time to remission (using life-table analysis), as well as change in Crohn’s disease endoscopic index of severity (CDEIS) from baseline, endoscopic response (CDEIS decrease >5), complete endoscopic remission (CDEIS <3), and adverse events (proportions were compared using χ2 test). Ghazi et al. evaluated remission (HBI<5) rate, HBI score, Short Inflammatory Bowel Disease Questionnaire (SIBDQ) score, number of hospitalizations, and surgeries.19 Differences between groups were analyzed using a χ2 test and t test. Finally, Rubin et al. evaluated corticosteroid use (per 6-month period) and Crohn’s disease surgery using multivariable logistic regression.22
The economic evaluation reported cost per quality-adjusted life-year (QALY).
Outcomes in Children
A description of scales used in the eligible studies is included at the beginning of Appendix 2.
Four studies examined remission rate.27–30 In these studies, remission was defined as a Pediatric CDAI (PCDAI) score less than 10, and authors compared the proportion achieving remission between groups. Walters et al.29 used propensity score analysis to analyze the relative risk of remission between groups. The other three studies mention Fisher’s exact test and a χ2 test but did not specify which was used to analyze remission rates. Three studies examined relapse rate (PCDAI > 10 after remission had been achieved) – they also mention Fisher’s exact test and a χ2 test but do not specify which was used.25,26,30 Four studies reported adverse events. Two studies27,28 compared PCDAI scores between groups (Kang et al. used a t test and Kim et al. [2011] did not specify). Kim et al. (2011)28 compared rates of fistula closure between groups (Fisher’s exact test and a χ2 test mentioned but did not specify which was used). Kang et al.27 compared both mucosal healing rate (Simple Endoscopic Score [SES-CD] = 0; Fisher’s exact test or χ2 test but not specified) and endoscopic findings (SES-CD score, compared using t test) between groups.
Summary of Critical Appraisal
Randomized controlled trials in adults
Details on the critical appraisal are in Appendix 3. Trials were appraised using Cochrane’s Risk of Bias 2.0 tool.12 Both RCTs16,17 had concerns related to the randomization process – in Khanna et al.17 allocation concealment was not described while in D’Haens et al.,16 allocation was not concealed. Both trials were rated as low risk for deviations from intended interventions – while both were unblinded, this did not appear to introduce deviations from the intended interventions. There were some concerns related to adherence to the interventions – co-interventions were not described in either trial. In Khanna et al., one center dropped out due to non-adherence with the early combined intervention algorithm. There were some concerns in the trial by Khanna et al. related to missing data as the number of withdrawals was higher in the early combined group compared to conventional treatment (reasons for withdrawal were not specified). In both RCTs,16,17 there were concerns related to measurement of the outcome. Both trials were open label and relied and patient- and investigator-completed rating scales. Thus, it is possible that knowledge of the treatment may have influenced outcome ratings. In Khanna et al., the outcome of composite adverse outcomes was not pre-specified in the protocol, which may reduce confidence in this result. In D’Haens et al., the authors reported outcomes (remission at multiple time points) which were not in the protocol and only presented CDAI and IBDQ data at certain timepoints. The long-term follow-up study18 of the D’Haens et al trial was at high risk of bias due to selective reporting of results as this was an unplanned and post-hoc analysis. Overall, D’Haens et al. was rated at high risk of bias and Khanna et al. was rated as having some concerns.
The trial by Khanna et al.17 included persons regardless of disease duration, severity, or activity. Approximately 55% of the participants were in steroid-free remission at baseline, the mean HBI score was 4 at baseline (a score <5 suggests remission) and the mean duration of disease was over 10 years. Given the population studied, the results may apply particularly to those with less severe and longer-standing disease. Conversely, the trial by D’Haens et al.16 included people early in the disease course who were treatment naïve. Thus, these results may be most applicable to newly diagnosed persons who have not received prior treatment.
Non-randomized studies in adults
Details on critical appraisal are in Appendix 3. Critical appraisal was completed using Downs and Black checklist.13
All four non-randomized studies clearly described their aim and main outcomes. The inclusion characteristics of patients were well described in three studies;19–21 however, Rubin et al.22 included limited information on patient characteristics (e.g. disease severity, type). None of the studies included information on socioeconomic status, marital status, education or family income. Interventions were well described except by Ghazi et al.19 who included limited detail. Results were comprehensively reported by D’Haens et al.(2017); however, the other studies had some concerns surrounding reporting, reducing confidence in the results. Fan et al.21 and Ghazi et al.19 did not report differences in proportions or mean differences between groups (and variability around these measurements, e.g. standard deviation or confidence intervals), which makes it challenging to interpret results. Fan et al.21 did not include any information on missing data in their study. Neither Ghazi et al. nor Rubin et al. reported adverse events.
D’Haens et al.(2017)20 was a registry study involving around 2,000 people thus it was likely reflective of typical persons with Crohn’s disease. However, it is unclear whether the population in the other three non-randomized studies reflects the typical patient population (for example, Ghazi et al.19 was conducted at a single tertiary care center and Rubin et al.22 was conducted using a specific drug plan’s administrative claim database). This may limit the generalizability of the findings. D’Haens et al. (2017) and Rubin et al. used regression models to analyze outcomes. D’Haens et al. (2017) used a multivariable Cox proportional hazards model adjusting for disease duration, severity, age and corticosteroid use, though residual confounding is possible. Rubin et al. used log binomial regression and described very limited information on confounders (sex, region, age location of disease)- it is unclear if these were adjusted for in their model. Thus, there are concerns surrounding confounding in both of these studies, limiting our confidence in the reported results. Fan et al.21 did not control for confounders in their analysis. They did show that age, sex, disease duration and severity were balanced at baseline, but residual confounding is possible in this study as well. Finally, Ghazi et al. did not adjust for confounders and their results suggest baseline imbalances and possible selection bias (e.g. patients in early biologic group were younger and had more severe disease compared to step-up patients).
Non-randomized studies in children
Details on critical appraisal are in Appendix 3. Critical appraisal was completed using Downs and Black checklist.13
All studies clearly described their aim, main outcomes, eligibility characteristics, and interventions. Three studies27,29,30 described the study population in detail; however, three of the studies included limited characteristics of the study population.25,26,28 All studies reported results of significance tests for outcomes, but three studies25,27,28 did not include information on random variability around outcome measurements comprehensively (e.g. CIs and SDs), which limits interpretability of the results. Only one non-randomized study adjusted for confounding in its analysis, the study by Walters et al. which used propensity score matching.29
For five of the studies, it is unclear whether the included persons reflect the typical patient population as they all included small samples (ranging from n = 28 to n = 76) from a single hospital.25–28,30 Further, since these studies took place at one hospital, it is unclear whether care received would reflect care at other centers. In contrast, the study by Walters et al. included data from a large observational study at 28 centers in North America, and thus likely reflects the typical patient population in Canada and the USA. There were concerns surrounding selection bias in four of the studies. In two studies26,29 patients were only included if they had one year of follow-up data available and in another study patients could only be included if they had three years of follow-up data.30 It is unclear whether systematic exclusion of patients who were not followed for the minimum time would introduce bias (e.g. if these patients tended to have worse or better outcomes this could create bias). Further, in three of the studies, people assigned to the different intervention groups may represent different populations of people with Crohn’s disease. For example, in the studies by Lee et al. (2012)25 and Kim et al.28 people assigned to the step-up group were only included after they had failed conventional therapy, while people in the top-down group were naïve to immunomodulator, anti-TNF, and corticosteroid treatment. Thus, these two groups did not represent the same population of persons at study baseline, signaling selection bias. Further, in three of the studies25,27,28 step-up patients were only included if they eventually received infliximab, which excludes the group of step-up people who did not require infliximab (also suggesting selection bias). These concerns raise the possibility that the estimates presented in these studies are biased, reducing confidence in the results. In the retrospective chart review studies,25,26,28 the procedure for identifying eligible participants was not described. It is unclear if these studies represent all persons treated with the interventions of interest or whether there was some sampling or selection procedure (and whether this was likely to have introduced bias). Five of the non-randomized studies involved small sample sizes (ranging from n = 28 to n = 76) and did not include power calculations.25–28,30 Therefore, it is unclear whether they were adequately powered to detect differences in outcomes.
Economic evaluation in adults
The economic evaluation23 clearly stated the research question, importance of the study, form of economic evaluation used, sources of data (costs, clinical data) and primary outcome. The time horizon, discount rate, and model details were provided. The discount rate was 3.5%, which is consistent with National Institute for Health and Clinical Excellence (NICE) guidance.31 Incremental analysis was reported, and the outcomes are presented in aggregated and disaggregated form. The choice of variables in sensitivity analyses were not described in detail, nor were the ranges over which the variables were varied, which raises questions about the validity of the sensitivity analyses. No confidence intervals were provided for stochastic data (limiting interpretability of the data and reducing confidence in these results), and the viewpoint of the analysis was not justified.
Summary of Findings
Appendix 4 presents a table of main study findings and authors’ conclusions.
Clinical effectiveness of early biologic therapy in adults
Remission
Data on remission were available from two RCTs16,17, long-term follow-up from one RCT,18 and one non-randomized study.21 In the Khanna et al. trial17 there was no significant difference between groups for the proportion of persons in remission at 12 or 24 months. In the D’Haens et al. RCT,16 there were significantly more persons in remission at both 26 and 52 weeks for early combined therapy than conventional treatment, but no significant difference at 78 and 104 weeks. Long-term follow-up of the D’Haens trial18 over 8 years found no difference in the proportion of persons in remission over time between the two groups. Fan et al.21 reported that significantly more persons were in deep remission (clinical remission plus mucosal healing) and clinical remission at week 30 for top-down therapy versus step-up therapy, but there was no significant difference at week 54. Fan et al. also reported that the median time to clinical remission was shorter for top-down versus step-up (7 weeks versus 14 weeks).
Relapse
Relapse was reported by one RCT and long-term follow-up from that RCT. D’Haens et al.16 evaluated the time to relapse after successful induction. The authors reported that the median time to relapse was statistically significantly longer for early combined therapy versus conventional therapy (329 versus 175 days). The long-term follow up study18 from the D’Haens et al. trial found that the median time to flare was statistically significantly shorter for the conventional therapy group versus early combined and that the proportion who experienced at least one flare was higher in the conventional therapy group compared to early combined therapy.
Disease activity scores
Disease activity scores were reported by both RCTs16,17 and one non-randomized study.19 Khanna et al.17 found no difference in HBI score between groups at 6, 18, or 24 months. D’Haens et al.16 found the mean reduction in CDAI score at 10 weeks was statistically significantly greater for early combined therapy compared to conventional treatment, but there was no difference between groups after 10 weeks. A non-randomized study by Ghazi et al.19 found no difference in HBI score between groups at 3, 6, and 12 months. When HBI change from baseline was compared between groups, there was a statistically significant difference for reduction in HBI score in favour of early biologic therapy at 3 months (suggesting greater improvement in symptoms), but not at 6 or 12 months. HBI scores were statistically significantly higher in the early biologic group at baseline.
Adverse outcomes of Crohn’s disease
Khanna et al.17 measured the time to adverse outcomes (a composite for surgery, hospital admission, or disease complication) and found a statistically significantly reduced rate of adverse outcomes in the early combined intervention group versus conventional therapy group. This was a secondary endpoint that was not pre-specified in the protocol and the authors note should be interpreted with caution.
Corticosteroid use
There were data from two RCTs16,17 and two non-randomized19,22 studies pertaining to corticosteroid use. Khanna et al.17 found the time to corticosteroid treatment was not different between early combined intervention and conventional therapy over 24 months. D’Haens et al.16 reported that persons in the early combined treatment group had lower exposure to corticosteroids compared to conventional treatment (statistical significance not reported). Long-term follow-up from D’Haens et al.18 found that the proportion of persons treated with corticosteroids at any point during follow-up was significantly lower for early combined therapy versus conventional therapy. The non-randomized study by Ghazi et al.19 found that the proportion of people receiving corticosteroids was statistically significantly lower in the early biologic group compared to step-up therapy at 3 months but there was no difference at 6 or 12 months. The non-randomized study by Rubin et al.22 found that the step-up therapy was associated with a significantly higher risk of receiving corticosteroids compared to early anti-TNF treatment at 6, 12, 18, and 24 months.
Quality of life
Khanna et al.17 reported no difference in EQ-5D or SF-36 scores between the early combined intervention and conventional treatment at any time point. In the D’Haens et al. RCT,16 there was a statistically significant increase in IBDQ (suggesting improved disease-related quality of life) for early combined therapy compared to conventional therapy at 10 weeks but there was no difference beyond this time point. The non-randomized study by Ghazi et al.19 reported no difference in SIBDQ between early biologic or step-up therapy at 3, 6, or 12 months. When change from baseline was measured, there was a statistically significantly greater improvement in SIBDQ at 6 months in the early combined group, but not at 3 or 12 months. The baseline SIBDQ was lower in the early biologic group at baseline.
Mortality
Khanna et al.17 found no difference in mortality between the early combined intervention and conventional treatment over 24 months.
Surgery for Crohn’s disease
Khanna et al.17 found a statistically signification reduction in the rate of surgery in the early combined intervention group versus conventional therapy. Long-term follow-up over 8 years from the D’Haens et al.18 RCT reported no difference in the proportion undergoing Crohn’s disease-related surgery between groups. Ghazi et al.19 found no difference in the number of surgeries between the early biologic and step-up groups, while Rubin et al.22 found an step-up therapy was associated with a statistically significantly increased risk of Crohn’s disease-related surgery in people receiving step-up therapy compared to early anti-TNF treatment.
Hospitalization for Crohn’s disease
Khanna et al.17 and the long-term follow-up18 from D’Haens et al. found no difference in the rate of hospitalization between early biologic therapy and conventional treatment. Ghazi et al.19 reported a significantly increased number of hospitalizations at 1 year in the early biologic group compared to the step-up group.
Serious complications
Khanna et al.17 found a statistically significant reduction in the rate of complications (new abscess, fistula, stricture, serious worsening of disease activity, extra-intestinal manifestations) for the early combined intervention group versus conventional therapy. Long-term follow-up from D’Haens et al.18 found no difference in the proportion of people developing fistulas between groups.
Endoscopic findings
The proportion of people with no ulcers at 104 weeks was significantly lower in the early combined group compared to conventional treatment in the D’Haens et al RCT.16 The endoscopy score was also significantly lower in the early combined group. The long-term follow-up study from this trial found no difference between groups in the proportion of people without ulcers over 8 years.18
Endoscopic remission and mucosal healing
The non-randomized study by Fan et al.21 found that a higher proportion of people receiving top-down therapy achieved complete endoscopic remission (CDEIS < 3) compared to step-up therapy at week 30 and week 54, though the differences were not statistically significant. There was a statistically significantly higher proportion of people with endoscopic remission (CDEIS < 6) at week 30 but not at week 54.
Fan et al.21 found that a significantly higher proportion of participants achieved mucosal healing in the top-down therapy group compared to the step-up group at week 30, but the difference was not significant at week 54 and week 102.
Safety
The RCT by Khanna et al.17 found a similar proportion of participants with adverse drug events. In the RCT by D’Haens et al.16, the proportion of participants with adverse events was higher in the early combined therapy group versus conventional management group (31% versus 25%), but there were no notable differences in individual events. Long-term follow-up of the D’Haens trial18 found that serious infections were more common in the early combined therapy persons compared to conventional treatment (22% versus 10%). The observational study by D’Haens et al. (2017)20 was designed to assess the safety of infliximab over 5 years. This study found that exposure to infliximab in the context of early biologic treatment was associated with a significantly increased risk of serious infections and hematological conditions with no increase in malignancy or lymphoproliferative disorders, compared to those receiving conventional treatment (and never receiving infliximab). Fan et al.21 examined the proportion of people with adverse events and found that 90% experienced an adverse event in the top-down group versus 85% in the step-up group (infusion reactions occurred in 13% of top-down versus 0% for step-up).
Clinical effectiveness of early biologic therapy in children
Remission
Four non-randomized studies reported remission.27–30 Kang et al.27 found that the proportion of those in clinical remission was no different between early combined and step-up therapy at week 14 versus 54. In the study by Lee et al. (2015),30 the proportion of people who achieved clinical remission was a statistically significantly higher at 1 year in the top-down group compared to the step-up group. Walters et al.29 found that the early biologic therapy was associated with a significantly higher corticosteroid-free clinical remission rate compared to the group receiving no immunosuppressant or early immunosuppressants. Kim et al.28 found that the remission rate in the top-down group was statistically significantly greater compared to step-up treatment at both 8 weeks and 1 year.
Relapse
Relapse was examined in three non-randomized studies. Lee et al. (2015)30 found that people receiving top-down therapy were significantly more likely to remain relapse-free over3 years to those receiving step-up therapy. Lee et al. (2012)25 found that the proportion of people who relapsed was lower in the top-down group compared to the step-up group at 1, 2, and 3 years, but the difference was only statistically significant at year 2. Lee et al. (2010)26 found that the proportion of people who relapsed was significantly lower in those receiving early combined therapy compared to those receiving AZA only or mesalamine, at 1 and 2 years.
Disease activity score
Kang et al.27 found there was no difference in PCDAI score between early combined and step-up treatment at 14 and 54 weeks. In the study by Kim et al.28 top-down treatment produced a statistically significantly greater improvement in PCDAI score compared to step-up treatment at both 8 weeks and 1 year.
Fistula closure
Kim et al.28 reported that in people with fistulas at baseline, the proportion with fistula closure at 8 weeks and 1 year was significantly greater in the top-down group compared to step-up.
Mucosal healing
Kang et al.27 found that the proportion of persons with mucosal healing was no different between groups at week 14 but was significantly higher at week 54 in the early combined group compared to the step-up group.
Endoscopic findings
Kang et al.27 reported that the SES-CD score was significantly lower in the early combined group compared to step-up group at both week 14 and 54 (suggesting lower mucosal involvement).
Safety
Kang et al.27 and Lee et al.(2015)30 reported no overall differences in adverse events between groups. Lee et al. (2015) found that more people in the top-down group experienced leukopenia as well as nausea and vomiting, while more participants in the top-down group experienced pancreatitis. Lee et al. (2010)26 found that fewer participants experienced an adverse event in the early biologic + AZA group (8%) compared to either AZA alone (39%) or mesalamine alone (30%); however, the number of people in each group was small. In the Kim et al.28 study, 36% of people experienced an adverse effect in the step-up group compared to 45% in the top-down group (there were no notable differences between groups).
Cost-effectiveness of early biologic therapy in adults
Top-down therapy improved quality-adjusted life expectancy and was cost-saving compared to step-up therapy.23 Time horizon and cost of infliximab had an important influence in sensitivity analysis. With a time horizon of 1 year the incremental cost-utility ratio was €92,440/QALY while at year 4 it was €1,462/QALY and dominant at year five.
Cost-effectiveness of early biologic therapy in children
No relevant evidence was identified and therefore no summary can be provided.
Limitations
Adults
One of the central challenges in interpreting the evidence is heterogeneity in the study populations and interventions tested. This makes it difficult to compare results across studies and draw conclusions about the body of evidence as a whole. For example, the two eligible RCTs comprising the highest quality evidence examined different populations and treatment approaches. Khanna et al.17 tested a treatment algorithm where early combined biologic therapy could be initiated after a 4- or 12-week course of corticosteroids (20% ended up on combined therapy). However, all people in the intervention group in D’Haens et al.16 received early combined therapy with a biologic from baseline. With regard to study population, some studies excluded persons with fistulizing disease while others did not, which also contributed to heterogeneity. Another challenge is that most studies focused only on a clinical definition of remission without endoscopic assessment (e.g. mucosal healing or endoscopic remission). Relying on a clinical definition of remission alone may be insufficient as this does not necessarily indicate that the natural course of the disease has been changed.32 Further, there were limited data on surgery and hospitalization (the avoidance of which are noted to be theoretical benefits to a top-down approach). It has been suggested that endoscopic findings are particularly important to evaluate as mucosal healing is important in changing disease course,32 reducing risk of complications and surgery (rather than relying on clinical symptoms alone).1–3
The cost-effectiveness study was based on people who were newly diagnosed and treatment naïve, thus it is unclear whether the results would apply to other patient populations. Analysis was conducted from the perspective of the Italian healthcare system using Italian healthcare and drug costs, thus it is unclear whether the findings are generalizable in a Canadian context. Finally, indirect costs were not considered in the model the authors used.
Children
Five of the six studies conducted in children were conducted at the same single hospital in South Korea. The generalizability of findings from these studies to the Canadian context is uncertain. These were small, studies (sample size ranging from n=28 to n=76) at high risk of bias due to concerns surrounding selection bias. There were also concerns surrounding heterogeneity in the studies conducted in children. The study population (e.g. severity and type of disease) and comparator intervention differed across studies making it challenging to compare results. As in adults, there was limited evidence on endoscopic findings and no evidence on surgery or hospitalization.
There were no cost-effectiveness data for children.
Conclusions and Implications for Decision or Policy Making
This report identified two RCTs (and one long-term follow-up study of one RCT) and four non-randomized studies examining the clinical effectiveness of early biologic therapy for Crohn’s disease in adults. There was one economic evaluation on the cost-effectiveness of early biologic therapy for Crohn’s disease in adults. The report also identified six non-randomized studies evaluating the clinical effectiveness of early biologic therapy for Crohn’s disease in children.
Adults
As discussed, the identified studies were heterogeneous in terms of both study populations and interventions, making it challenging to draw conclusions across studies. The results from the two eligible RCTs were conflicting. One study17 by Khanna et al. found that an early biologic treatment algorithm made no difference in clinical remission rates or symptom severity scores at one year compared to conventional treatment, while the other16 reported that early biologic therapy increased the rate of clinical remission compared to conventional treatment at one year. The RCT by D’Haens et al. reporting benefit on remission rates16 also reported improved symptom severity scores and disease-related quality of life at 10 weeks, but there was no difference thereafter. There were differences in the conduct of these two trials. The RCT demonstrating benefit in remission rates for early combined therapy was conducted in treatment-naïve persons with a two-week duration between diagnosis and treatment. All people received early combined therapy from baseline in this trial. In comparison, for the trial reporting no benefit on remission,17 people had longer standing disease (mean disease duration approximately 10 years), less severe disease, and over half were in remission at baseline. Further, as part of the early biologic treatment algorithm, there was a corticosteroid induction phase of 4 or 12 weeks, such that treatment biologics was delayed from baseline (further, not all participants in the intervention group actually received biologics).17 While the evidence on clinical remission rates and symptom severity scores is limited and conflicting, it is possible that the clinical benefit of early combined therapy may depend on the patient population and timing of initiation of therapy. This has also been suggested by a recent narrative review on the topic.8
The effect of early biologic therapy on Crohn’s-related surgery and complications was also conflicting and uncertain, though one RCT pointed to a possible benefit in favour of early biologic therapy. The rate of Crohn’s-related surgery and the rate of complications were both lower in the early combined intervention group in the RCT by Khanna et al.17 However, long-term follow-up of people from another RCT over 8 years18 found no difference in the proportion undergoing surgery, being hospitalized, or developing fistulas. There was conflicting evidence surrounding the effect of early biologics on rates of surgery in non-randomized studies.
There were limited data on endoscopic remission from two studies. One non-randomized study21 found that rates of endoscopic remission were higher with early biologic treatment, but the difference was not significant. This study also reported that the proportion of people with mucosal healing was higher with early biologic therapy at week 30, but not different at 2- or 3-years follow-up. However, this was a small (n=77), open-label study with concerns surrounding confounding. The proportion of participants with no ulcer was higher with early biologic treatment in the D’Haens et al. RCT.16 These results point to possible positive effects on endoscopic findings, but given the limitations in evidence the benefits are uncertain.
A large, long-term observational study found that infliximab in the context of early biologic therapy increased the risk of serious infections and hematological conditions, with no increase in malignancy or lymphoproliferative disorders, compared to people on conventional therapy (that did not include infliximab).20 The authors noted that these findings appear consistent with the known side effect profile of anti-TNF agents.
Overall, clinical evidence in adults was limited in that there were few studies, with concerns related to design, methods, and generalizability. There were concerns surrounding risk of bias in both RCTs related to allocation concealment and an open label design (and influence on outcome measurement). In the non-randomized studies, there was possible selection bias and confounding in many of the studies, reducing confidence in results. Another limitation is that most studies focused on clinical endpoints, which may be inappropriate, since it is suggested that the effectiveness of a treatment strategy should be based on its ability to modify natural course of the disease. The limitations of these studies should also be kept in mind when interpreting the results.
The results of one cost-effectiveness study in newly-diagnosed adult persons who were treatment naïve suggested that early biologic therapy was cost-saving and more effective compared to step-up therapy. This study suggests that early biologic therapy could be cost-effective. The study was conducted from the Italian healthcare system perspective; thus, the extent to which this analysis applies in Canada is unclear, particularly as drug cost had an important impact on cost-effectiveness estimates in sensitivity analyses.
Children
Evidence in children was limited, as noted above. Only non-randomized studies were identified, all of which had methodological concerns. Five of the studies had small sample sizes and were conducted at one hospital in South Korea, limiting the generalizability of the findings. Three studies28–30 reported that early biologic therapy increased rate of remission, while one study27 found no difference between early biologic therapy and conventional treatment. Three studies25,26,30 also found that the rate of relapse was lower with early biologic therapy compared to conventional treatment. There was conflicting evidence on disease activity scores. In one study, the endoscopic severity of disease was lower with early biologic therapy compared to step-up therapy at 14 weeks and 54 weeks.27 In this study, the proportion of participants with mucosal healing was higher with early biologic therapy at 54 weeks but not at 14 weeks. There was no evidence surrounding surgery or hospitalizations. Adverse event reporting was limited in all studies.
Conclusions
In conclusion, whether early biologic therapy is more effective than conventional therapy for Crohn’s disease in adults is unclear due to a limited number of studies, insufficient data on endoscopic remission, and heterogeneity of existing studies. Available evidence does not suggest a clear and consistent benefit for early biologic therapy across outcomes. Existing evidence points to possible benefits; however, future randomized controlled trials are likely required to clarify the clinical effectiveness of early biologic therapy (and also to clarify in which patient groups it is most appropriate).
Given the size and quality of the evidence base in children, no firm conclusions can be drawn about the comparative effectiveness of early biologic therapy in this group. Further research, preferably in the form of an RCT, is required to assess the effectiveness and safety of early biologic therapy compared to conventional therapy for Crohn’s disease in children.
The results of this report are consistent with a recent narrative review.8 The authors of the review also concluded that based on current evidence, early biologic treatment does not appear to have a clear benefit over conventional (step-up) therapy, though some studies suggest possible benefits. This review also noted that available evidence was limited (both in number and design of studies) and that the evidence base in children was particularly inconclusive.
References
- 1.
Baumgart
D, Sandborn
W. Crohn’s disease.
Lancet. 2012;380(9853):1590–1605. [
PubMed: 22914295]
- 2.
Kalla
R, Ventham
N, Satsangi
J, Arnott
I. Crohn’s disease.
BMJ. 2014;349. [
PubMed: 25409896]
- 3.
Feuerstein
J, Cheifetz
A. Crohn Disease: epidemiology, diagnosis, and management.
Mayo Clin Proc. 2017;92(7):1088–1103. [
PubMed: 28601423]
- 4.
- 5.
Molodecky
N, Soon
I, Rabi
D, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review.
Gastroenterol. 2012;142(1):46–54.e42; quiz e30. [
PubMed: 22001864]
- 6.
Benchimol
E, Fortinsky
K, Gozdyra
P, Van den Heuvel
M, Van Limbergen
J, Griffiths
A. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends.
Inflamm Bowel Dis. 2011;17(1):423–439. [
PubMed: 20564651]
- 7.
Rogler
G. Top-down or step-up treatment in Crohn’s disease?
Dig Dis. 2013;31(1):83–90. [
PubMed: 23797128]
- 8.
- 9.
Cozijnsen
MA, van Pieterson
M, Samsom
JN, Escher
JC, de Ridder
L. Top-down Infliximab Study in Kids with Crohn’s disease (TISKids): an international multicentre randomised controlled trial.
BMJ Open Gastroenterol. 2016;3(1):e000123. [
PMC free article: PMC5223648] [
PubMed: 28090335]
- 10.
Baert
F, Moortgat
L, Van Assche
G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease.
Gastroenterol. 2010;138(2):463–468; quiz e410-461. [
PubMed: 19818785]
- 11.
Colombel
JF, Sandborn
WJ, Reinisch
W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s Disease.
New England J Med. 2010;362:1383–1395. [
PubMed: 20393175]
- 12.
Higgins
J, Sterne
J, Savović
J, et al. RoB 2.0: a revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10(1).
- 13.
- 14.
- 15.
Liberati
A, Altman
DG, Tetzlaff
J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration.
J Clin Epidemiol. 2009;62(10):e1–e34. [
PubMed: 19631507]
- 16.
D’Haens
G, Baert
F, van Assche
G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial.
Lancet. 2008;371(9613):660–667. [
PubMed: 18295023]
- 17.
Khanna
R, Bressler
B, Levesque
BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial.
Lancet. 2015;386(10006):1825–1834. [
PubMed: 26342731]
- 18.
Hoekman
DR, Stibbe
JA, Baert
FJ, et al. Long-term Outcome of Early Combined Immunosuppression Versus Conventional Management in Newly Diagnosed Crohn’s Disease.
J Crohn Colitis. 2018;12(5):517–524. [
PubMed: 29401297]
- 19.
Ghazi
LJ, Patil
SA, Rustgi
A, Flasar
MH, Razeghi
S, Cross
RK. Step up versus early biologic therapy for Crohn’s disease in clinical practice.
Inflamm Bowel Dis. 2013;19(7):1397–1403. [
PubMed: 23598813]
- 20.
D’Haens
G, Reinisch
W, Colombel
JF, et al. Five-year Safety Data From ENCORE, a European Observational Safety Registry for Adults With Crohn’s Disease Treated With Infliximab [Remicade] or Conventional Therapy.
Journal of Crohn’s & colitis. 2017;11(6):680–689. [
PubMed: 28025307]
- 21.
Fan
R, Zhong
J, Wang
ZT, Li
SY, Zhou
J, Tang
YH. Evaluation of “top-down” treatment of early Crohn’s disease by double balloon enteroscopy.
World J Gastroenterol. 2014;20(39):14479–14487. [
PMC free article: PMC4202377] [
PubMed: 25339835]
- 22.
Rubin
D, Uluscu
O, Sederman
R. Response to biologic therapy in Crohn’s disease is improved with early treatment: an analysis of health claims data.
Inflamm Bowel Dis. 2012;18(12):2225–2231. [
PubMed: 22359399]
- 23.
Marchetti
M, Liberato
N, Di Sabatino
A, Corazza
G. Cost-effectiveness analysis of top-down versus step-up strategies in patients with newly diagnosed active luminal Crohn’s disease.
Eur J Health Economics. 2013;14(6):853–861. [
PubMed: 22975794]
- 24.
Kim
M, Lee
W, Choi
K, Choe
Y. Effect of infliximab top-down therapy on weight gain in pediatric Crohns disease.
Indian Ped. 2012;49(12):979–982. [
PubMed: 22728632]
- 25.
Lee
Y, Baek
S, Kim
M, Lee
Y, Lee
Y, Choe
Y. Efficacy of early infliximab treatment for pediatric Crohn’s Disease: a three-year follow-up.
Ped Gastroenterol Hepatol Nutr. 2012;15(4):243–249. [
PMC free article: PMC3746055] [
PubMed: 24010094]
- 26.
- 27.
Kang
B, Choi
S, Kim
H, Kim
K, Lee
Y, Choe
Y. Mucosal healing in paediatric patients with moderate-to-severe luminal Crohn’s Disease under combined immunosuppression: escalation versus early treatment.
J Crohn Colitis. 2016;10(11):1279–1286. [
PubMed: 27095752]
- 28.
Kim
M, Lee
J, Lee
J, Kim
J, Choe
Y. Infliximab therapy in children with Crohn’s disease: a one-year evaluation of efficacy comparing ‘top-down’ and ‘step-up’ strategies.
Acta Paediatr. 2011;100(3):451–455. [
PubMed: 20626362]
- 29.
Walters
TD, Kim
MO, Denson
LA, et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-alpha vs an immunomodulator in children with Crohn’s disease.
Gastroenterol. 2014;146(2):383–391. [
PubMed: 24162032]
- 30.
Lee
Y, Kang
B, Lee
Y, Kim
M, Choe
Y. Infliximab “top-down” strategy is superior to “step-up” in maintaining long-term remission in the treatment of pediatric Crohn Disease.
J Ped Gastroenterol Nutr. 2015;60(6):737–743. [
PubMed: 25564801]
- 31.
- 32.
Baert
F, Moortgat
L, Van Assche
G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease.
Gastroenterol. 2010;138(2):463–468; quiz e410-461. [
PubMed: 19818785]
- 33.
Best
WR, Becktel
JM, Singleton
JW, Kern
F, Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study.
Gastroenterol. 1976;70(3):439–444. [
PubMed: 1248701]
- 34.
- 35.
Guyatt
G, Mitchell
A, Irvine
EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease.
Gastroenterol. 1989;96(3):804–810. [
PubMed: 2644154]
- 36.
Hyams
J, Markowitz
J, Otley
A, et al. Evaluation of the pediatric crohn disease activity index: a prospective multicenter experience.
J Pediatr Gastroenterol Nutr. 2005;41(4):416–421. [
PubMed: 16205508]
- 37.
Irvine
EJ, Zhou
Q, Thompson
A. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial.
Am J Gastroenterol. 1996;91(8):1571–1578. [
PubMed: 8759664]
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Table 2Description of Scales
View in own window
| Scale | Scoring and definitions |
|---|
| Crohn’s Disease Activity Index (CDAI)33 | Measure of disease activity – includes patient reported information on bowel movements, abdominal pain, wellbeing, extra-intestinal symptoms, medication use, weight Score ranges from 0 to 600, remission < 150, 200 to 300 = moderate to severe disease, >300 = severe disease; clinical response is a fall of >70 points |
| Crohn’s Disease Endoscopic Index of Severity (CDEIS)34 | For determining severity of Crohn’s disease endoscopically Remission is categorized as <3, a score of 3 to 8 is mild endoscopic activity, a score of 9 to 12 is moderate endoscopic activity, and >12 severe activity |
| Harvey-Bradshaw Index (HBI)34 | Patient and physician completed questionnaire on general well-being, abdominal pain, bowel movements, and other symptoms Remission categorized as <5, mild disease 5 to 7, moderate disease 8 to 16, severe disease >16 |
| Inflammatory Bowel Disease Questionnaire (IBDQ)35 | Disease-related quality of life instrument with 32 questions Higher scores suggest improved quality of life (range from 32 to 224) |
| Pediatric Crohn’s Disease Activity Index (PCDAI)36 | Measure of disease activity incorporating symptoms, laboratory measurements, and physical findings Scores range from 0 to 100, higher scores represent more severe disease Remission categorized as PCDAI < 10, mild disease 11 to 30, moderate to severe disease >30, and a decrease of 12.5 points or more suggests an improvement in disease |
| Simple Endoscopic Score for Crohn’s Disease (SES-CD)34 | Assess mucosal ulcers, presence of stenosis Score of 0 to 2 suggest remission, 3 to 6 mild endoscopic activity, 7 to 15 moderate endoscopic activity, >15 severe activity (a decrease of 50% has been suggested as clinically significant) |
| Short Inflammatory Bowel Disease Questionnaire (SIBDQ)37 | Shortened 10-item version of IBDQ scored from 10 to 70 points where higher scores suggest improved quality of life |
Table 3Characteristics of Included Randomized Studies in Adults
View in own window
| First Author, Publication Year, Country | Study Design | Population Characteristics | Intervention and Comparator(s) | Clinical Outcomes, Length of Follow-Up |
|---|
| Khanna 2015; Belgium and Canada17 | Open-label cluster randomized controlled trial Gastroenterology practices were randomized 1:1 to use either an early combined immunotherapy algorithm or conventional treatment; a minimization procedure was used to balance by country and practice size; there were 21 early combined practices and 18 conventional treatment practices, around 87% were in Canada | n=1982, ~42% male ≥18 years old (mean age 44) Crohn’s disease regardless of disease activity or existing treatments (56% classified as being in steroid-free remission at baseline); 7% had active fistula Mean disease duration 149 months for early combined versus 158 for conventional | Early combined immunosuppression algorithm: if continuing disease activity (HBI >4) after steroid induction of 4 or 12 weeks, patients received combined anti-TNF and antimetabolite (choice of investigator); treatment could be modified every 12 weeks after reassessment Conventional management: usual practice | Primary outcome: mean proportion of patients in corticosteroid-free remission (HBI < 5) at month 12 Secondary outcomes: mean proportion of patients in remission at 6, 8 and 24 months; difference in mean HBI at 6, 8 and 24 months; time to first adverse outcome (surgery, complication or hospital admission); time to corticosteroids; adverse events, quality of life, mortality |
| D’Haens 2008; Belgium, Netherlands, Germany16 | Open-label randomized controlled trial Patients were randomized into two groups in blocks of four; a minimization procedure was used | n = 133, 62% female Age 18 to 75 (mean~30) diagnosed with Crohn’s in past 4 years (mean 2 weeks from diagnosis to treatment) and had clinically active disease; mean CDAI score was 330 in the combined immunosuppression group and 306 in the conventional therapy group Had not received biologics, corticosteroids or antimetabolites; patients with stenosis or strictures were excluded | Combined immunosuppression: infliximab 5 mg/kg at 0, 2 and 6 weeks in combination with AZA 2 to 2.5mg/kg per day (patients intolerant to AZA were given methotrexate 25 mg per week for 12 weeks then 15 mg per week); if responded, continued for rest of trial; if symptoms worsened additional infusions of infliximab were given Conventional: induction treatment with methylprednisolone (32 mg daily for 3 weeks then tapered over 4 weeks) or budesonide (9 mg per day for 8 weeks then tapering); if symptoms worsened the corticosteroid course was repeated at higher dose; if symptoms continued to worsen, AZA was added; if relapse patients received another course of corticosteroids; if symptomatic after 16 weeks with AZA, received infliximab | Patients were assessed at week 2, 6, 10, 14, 26 and then every 12 weeks until 104 weeks Primary outcome: remission defined as CDAI score of less than 150, absence of corticosteroids and no intestinal resection at 26 and 52 weeks Secondary outcomes: time to relapse (CDAI increase >50), treatment with corticosteroids, CDAI and IBDQ scores, mean endoscopic severity scores, proportion in remission at week 14 (CDAI and no corticosteroid); proportion without ulcers after 24 months, adverse events |
| Hoekman 2018 (follow-up from D’Haens 2008)18 | Retrospective review of patients included in D’Haens 2008 open-label randomized controlled trial; data were collected between 2003 and 2014 to allow for an 8-year follow-up from patients in the original trial | Data available for 119/133 patients | See D’Haens 2008 | Follow up over 8 years (split into 16 semesters) Primary outcome: proportion of patients in remission (not defined) Secondary outcomes: hospitalization, surgery, new fistula, rescue treatment, flares, disease activity |
Abbreviations: AZA = azathioprine; CDAI = Crohn’s disease activity index; HBI = Harvey-Bradshaw Index; IBDQ = inflammatory bowel disease questionnaire
Table 4Characteristics of Included Non-Randomized Studies in Adults
View in own window
| First Author, Publication Year, Country | Study Design | Population Characteristics | Intervention and Comparator(s) | Clinical Outcomes, Length of Follow-Up |
|---|
| D’Haens 2017, Austria, Belgium, France, Germany, Italy, Netherlands, Spain, Sweden, UK20 | Observational prospective registry study Patients from ENCORE registry, a prospective registry that enrolled from 2003 to 2008 and followed patients for 5 years; patients were eligible when Crohn’s disease treatment (e.g. corticosteroid, immunomodulator, anti-TNF) intensification was indicated; authors identified three groups of patients to compare | n = 2662, ~60% female, 95% Caucasian Age 16 to 86 (median age ~35) with active luminal or fistulizing Crohn’s and no previous exposure to anti-TNF treatment Mean time since diagnosis ~8.5 years; 28% had a fistula in conventional group, 43% in early infliximab group, 27% in switch to infliximab group | Infliximab within 30 days of enrolment visit Conventional therapy: treatment without anti-TNF agents Started with conventional therapy and switched to infliximab during follow up
| Adverse events as reported by the treating physician, assessed every 6 months according to 7 pre-specified categories Safety data were reported as: (1) frequency of all observed adverse events, (2) incidence per 1000 person-years, (3) multivariable analysis by treatment, (4) multivariable analysis accounting for latest infliximab dose relative to onset of adverse events Some analyses were done based on exposure versus non-exposure to infliximab (patients in the step-up group who received infliximab were combined with the early infliximab group); only analyses comparing conventional to early infliximab are reported |
| Fan 2014, China21 | Controlled before-after (prospective study where one group received “top-down” therapy [group 1] and other group [group 2] received corticosteroid then azathioprine in a non-randomized open-label manner) | n=77, 47% male ≥18 years old (median age at onset was 25 years), newly diagnosed with moderate to severe Crohn’s disease and naïve to treatment (median disease duration was 10 weeks) Baseline CDAI score between 220 and 450 (median CDAI 279) with ileal and colonic mucosal ulcerations; excluded if fibrostenotic, fistulizing or penetrating disease | (1) Induction with infliximab 5 mg/kg at 0, 2, 6, 14, 22 and 30 weeks plus azathioprine 1 to 2.5 mg/kg daily from week 6 onwards (titrated to maximum tolerated dose) (2) prednisone 1 mg/kg daily for 7 to 14 days and tapered over 6 to 12 weeks plus AZA 1 to 2.5 mg/kg daily from week 6 onwards (titrated to maximum tolerated dose) | Primary endpoint: deep remission at week 30, 54 and 102 (CDAI score < 150 plus complete absence of mucosal ulcerations observed at baseline) Secondary endpoints: time to clinical remission; clinical remission at week 2, 6, 14, 22, 30, 54, 102; change from baseline for CDEIS score at week 30 and 54; complete endoscopic remission (CDEIS <3); endoscopic remission (CDEIS<6) Adverse events |
| Ghazi 2013, USA19 | Retrospective chart review from the University of Baltimore Inflammatory Bowel Disease Program Charts were reviewed from 2004 to 2010 in patients who were anti-TNF naïve and patients were grouped into two groups: early biologic or step-up | n=93, 59% female Mean age at diagnosis was 28 and mean duration of disease was 11 years 48% of patients had penetrating disease Mean baseline HBI was 3.7 in the step up group and 6.3 in the early biologic group | Early biologic: initial treatment with infliximab 5 mg/kg at week 0, 2, 6 and maintenance every 8 weeks or adalimumab 160 mg at week 0, 80 mg at week 2, then 40 mg every 2 weeks or certolizumab 400 mg at week 0, 2, and 4 then every 4 weeks (the dose interval could be increased or decreased) with or without an immunosuppressant Step-up: immunosuppressant alone or starting an immunosuppressant before an anti-TNF | Response and remission rates (HBI<5), HBI, SIBDQ, steroid free-remission, number of hospitalizations and surgeries at 0, 3, 6 and 12 months after initiation, corticosteroid use |
| Rubin 2012, USA22 | Retrospective cohort study Patients were identified using from an administrative claim database (PharMetrics) between 2000 and 2009 using ICD-9 codes and drug claims; patients had to be continuously enrolled in the same health plan for 6 months prior to their first claim and remain enrolled 12 months after anti-TNF claims; 3 groups were created based on treatment | n=3750, 60% female Median age 40 11% had an anal fistula | Step-up: 5-ASA and/or corticosteroids and/or immunosuppressants prior to anti-TNF “IS-to-TNF”: immunosuppressants only prior to anti-TNF Early-TNF: initiation of anti-TNF within 30 days of first prescription for Crohn’s disease | Concomitant corticosteroid use, Crohn’s disease surgery At 6, 12, 18 and 24 months |
Abbreviations: ASA = aminosalicylicacid; AZA = azathioprine; CDAI = Crohn’s disease activity index; CDEIS = Crohn’s disease endoscopic index of severity; HBI = Harvey-Bradshaw Index; IS = immunosuppressant; SIBDQ = Short Inflammatory Bowel Disease Questionnaire; TNF = tumor necrosis factor
Table 5Characteristics of Included Non-Randomized Studies in Children
View in own window
| First Author, Publication Year, Country | Study Design | Population Characteristics | Intervention and Comparator(s) | Clinical Outcomes, Length of Follow-Up |
|---|
| Kang 2016, South Korea27 | Prospective observational study comparing two groups of patients who received infliximab: either as part of step-up or top-down Patients prospectively enrolled and could choose to receive step-up or top-down with their guardian after explanation of options Study was specifically interested in those who received infliximab (either early or as part of step-up) and therefore only included in step-up group if eventually received infliximab after failure of conventional therapy | n=76, median age at diagnosis 14 years for step-up and 15 year for top-down, 64% male, median PCDAI = 35, median duration to infliximab treatment 8 months in step-up and 0.7 months in top-down Pediatric patients (< 18 years of age) with moderate to severe luminal disease were included (excluded if mild disease, penetrating disease, strictures, refractory perianal strictures or history of bowel surgery) | Top-down: within 1 month from diagnosis, infliximab 5mg/kg at 0, 2, 6, 14 weeks then every 8 weeks plus AZA 0.5 to 1 mg/kg/day (adjusted as required) plus mesalazine 50 mg/kg/day Step-up: oral corticosteroid (not specified) 1 mg/kg/day tapered over 8 weeks plus AZA 0.5 to 1 mg/kg/day (adjusted as required) plus mesalazine 50 mg/kg/day; if disease relapsed then infliximab initiated (as in top-down group) | Primary outcome: mucosal healing (SES-CD score=0) at 14 and 54 weeks from baseline infliximab Secondary outcomes: clinical remission (PCDAI<10), PCDAI, adverse events, SES-CD score Baseline was first infliximab dose |
| Lee 2015, South Korea30 | Retrospective review of a prospective study Conducted at one hospital based on patients from 2008 to 2012; 2 groups depending on treatment received (therapy decisions made by guardians after explanation of treatment options) | n = 51, median age at diagnosis 15 years old, sex not described Patients had moderate to severe Crohn’s (PCDAI 39 in top-down and 34 in step-up at baseline) Disease duration 1 month in top-down versus 10.8 months in step-up 71% had fistula in top-down and 55% in step-up Patients in step-up initially achieved remission on conventional but eventually required infliximab | Top-down: patients who started infliximab at the time of diagnosis (in combination with AZA, mesalazine and corticosteroids) Step-up: patients who started infliximab after relapse (and where remission had been achieved with conventional therapy) Infliximab given 5 mg/kg week 0, 2, 6 then every 8 weeks | Disease remission (PCDAI < 10 points) Relapse free rate (relapse defined as PCDAI > 10 points after clinical remission), adverse events Follow-up over 3 years |
| Walters 2014, USA and Canada29 | Prospective observational study (data from 28 pediatric gastroenterology centres in North America from 2008 to 2012); propensity score matching to create three groups: (1) early anti-TNF, (2) early immunomodulator, (3) no early immunotherapy | n=552 (propensity score matched sample n=204); median age 13.8 years for anti-TNF, 12.6 years for immunomodulator, 12.0 years for no therapy; 62% male; 45% with moderate to severe Crohn’s disease Patients <17 years of age newly diagnosed with non-penetrating, non-stricturing disease were included; patients receiving early anti-TNFα in combination with an early immunomodulator were excluded | Three groups:
- (1)
early anti-TNF - (2)
early immunomodulator - (3)
no early immunotherapy
Early = within 3 months of diagnosis; all patients could receive corticosteroids or mesalamine | Primary outcome: corticosteroid free clinical remission (PCDAI < 10) at 1 year after diagnosis |
| Lee 2012, South Korea25 | Retrospective chart review Charts were reviewed at one hospital in patients followed for at least 36 months between 1998 and 2009; patients were put into 2 groups depending on treatment received | n = 28, 14% female Mean age 13 years Mean PCDAI score at diagnosis 40.5 Patients either had moderate or severe CD (top-down group) or had therapy-resistant CD (step-up group) | Top-down: infliximab 5 mg/kg at 0, 2, 6 weeks then every 8 weeks for 10 months in combination with AZA daily Step-up: oral prednisolone 1 to 2 mg/kg/day and mesalamine (50 to 80 mg/kg/day) or AZA (2 go 3 mg/kg/day), then infliximab | Relapse (PCDAI > 10 points after remission) Evaluated at 1, 2, and 3 years |
| Kim 2011, South Korea28 | Researchers identified patients given infliximab for therapy resistant or severe Crohn’s disease between 2001 and 2008; these patients were identified from two hospitals and had to have follow-up data for 1 year; patients grouped according to therapy received | n = 29, 17% female Mean age 14 Patients had therapy resistant (step-up group) or severe Crohn’s (top-down), mean PCDAI score 41; 62% of patients had a fistula | Top-down: infliximab 5 mg/kg at 0, 2, 6 weeks then every 8 weeks for 10 months in combination with AZA daily Step-up: oral prednisolone 1 to 2 mg/kg/day and mesalamine (50 to 80 mg/kg/day) or AZA (2 go 3 mg/kg/day), then infliximab | Disease remission (PCDAI score < 10), adverse events, clinical status (PCDAI score), fistula closure, adverse events, at 8 weeks and 1 year |
| Lee 2010, South Korea26 | Retrospective chart review Charts were reviewed at one hospital for newly diagnosed patients naïve to treatment and followed for at least 24 months from 2001 to 2007; patients were divided by 3 groups depending on which treatment they received | n = 36, 25% female Age 1 to 16 (median 13) Newly diagnosed with Crohn’s disease (mean PCDAI at diagnosis 33) | A: oral prednisolone (1 to 2 mg/kg/day) then mesalamine (50 to 80 mg/kg/day) B: oral prednisolone then AZA (2 to 3 mg/kg/day) C: infliximab 5 mg/kg at 0, 2, 6 weeks then every 8 week for 10 months, plus AZA | Relapse (PCDAI > 10 after remission) Adverse events Follow up at 12 and 24 months |
AZA = Azathioprine; CRP = C-reactive protein; PCDAI = Pediatric Crohn’s Disease Activity Index; SES-CD = Simple endoscopic score for Crohn’s disease; TNF = tumor necrosis factor
Table 6Characteristics of Included Economic Evaluations
View in own window
| First Author, Publication Year, Country | Type of Analysis, Time Horizon, Perspective | Decision Problem | Population Characteristics | Intervention and Comparator(s) | Approach | Clinical and Cost Data Used in Analysis | Main Assumptions |
|---|
Marchetti 201323 Italy | Cost-effectiveness analysis Time horizon = 5 years Perspective = Italian healthcare system (third party payer) | Evaluate if a top-down approach using infliximab is cost-effective compared to step-up therapy for newly diagnosed active luminal Crohn’s disease in the Italian context | Population based on D’Haens et al. (2008)16 n = 133 patients, age 16 to 75 (mean ~30 years), diagnosed with Crohn’s in previous 4 years and treatment naïve | Top-down: infliximab plus AZA, if relapse additional infliximab infusions and, if necessary, corticosteroids Step-up: corticosteroids, if relapse add AZA, if relapse again add infliximab | Trial-based | Clinical data: mild flare (requiring additional drug), severe flare requiring surgery, surgery-related mortality, mucosal healing at second year, severe infliximab adverse event Cost data: inpatient care for surgery, purchase cost of drug for hospital, cost of imaging Indirect costs were not considered | In patients requiring surgery, 20% would have early complications after surgery Average patient weight 60 kg (drug costs based on this) Purchase cost of 1 vial of infliximab = €512 Bowel resection surgery required by 6/65 receiving top-down and 8/64 receiving step-up Probability of mild flare (requiring additional drug treatment) = 8% per month for top-down and 12% per month for step-up |
Abbreviations: AZA = azathioprine
Appendix 3. Critical Appraisal of Included Publications
Table 7Critical Appraisal for Randomized Studies Using Cochrane Risk of Bias 2.0 Tool12
View in own window
| Item | Khanna 201515 | D’Haens 200814 | Hoekman 201816 (long-term follow-up from D’Haens 2008) |
|---|
| Risk of bias arising from randomization process | Some concerns | High | N/A |
| Deviations from intended interventions (effect of assignment to intervention) | Low | Low | N/A |
| Deviations from intended interventions (effect of adhering to intervention) | Some concerns | Some concerns | N/A |
| Missing outcome data | Some concerns | Low | Low |
| Measurement of the outcome | Some concerns | Some concerns | Low |
| Selection of reported results | Some concerns | High | High |
| Overall judgement | Some concerns | High | N/A |
Table 8Critical Appraisal for Non-Randomized Studies in Adults Appraised Using Downs and Black Checklist
View in own window
| Item | D’Haens 201718 | Fan 201419 | Ghazi 201317 | Rubin 201220 |
|---|
| Is the hypothesis/aim clearly described? | Yes | Yes | Yes | Yes |
| Are the main outcomes clearly described in the introduction or methods? | Yes | Yes | Yes | Yes |
| Are the characteristics of patients in the study clearly described? | Yes | Yes | Yes | No |
| Are the interventions of interest clearly described? | Yes | Yes | No | Yes |
| Are the distributions of principal confounders in each group of patients clearly described? | Yes | Partially | Yes | No |
| Are the main findings of the study clearly described? | Yes | Yes | Yes | No |
| Does the study provide estimates of the random variability in the data for the main outcomes? | Yes | No | No | Yes |
| Have all important adverse events that may be a consequence of the intervention been reported? | Yes | Yes | No | No |
| Have the characteristics of patients lost to follow-up been described? | Yes | No | N/A | N/A |
| Have actual probability values been reported for the main outcomes? | Yes | Yes | Yes | No |
| Were the subjects asked to participate in the study representative of the entire population from which they were recruited? | Yes | Unable to determine | Unable to determine | Unable to determine |
| Were those subjects who were prepared to participate representative of the entire population from which they were recruited? | Yes | Unable to determine | Unable to determine | Unable to determine |
| Were the staff, places, and facilities where the patients were treated representative of the treatment the majority of patients receive? | Yes | Unable to determine | Unable to determine | Unable to determine |
| Was an attempt made to blind study subjects to the intervention they have received? | No | No | No | No |
| Was an attempt made to blind those measuring the main outcomes of the intervention? | No | No | No | No |
| If any of the results of the study were based on data dredging, was this made clear? | Unable to determine | Unable to determine | Unable to determine | Unable to determine |
| In trials and cohort studies, do the analyses adjust for different lengths of follow-up of patients, or in case-control studies, is the time period between the intervention and outcome the same for cases and controls? | Yes | Yes | Yes | Yes |
| Were the statistical tests used to assess the main outcomes appropriate? | Yes | No | No | No |
| Was compliance with the intervention(s) reliable? | Unable to determine | Unable to determine | Unable to determine | Unable to determine |
| Were the main outcome measures used accurate (valid and reliable)? | Yes | Yes | Yes | Unable to determine |
| Were patients in different intervention groups recruited from the same population? | Yes | Yes | Yes | Yes |
| Were subjects in different intervention groups recruited over the same time period? | Yes | Yes | Yes | Yes |
| Were study subjects randomized to intervention groups? | No | No | No | No |
| Was the randomized intervention assignment concealed from patients and staff until recruitment was complete and irrevocable? | N/A | N/A | N/A | N/A |
| Was there adequate adjustment for confounding in the analyses from which main findings were drawn? | Yes | No | No | No |
| Were losses to follow-up taken into account? | Yes | Unable to determine | N/A | N/A |
Table 9Critical Appraisal for Non-Randomized Studies in Children appraised using Downs and Black Checklist
View in own window
| Item | Kang 201625 | Lee 201528 | Walters 201427 | Lee 201223 | Kim 201126 | Lee 201024 |
|---|
| Is the hypothesis/aim clearly described? | Yes | Yes | Yes | Yes | Yes | Yes |
| Are the main outcomes clearly described in the introduction or methods? | Yes | Yes | Yes | Yes | Yes | Yes |
| Are the characteristics of patients in the study clearly described? | Yes | Yes | Yes | Yes | Yes | Yes |
| Are the interventions of interest clearly described? | Yes | Yes | Yes | Yes | Yes | Yes |
| Are the distributions of principal confounders in each group of patients clearly described? | Yes | Yes | Yes | No | No | No |
| Are the main findings of the study clearly described? | Yes | Yes | Yes | Yes | Yes | Yes |
| Does the study provide estimates of the random variability in the data for the main outcomes? | No | Yes | Yes | No | No | Yes |
| Have all important adverse events that may be a consequence of the intervention been reported? | Yes | Yes | No | No | Yes | Yes |
| Have the characteristics of patients lost to follow-up been described? | Yes | Unable to determine | N/A | N/A | N/A | No |
| Have actual probability values been reported for the main outcomes? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the subjects asked to participate in the study representative of the entire population from which they were recruited? | Unable to determine | Unable to determine | Yes | Unable to determine | Unable to determine | Unable to determine |
| Were those subjects who were prepared to participate representative of the entire population from which they were recruited? | Unable to determine | Unable to determine | Unable to determine | Unable to determine | Unable to determine | Unable to determine |
| Were the staff, places, and facilities where the patients were treated representative of the treatment the majority of patients receive? | Unable to determine | Unable to determine | Yes | Unable to determine | Unable to determine | Unable to determine |
| Was an attempt made to blind study subjects to the intervention they have received? | No | No | No | No | No | No |
| Was an attempt made to blind those measuring the main outcomes of the intervention? | Unable to determine | No | No | No | No | No |
| If any of the results of the study were based on data dredging, was this made clear? | Unable to determine | Unable to determine | Unable to determine | Unable to determine | Unable to determine | Unable to determine |
| In trials and cohort studies, do the analyses adjust for different lengths of follow-up of patients, or in case-control studies, is the time period between the intervention and outcome the same for cases and controls? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the statistical tests used to assess the main outcomes appropriate? | No | Yes | Yes | No | No | No |
| Was compliance with the intervention(s) reliable? | Unable to determine | Unable to determine | Unable to determine | Unable to determine | Unable to determine | Unable to determine |
| Were the main outcome measures used accurate (valid and reliable)? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were patients in different intervention groups recruited from the same population? | No | Yes | Yes | No | No | Yes |
| Were subjects in different intervention groups recruited over the same time period? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were study subjects randomized to intervention groups? | No | No | No | No | No | No |
| Was the randomized intervention assignment concealed from patients and staff until recruitment was complete and irrevocable? | N/A | N/A | N/A | N/A | N/A | N/A |
| Was there adequate adjustment for confounding in the analyses from which main findings were drawn? | No | No | Yes | No | No | No |
| Were losses to follow-up taken into account? | Unable to determine | Unable to determine | Unable to determine | Unable to determine | Unable to determine | Unable to determine |
Table 10Strengths and Limitations of Economic Studies using the Drummond Checklist14
View in own window
| Item | Marchetii 201321 |
|---|
| The research question is stated | Yes |
| The economic importance of the research question is stated. | Yes |
| The viewpoint(s) of the analysis are clearly stated and justified. | No |
| The rationale for choosing alternative programs or interventions compared is stated. | Yes |
| The alternatives being compared are clearly described | Yes |
| The form of economic evaluation used is stated | Yes |
| The choice of form of economic evaluation is justified in relation to the questions addressed. | Yes |
| The source(s) of effectiveness estimates used are stated. | Yes |
| Details of the design and results of effectiveness study are given (if based on a single study). | Yes |
| The primary outcome measure(s) for the economic evaluation are clearly stated. | Yes |
| Details of the subjects from whom valuations were obtained were given. | No |
| Quantities of resource use are reported separately from their unit costs. | Yes |
| Methods for the estimation of quantities and unit costs are described. | Yes |
| Currency and price data are recorded. | Yes |
| Details of currency of price adjustments for inflation or currency conversion are given | No |
| Details of any model used are given. | Yes |
| The choice of model used and the key parameters on which it is based are justified. | Yes |
| Time horizon of costs and benefits is stated. | Yes |
| The discount rate(s) is stated. | Yes |
| The choice of discount rate(s) is justified. | Yes |
| Details of statistical tests and confidence intervals are given for stochastic data. | No |
| The approach to sensitivity analysis is given. | Yes |
| The choice of variables for sensitivity analysis is justified | Unclear |
| The ranges over which the variables are varied are justified. | Unclear |
| Relevant alternatives are compared. | Yes |
| Incremental analysis is reported. | Yes |
| Major outcomes are presented in a disaggregated as well as aggregated form. | Yes |
| The answer to the study question is given. | Yes |
| Conclusions follow from the data reported. | Yes |
| Conclusions are accompanied by the appropriate caveats | Yes |
Note: items which were not appropriate for the study were removed from the table
Appendix 4. Main Study Findings and Authors’ Conclusions
Table 11Summary of Findings of Included Randomized Controlled Trials in Adults
View in own window
| First author, year | Description | Findingsa | Author’s conclusions |
|---|
| Remission |
|---|
| Khanna 201517 | Primary outcome at 12 months was remission rate (adjusted for cluster size, practice size, country, baseline remission rate) | At 12 months, crude remission rate was 66.0% for early combined intervention versus 61.9% for conventional Adjusted difference was 2.5% (95% CI −5.2 to 10.2) At 24 months, crude remission rate was 73.1% for early combined intervention and 65.1% for conventional Adjusted difference was 6.4% (95% CI −0.9% to 13.6%) | Early combined intervention was not more effective than conventional management at controlling symptoms; there was a modest and non-significant benefit in clinical remission17 |
| D’Haens 200816 | Proportion of patients in remission at 26 and 52 weeks Secondary outcome: proportion of patients in remission at week 78 and 104 | 26 weeks: 60.0% of patients in early combined group in remission versus 35.9% in conventional treatment (absolute difference 24.1%, 95% CI 7.3 to 40.8) 52 weeks: 61.5% of patients in combined group versus 42.2% in conventional (absolute difference 19.4%, 95% CI 2.4 to 36.3) No significant difference in proportion in remission at week 78 and 104 (individual proportions not reported) | Early combined therapy was more effective at inducing remission and resulted in remission faster than conventional treatment16 |
| Hoekman 201818 | Proportion of semesters (6 months) in clinical remission per patient over 8 years in patients from D’Haens 2008 | Proportion of semesters in clinical remission was 73% for early combined and 70% for conventional (P = 0.85) | There was no difference in rate of remission long-term (authors also noted that the possible benefits of early combined immunosuppression may not alter long-term course of Crohn’s if regimen not maintained)18 |
| Relapse |
|---|
| D’Haens 200816 | Time to relapse after successful induction at week 14 | Median time to relapse longer in early combined group compared to conventional therapy (329 days versus 175 days, P = 0.031) | Time to relapse was longer in patients receiving early combined therapy16 |
| Hoekman 201818 | Time to flare over 8 year follow up from D’Haens 2008 Proportion who experienced at least one flare during 8-year follow-up | Median time to flare was shorter for step-up versus early combined (5 semesters versus 9 semesters, P = 0.02) Proportion who experienced at least one flare was higher in step-up versus early combined (78% versus 58%, P = 0.02) | Top-down treatment was associated with a lower relapse rate compared to conventional treatment18 |
| Disease severity score |
|---|
| Khanna 201517 | HBI score at 6, 18, 24 months | No difference between groups at any time point (individual mean differences not provided) | There was no difference in clinical symptoms between groups17 |
| D’Haens 200816 | CDAI at 10 weeks | CDAI: mean reduction was 231 points in early combined group versus 178 in conventional (mean difference 53.3, 95% CI 9.2 to 97.4) No differences were observed beyond 10 weeks (no data provided) | There was a more rapid drop in symptom scores up until week 10, then the scores in both groups were similar16 |
| Adverse outcome of Crohn’s disease |
|---|
| Khanna 201517 | Time to adverse outcome Adverse outcome defined as composite of surgery or hospital admission for Crohn’s disease, disease complication | Reduced rate of adverse outcomes in early combined intervention group compared to conventional therapy (HR 0.73%, 95% CI 0.62 to 0.86; NNT at 24 months 14) | There was a reduction in adverse outcomes for early combined therapy suggesting that it may alter the natural history of Crohn’s disease; this difference should be interpreted cautiously since it was a secondary endpoint17 |
| Corticosteroid use |
|---|
| Khanna 201517 | Time to treatment with corticosteroids | No difference between early combined intervention and conventional over 24 months (HR 0.97, 95% CI 0.77 to 1.21) | There was no difference in corticosteroid use17 |
| D’Haens 200816 | Exposure to methylprednisolone in 4-week windows over 104 weeks | Lower exposure to methylprednisolone in early combined group (no quantitative measure provided) | Patients with early combined therapy were exposed to substantially less corticosteroid than the conventional treatment group16 |
| Hoekman 201818 | Ever-treated with corticosteroid during 8-year follow-up from D’Haens 2008 Proportion of semesters in corticosteroid-free remission | 41% of early combined versus 62% of conventional were treated with corticosteroids (p=0.02) No difference in proportion of semesters in corticosteroid-free remission (69% in early combined versus 63% in conventional, P = 0.71) | Conventional treatment patients were treated with corticosteroids more frequently than early combined18 |
| Quality of life |
|---|
| Khanna 201517 | SF-36 physical and mental, at baseline, 6, 12, 18 and 24 months EQ-5D at baseline, 6, 12, 18 and 24 months | No difference at any time point for mental or physical quality of life No difference at any time point | There was no difference in quality of life between treatments17 |
| D’Haens 200816 | IBDQ scores at 10 weeks | Mean increase of 59.2 in early combined group versus 37.4 in conventional (mean difference 21.8, 95% CI 8.7 to 34.9) No difference between groups beyond 10 weeks | There was a more rapid improvement in quality of life scores for early combined therapy versus conventional16 |
| Mortality |
|---|
| Khanna 201517 | Mortality over 24 months | No difference between groups; for early combined intervention compared to conventional HR 0.62 (95% CI 0.24 to 1.63) | There was no difference in mortality17 |
| Surgery for Crohn’s disease |
|---|
| Hoekman 201818 | Proportion undergoing Crohn’s disease related surgery during follow-up (8 years) | 12% in early combined versus 20% in conventional (P = 0.32) | There was no difference in the rate of surgery18 |
| Khanna 201517 | Time to surgery | Reduced rate of surgery in early combined intervention group versus conventional therapy (HR 0.69, 95% CI 0.50 to 0.97; NNT at 24 months 35) | There was a reduction in need for surgery for early combined treatment17 |
| Hospitalization for Crohn’s disease |
|---|
| Hoekman 201818 | Proportion of Crohn’s disease-related hospitalizations during follow-up | Hospitalization occurred in 25% of early combined and 35% of conventional (P = 0.32) | There was no difference in the rate of hospitalization18 |
| Khanna 201517 | Time to hospitalization | No difference for early combined intervention compared to conventional (HR 0.84, 95% CI 0.65 to 1.08) | There was no difference in hospitalization rates between groups17 |
| Serious complication |
|---|
| Hoekman 2018 | Proportion with fistula during follow-up (8 years) | 10% of early combined versus 18% of conventional (P = 0.20) | There was no difference in the rate of new fistulas |
| Khanna 201517 | Time to serious complication; complication defined as new abscess, fistula or stricture, serious worsening of disease activity or extra-intestinal manifestations | Reduced rate of complications for early combined intervention compared to conventional treatment (HR 0.73, 95% CI 0.61 to 0.87; NNT at 24 months 16) | There was a reduction in serious complications with early combined treatment17 |
| Endoscopic findings |
|---|
| D’Haens 200816 | Proportion of patients with no ulcers at week 104 Endoscopy score at 104 weeks | No ulcers for 73.1% of patients in early combined versus 30.4% in conventional (P = 0.0028) Endoscopy scores 0.7 in early combined versus 3.1 in conventional (P < 0.001) | “Patients with early combined therapy were less likely to have ulcerations after 2 years of treatment”16 |
| Hoekman 201818 | Proportion with absence of ulcers over follow up (8 years) | Absence of ulcers in 44% of early combined versus 33% of conventional therapy (P = 0.55) | There was no difference in absence of ulcers18 |
| Safety |
|---|
| Khanna 201517 | Proportion of patients with serious drug-related adverse events | 10/1084 for early combined versus 10/898 for conventional | The proportion of serious drug related adverse events was not different between groups17 |
| D’Haens 200816 | Proportion of patients with adverse events | 30.8% in early combined versus 25.3% in conventional management group | There were no important differences in adverse effects but authors noted that study was inadequate to address safety differences16 |
| Hoekman 201818 | Proportion of patients with adverse events (over 8 years) | Infusion reactions occurred in 14% of early combined patients and 10% of conventional patients; serious infections occurred in 22% of early combined patients and 10% of conventional therapy | Was not commented on specifically18 |
Abbreviations: CDAI = Crohn’s Disease Activity Index; CI = confidence interval; EQ-5D = EuroQoL 5-dimension; HBI = Harvey Bradshaw Index; HR = hazard ratio; IBDQ = Inflammatory Bowel Disease Questionnaire; NNT = number needed to treat; SF-36 = Short Form 36-item Health Survey
- a
Confidence intervals are provided where available but were not provided in all studies
Table 12Summary of Findings of Included Non-Randomized Studies in Adults
View in own window
| First author, year | Description | Findingsa | Author’s conclusions |
|---|
| Remission |
|---|
| Fan 201421 | Proportion in deep remission at week 30 and 54 Median time to clinical remission Proportion in clinical remission at week 30 and 54 | Week 30: proportion in deep remission for top-down 44.7% versus 17.9% in step-up (P = 0.011) Week 54: proportion with deep remission for top-down 52.6% in top-down versus 35.9% in step-up (P = 0.13) Median time to clinical remission was 6.8 weeks for top-down versus 14.2 for step-up (P = 0.009) Clinical remission at 30 weeks: 68.4% in top-down versus 43.6% in step-up (p=0.028) Clinical remission at 54 weeks: 71.1% in top-down versus 56.4% in step-up (P = 0.182) | There were significantly higher rates of clinical remission at week 30 and a higher proportion in deep remission at week 54 and 102, though the differences were not significant Induction therapy with infliximab provided important benefits compared to induction with corticosteroids in patients with early Crohn’s disease21 |
| Disease activity score |
|---|
| Ghazi 201319 | Mean difference in HBI from baseline at 3, 6 and 12 months Mean score at 3, 6, and 12 months | 3 months: mean difference from baseline −3.6 (SD 4.5) in early biologic group versus −1.3 (SD 2.7) for step-up (P = 0.01) 6 months: mean difference from baseline −2.6 (SD 4.3) in early biologic group versus −1.0 (SD 2.7) for step-up (P = 0.08) 12 months: mean difference from baseline −3.4 (SD 4.9) in early biologic group versus −1.3 (SD 2.7) for step-up (P = 0.07) Mean scores at 3, 6 and 12 months were no different across groups but early biologic group had more severe disease (HBI 6.3 versus 3.7 in step-up) at baseline | There was no difference in disease activity between groups When comparing to score at 3, 6, 9 months to baseline score, patients in early biologic therapy group experienced greater improvements in disease activity from baseline compared to step-up group19 |
| Safety |
|---|
D’Haens 201720 Some analyses in this study were based on exposure versus non-exposure to infliximab (patients in the step-up group who received infliximab were combined with the early infliximab group); only analyses comparing conventional to early infliximab are reported | Proportion of patients with adverse events over 5 year follow up | Greater percentage for early infliximab versus switch or conventional (84.4% versus 75.8% versus 64.6%) Proportion (%) for early versus switch versus conventional: Serious infection 8.6 vs. 6.0 vs. 4.2 Infusion reaction 11.2 vs. 9.4 vs. 0.1 Hemotological condition 3.2 vs. 2.3 vs. 1.0 Congestive heart failure 0.1 vs. 0 vs. 0.1 Demyelinating neurological disorder 0.3 vs. 0 vs. 0.1 Lymphoproliferative conditions and malignancy 3.2 vs. 2.7 vs 1.9 Fatality 1.9 vs. 1.3 vs. 1.2 | Infliximab exposure was associated with an increased risk of serious infections and hematological conditions with no increased risk of malignancy or lymphoproliferative disorders “The data confirm the risk profile of infliximab in Crohn’s disease”20 (page 689) |
| Risk of serious infection for exposure to infliximab versus conventional therapy using Cox-proportional hazard model | Infliximab associated with higher risk of serious infection compared to conventional treatment (HR 1.64, 95% CI 1.17 to 2.31) | Infliximab was associated with increased risk of serious infection compared to conventional treatment20 |
| Risk of hematological condition for infliximab versus conventional therapy using Cox-proportional hazard model | Infliximab associated with higher risk of hematological condition compared to conventional treatment (HR 2.91, 95% CI 1.51 to 5.59) | Infliximab was associated with increased risk of hematological conditions compared to conventional treatment20 |
| Risk of lymphoproliferative conditions and malignancy for infliximab versus conventional therapy using Cox-proportional hazard model | Infliximab associated with no increased risk of lymphoproliferative conditions and malignancy compared to conventional treatment (HR 1.44, 95% CI 0.86 to 2.42) | There was no difference in lymphoproliferative conditions and malignancy compared to conventional treatment20 |
| Risk of fatality for infliximab versus conventional therapy using Cox-proportional hazard model | Early infliximab associated with no difference in fatality compared to conventional treatment (HR 1.22, 95% CI 0.63 to 2.36) | There was no difference in fatality for infliximab compared to conventional treatment20 |
| Fan 201421 | Proportion with adverse events | 89.5% of patients in top-down experienced an adverse event versus 84.6% in step-up group Infusion reactions occurred in 13.2% on top-down versus 0% on step-up Adverse events leading to discontinuation occurred in 13.2% on top-down and 10.3% for step-up; no serious infections in either group | No conclusions made21 |
| Endoscopic remission and mucosal healing |
|---|
| Fan 201421 | Proportion with mucosal healing at week 30, 54, 102 | Week 30: 44.7% in top-down versus 17.9% in step-up (P = 0.011) Week 54: 52.6% in top-down versus 35.9% in step-up (P = 0.139) Week 102: 57.9% in top-down versus 38.5% in step-up (P = 0.088) | There was a higher mucosal healing rate at week 30 in the top-down group21 |
| Fan 201421 | Proportion with complete endoscopic remission (CDEIS < 3) at week 30 and 54 | Week 30: 36.8% for top-down versus 20.5% for step-up (P = 0.113) Week 54: 47.4% for top-down versus 46.2% for step-up (P = 0.915) | Proportion with endoscopic response was higher for top-down versus step-up at week 30 but not at week 5421 |
| Proportion with endoscopic remission (CDEIS < 6) at week 30 and 54 | Week 30: 78.9% for top-down versus 48.7% for step-up (P = 0.006) Week 54: 81.6% for top-down versus 74.4% for step-up (P = 0.915) |
| Quality of life |
|---|
| Ghazi 201319 | Mean difference in SIBDQ from baseline at 3, 6, 12 months Mean score at 3, 6, and 12 months Higher scores suggest improved quality of life (ranges from 10 to 70) | 3 months: mean difference from baseline 8.0 (SD 13) in early biologic group versus 4.0 (SD 8) for step-up (P = 0.11) 6 months: mean difference from baseline 9.6 (SD 14) in early biologic group versus 3.3 (SD 8) for step-up (P = 0.002) 12 months: mean difference from baseline 9.9 (SD 13) in early biologic group versus 4.8 (SD 10) for step-up (P = 0.09) Mean scores at 3, 6 and 12 months were no different across groups but early biologic group had lower quality of life (SIBDQ 43 versus 52 in step-up) at baseline | There was no difference in quality of life scores, but comparing to baseline: patients in early biologic therapy group experienced greater improvements in quality of life compared to step-up group19 |
| Corticosteroid use |
|---|
| Ghazi 201319 | Proportion of patients on corticosteroids at 3, 6, 12 months | 3 months: 30% in early biologic versus 53% in step-up (P = 0.03) 6 months: 18% in early biologic versus 18% in step-up (P = 0.93) 12 months: 15% in early biologic versus 16% in step-up (P = 0.92) | There was an overall high rate of steroid withdrawal at 1 year19 |
| Rubin 201222 | Relative risk of corticosteroid use for step-up compared to early anti-TNF at 6, 12, 18, 24 months | 6 months: RR 1.85 (95% CI 1.64 to 2.10) 12 months: RR 1.91 (95% CI 1.66 to 2.20) 18 months: RR 1.99 (95% CI 1.67 to 2.37) 24 months: RR 1.79 (95% CI 1.47 to 2.18) | Early anti-TNF treatment reduced rates of corticosteroid use compared to step-up treatment22 |
| Surgery |
|---|
| Ghazi 201319 | Reported as “Number of surgeries” at 1 year (unclear what measurement is reported) | 0.50 (SD 0.8) in early biologic group compared to 0.28 (SD 0.8) surgeries in step-up (p=not significant) | There was no difference in the number of surgeries between groups19 |
| Rubin 201222 | Relative risk of Crohn’s disease-related surgery for step-up group compared to early anti-TNF at 6, 12, 18, 24 months | 6 months: RR 1.33 (95% CI 0.93 to 1.90) 12 months: RR 1.44 (95% CI 1.08 to 1.92) 18 months: RR 1.50 (95% CI 1.16 to 1.93) 24 months: RR 1.43 (95% CI 1.12 to 1.81) | Patients receiving early anti-TNF treatment had fewer surgeries compared to those on step-up treatment22 |
| Hospitalization |
|---|
| Ghazi 201319 | Reported as “Number of hospitalizations” at 1 year (unclear what measurement is reported) | 0.81 (SD 1.12) in early biologic group compared to 0.38 (SD 0.8) in step-up group (P = 0.04) | There was a higher need for hospitalization in the early biologic group19 |
Abbreviations: AZA = Azathioprine; CDAI = Crohn’s Disease Activity Index; CDEIS = Crohn’s Disease Endoscopic Index of Severity; CI = confidence interval; HBI = Harvey Bradshaw Index; HR = hazard ratio; RR = relative risk; SD = standard deviation; TNF = tumor necrosis factor
- a
Confidence intervals are provided where available but were not provided in all studies
Table 13Summary of Findings of Included Non-Randomized Studies in Children
View in own window
| First author, year | Description | Findingsa | Author’s conclusions |
|---|
| Remission |
|---|
| Kang 201627 | Proportion in clinical remission at week 14 and week 54 from baseline infliximab | Week 14: 91% in early combined versus 80% in step-up (P = 0.26) Week 54: 89% in early combined versus 79% in step-up (P = 0.29) | There was no statistically significant difference in remission rate between groups27 |
| Lee 201530 | Proportion in clinical remission at 1 year | 84% of patients in top-down versus 40% in step-up (P = 0.0006) | ‘‘Top-down strategy had a longer remission period compared with the ‘‘step-up’’ strategy in pediatric Crohn disease during a study period of 3 years, based on relapse-free rate and remission period rate. Earlier introduction of infliximab is recommended in pediatric patients with moderate to severe Crohn disease.”30 (page 742) |
| Walters 201429 | Corticosteroid-free clinical remission rate at 1 year | Remission rate was 85% with early anti-TNF versus 60% with early immunosuppressant and 54% with no early immunosuppressant (P = 0.0003) Relative risk of being in remission was 1.41 (95% CI 1.14 to 1.76) for anti-TNF compared to immunosuppressant only Relative risk of being in remission was 1.57 (95% CI 1.23 to 1.99) for anti-TNF compared to no early therapy | Early anti-TNF therapy was associated with improved clinical outcomes during the first year compared to early immunosuppressant in patients newly diagnosed with comparably severe Crohn’s disease29 |
| Kim 201128 | Remission rate at 8 weeks and 1 year | 8 weeks: 16/18 (89%) in top-down versus 3/11 (27%) in step-up (P = 0.001) 1 year: 15/18 (83%) in top-down versus 5/11 (45%) in step-up (P = 0.048) | “In paediatric patients with Crohn’s disease, the infliximab ‘top-down’ strategy resulted in superior outcomes when compared to the ‘step-up’ strategy for inducing and maintaining remission at 8 weeks and 1 year posttreatment.”28 (page 454) |
| Relapse |
|---|
| Lee 201530 | Relapse free rate at 1, 2, 3 years | 1 year: 83.9% (95% CI 65.5 to 93.0) in top-down versus 45.0% (95% CI 23.1 to 64.7) in step-up 2 years: 58.1% (95% CI 39.0 to 73.1) in top-down versus 20.0% (95% CI 6.2 to 39.3) in step-up 3 years: 35.5% (95% CI 19.4 to 51.9) in top-down versus 15.0% (95% CI 3.7 to 33.5) in step-up Difference in rate between groups estimated using Kaplan Meier method, P = 0.0094 | ‘‘Top-down strategy had a longer remission period compared with the ‘‘step-up’’ strategy in pediatric Crohn disease during a study period of 3 years, based on relapse-free rate and remission period rate. Earlier introduction of infliximab is recommended in pediatric patients with moderate to severe Crohn disease.”30 (page 742) |
| Lee 201225 | Proportion of patients who relapse at 1, 2, 3 years | 1 year: 16.7% in top-down group versus 50% in step-up (absolute difference 33.3%, P = 0.091) 2 years: 50% in top-down versus 90% in step-up (absolute difference 40%, P = 0.048) 3 years: 61.1% in top-down versus 90% in step-up (absolute difference: 29.9%, P = 0.194) | Early use of infliximab was more effective than step-up therapy for treatment of moderate to severe Crohn’s disease over 3 years25 |
| Lee 201026 | Proportion who relapse at 1 year and 2 years Group C: early biologic + AZA Group B: AZA Group A: mesalamine | 1 year: 23.1% (95% CI 0.2 to 46) in group C versus 61.5% (95% CI 35.1 to 88.0) in group B versus 80.0% (95% CI 55.2 to 100%) in group A Difference between groups C to A = 56.9% (95% CI 23.2 to 90.7) and difference between group C and B = 38.5% (95% CI 3.5 to 73.4) 2 years: 38.5% (95% CI 12.0 to 64.9) in group C versus 76.9% (95% CI 54.0 to 99.8) in group B and 90% (95% CI 71.4 to 100) in group A Difference between C and A = 51.5% (95% CI 19.2 to 83.9) and between C and B = 38.5% (95% CI 3.5 to 73.4) | “Induction with infliximab and AZA was effective for reducing relapse rate compared to conventional therapies for at least 2 years”26 (page 1780) |
| Disease activity score |
|---|
| Kang 201627 | Median (range) PCDAI at week 14 and week 54 from baseline infliximab | Week 14: 5 (0 to 15) in early combined versus 5 (0 to 15) in step-up (P = 0.52) Week 54: 0 (0 to 22.5) in early combined group versus 3.8 (0 to 35) in step-up (P = 0.049) | There were no statistically significant differences between groups27 |
| Kim 201128 | PCDAI score between groups at 8 weeks and 1 year | 8 weeks: Mean PCDAI was 3.8 (SD 3.4) in top-down group versus 14.3 (SD 11.7) in step-up (P = 0.002) 1 year: 1.0 (SD 0.9) in top-down versus 12.7 (SD 10.1) in step-up (P <0.001) | The improvement in PCDAI score at both 8 weeks and 1 year was superior for top-down compared to step-up therapy28 |
| Fistula closure |
|---|
| Kim 201128 | Complete fistula closure at 8 weeks and 1 year (in patients who had fistulas at baseline) | 8 weeks: Closure in 58.3% of top-down group compared to 16.7% in step-up (p=0.029) 1 year: Closure in 100% of top-down compared to 50% of step-up (P = 0.015) | Top-down therapy was superior in terms of fistula presence compared to step-up therapy28 |
| Safety (adverse events) |
|---|
| Kang 201627 | Adverse events during study period | No difference in adverse events between groups (p=0.804) (no further data provided) | There was no significant difference between groups in terms of adverse effects27 |
| Lee 201530 | Adverse events during study period | Leukopenia in 6 patients in top-down group versus 3 patients in step-up group Nausea and vomiting in 4 patients in top-down versus 1 patient in step-up Pancreatitis in 1 patient in top-down versus 2 patients in step-up No difference in adverse events between groups (p=0.97) | Was not commented on |
| Lee 201026 | Proportion with adverse events during study period Group C: early biologic + AZA Group B: AZA Group A: mesalamine | 1 (8.3%) adverse event in group C (dyspnea and tachycardia) 5 (38.5%) adverse events in group B (anorexia in 1 patient, pancytopenia in 3 patients, pancreatitis in 1 patient) 3 (30%) adverse events in group A (anorexia in 2 patients in pancreatitis in 1 patient) | There were fewer adverse events in group C compared to group A and B26 |
| Kim 201128 | Proportion with adverse events | 5/11 (45%) in top-down versus 4/11 (36%) patients in step-up Broken down by medication: Mesalamine: anorexia in 2/6 in step-up (none in top-down); pancreatitis in 1/11 in top-down (none in step-up) AZA: Leukopenia in 2/18 in top-down group versus 1/11 in step-up; anorexia in 1/18 in top-down versus 0/11 in step-up; pancreatitis in 1/18 top-down versus 0/18 in step-up Infliximab: dyspnea in 0/18 in top-down versus 1/11 in step-up | Was not commented on specifically |
| Mucosal healing |
|---|
| Kang 201627 | Proportion with mucosal healing at week 14 and 54 from baseline infliximab | Week 14: 51% in early combined group versus 32% in step-up (P = 0.121) Week 54: 74% in early combined group versus 42% in step-up (P = 0.007) | Mucosal healing rates were higher in patients treated with early combined therapy27 |
| Endoscopic findings |
|---|
| Kang 201627 | Median (range) SES-CD at week 14 and week 54 from baseline infliximab | Week 14: 2 (0 to 18) in early combined versus 4 (0 to 21) in step-up (P = 0.012) Week 54: 0 (0 to 19) in early combined versus 3 (0 to 22) in step-up (P = 0.03) | Was not commented on specifically27 |
Abbreviations: AZA = Azathioprine; CI = confidence interval; PCDAI = Pediatric Crohn’s Disease Activity Index; SES-CD = simple endoscopic score for Crohn’s disease; SD = standard deviation; TNF = tumor necrosis factor
- a
Confidence intervals are provided where available but were not provided in all studies
Table 14Summary of Findings of Included Economic Evaluations
View in own window
| Main Study Findings | Authors’ Conclusion |
|---|
| Marchetti 201323 |
|---|
Top-down therapy improved quality-adjusted life expectancy from 3.76 to 3.90 QALYs with a cost savings of €773 compared to step-up therapy Top-down therapy was more effective than step-up in 99% of simulations and dominant in 66% 84% of replicates were below a threshold of €20,000/QALY Sensitivity analyses showed that time horizon impacted ICUR: €92,440/QALY at year 1, €26,878/QALY at year 2, €9,983 at year 3, €1,462 at year 4 and dominant at year 5 | Top-down therapy was cost-saving and more effective than step-up therapy in treatment naïve patients, likely due to a lower percentage of relapse and less hospitalization “Our analysis showed that a strategy involving the early use of infliximab in the treatment of luminal Crohn’s disease patients with moderate or severe disease is highly cost-effective”23 (page 860) |
Abbreviations: ICUR = incremental cost-utility ratio; QALY = quality-adjusted life-year
Tables
Table 1Selection Criteria
View in own window
| Population | Adult and pediatric patients with Crohn’s disease (regardless of activity or severity) |
|---|
| Intervention | Biologics: adalimumab, infliximab, vedolizumab, ustekinumab given in the context of an early initiation algorithm May be given in combination with immunosuppressants (azathioprine, 6-mercaptopurine, methotrexate, cyclosporine) |
|---|
| Comparator | Conventional management sequence (“step-up”) typically consisting of giving steroids, then switching to or adding immunosuppressants when remitting or not responding, and then switching to or adding biologics if not responding to previous drugs. |
|---|
| Outcomes | Q1. Clinical effectiveness based on:
Commonly accepted disease activity scales such as CDAI or the Harvey–Bradshaw Index, clinical response rate, clinical remission, steroid-free remission, endoscopic remission (mucosal healing), corticosteroid use, need for surgery, hospitalization, mortality, quality of life, safety outcomes (harms including infections and malignancies, discontinuation)
Q2. Cost-effectiveness |
|---|
| Study Designs | HTA, systematic reviews/meta-analyses, RCTs, non-randomized studies, economic evaluations |
|---|
Abbreviations: CDAI = Crohn’s disease activity index; HTA = health technology assessment; RCT = randomized controlled trial
About the Series
CADTH Rapid Response Report: Peer-Reviewed Summary With Critical Appraisal
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Suggested citation:
Early biologic treatment versus conventional treatment for the management of Crohn’s Disease: A review of comparative clinical effectiveness and cost-effectiveness. Ottawa: CADTH; 2019 Feb. (CADTH rapid response report: peer-reviewed summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.