Background
Pancreatic Adenocarcinoma
Pancreatic cancer is the fourth most common cause of cancer death among men and women in the United States.1,2 In 2013 in the United States, about 46,000 people received a diagnosis of pancreatic cancer and 40,000 died of the disease.3 The median age at diagnosis is 71 years, the overall 5-year survival is 5.8 percent, and the overall age-adjusted mortality rate is 10.8 per 100,000 people per year.4,5 The most common type of pancreatic cancer is adenocarcinoma (approximately 90% of all pancreatic malignancies).2 Based on rates from 2007 to 2009, the lifetime risk of receiving a diagnosis of pancreatic cancer is 1.47 percent.5
Risk factors for pancreatic cancer include tobacco use; personal history of chronic pancreatitis, diabetes, obesity; and a family history of pancreatic cancer.1 About 10 percent of patients with pancreatic cancer have a positive family history for the disease.4 Pancreatic cancer is also a disease of aging and median age at diagnosis is 71.
Pancreatic cancer incidence rates were reportedly highest among African-American men (21.3 per 100,000) and women (17.6 per 100,000) during 2004 and 2008.1 The second highest rates were reported for Caucasian men (16.8 per 100,000) and women (12.8 per 100,000).1 The differences between these populations and burden of disease may be related to higher rates of cigarette smoking and diabetes mellitus among African American men than for Caucasian men and higher body mass indices among African American women than for Caucasian women.1
Diagnosis and Staging
Patients often remain asymptomatic or have only nonspecific symptoms such as malaise, fatigue, and loss of appetite until relatively late in the course of the disease, often with extensive spread, when weight loss, jaundice, and severe abdominal pain often appear. Due to late diagnosis, approximately 80 percent to 85 percent of cases are unresectable (i.e., too advanced to permit surgical resection),6 and the median survival of patients with unresectable tumors is only 6–10 months.7
Common symptoms leading to suspicion of pancreatic cancer are jaundice, epigastric pain, and weight loss;8 however, these symptoms are not specific. For example, in one study of 70 patients suspected of having pancreatic cancer, only 30 actually had pancreatic cancer; of the other 40, 16 had irritable bowel syndrome, 9 had other intra-abdominal cancers, 8 had pancreatitis, and 7 had other conditions.9 Thus, additional clinical information, including imaging tests, laboratory values, and biopsies, are important to differentiate these conditions from pancreatic cancer.
For the patient, given the poor prognosis of most cases, the differences in modalities and the consequences of their use are important to understand. Currently, there are no widely-accepted clinical practice guidelines that address which imaging modalities to use in the diagnosis and staging of pancreatic cancer. Also, elucidating patients’ experience and tolerance of various imaging modalities may help future patients weigh the benefits and harms of the tests and allow them to incorporate their values and priorities. Once pancreatic adenocarcinoma is diagnosed, the stage of disease is a key determinant of clinical management, as well as a key predictor of survival. Most cases are diagnosed at an advanced stage, precluding surgical resection.1 For localized disease, the 5-year survival is approximately 22 percent.1 When pancreatic adenocarcinoma is diagnosed at an advanced stage, the 5-year survival is approximately 2 percent.1
The most commonly used system for staging pancreatic adenocarcinoma is the 2010 American Joint Committee on Cancer (AJCC) system:10
- Stage 0: carcinoma in situ, with neither lymph node involvement nor metastasis
- Stage IA: a ≤2 cm tumor limited to the pancreas, with neither lymph node involvement nor metastasis
- Stage IB: a >2 cm tumor limited to the pancreas, with neither lymph node involvement nor metastasis
- Stage IIA: any size tumor that extends beyond the pancreas but does not involve either the celiac axis or the superior mesenteric artery (SMA), and with neither lymph node involvement nor metastasis
- Stage IIB: the same as IIA, except the lymph nodes are involved
- Stage III: any size tumor that involves the celiac axis or SMA, any lymph node status, and no metastases
- Stage IV: any size tumor and any lymph node involvement, and metastasis
An exact staging process before surgery (i.e., assigning the patient to stage I/II/III/IV) for pancreatic adenocarcinoma might not be performed, and the disease is often deemed resectable or unresectable intraoperatively. For unresectable cases, however, a biopsy is taken and a formal stage is determined to guide the planning of treatment modalities such as chemotherapy and potentially radiation.
Resectability
Surgical resection offers the only hope of cure and is decided via multidisciplinary consultation (e.g., surgeon, gastroenterologist, radiologist, oncologist, radiation oncologist). The two key factors in assessment of resectability are distant metastasis (which usually indicates unresectability) and blood vessel involvement (which sometimes indicates unresectability, depending on the degree of involvement). The major blood vessels of focus are the superior mesenteric vein (SMV), portal vein, celiac artery, common hepatic artery, and SMA. According to the 2012 guideline from the National Comprehensive Cancer Network (NCCN) on pancreatic adenocarcinoma:11
- A resectable tumor shows no involvement of either the SMV or portal vein and shows “clear fat planes” around the celiac axis, hepatic artery, and SMA, and there are no distant metastases.
- An unresectable tumor has >180 degrees SMA encasement or any celiac abutment, or an unreconstructible SMV/portal vein occlusion, or any aortic invasion/encasement, or any distant metastases.
- A “borderline” resectable tumor fits neither of the above two categories (e.g., some abutment of SMV/portal vein, <180 SMA abutment). For these cases, NCCN recommends biopsy and possible neoadjuvant chemotherapy, which may shrink the tumor and permit subsequent resection.
These criteria continue to evolve, as surgical techniques advance and more tumors are resectable via reconstruction of blood vessels.12
Regarding the interface between stage and resectability, AJCC and others state that stages I and II are resectable, but stages III and IV are not.10,13 However, others believe that minor arterial involvement (stage III) may still permit resection.12,14 Vincent et al. (2011)14 argued that some stage III cases are borderline resectable and may be appropriate targets for neoadjuvant therapy followed by consideration of resection.
In judging resectability, multidisciplinary teams may perform optimally if the radiology reports present all of the key information in a standardized format. Such a format, specific to staging for pancreatic adenocarcinoma, was proposed in a 2014 consensus statement from the Society of Abdominal Radiology and the American Pancreatic Association.15 This statement provides a comprehensive list of the critical imaging findings, using common terminology that should be summarized by radiologists.
Screening
Screening for pancreatic adenocarcinoma is not recommended for the general population (e.g., the U.S. Preventive Services Task Force gives a D recommendation).16 However, some recommend screening those who are at high risk of developing pancreatic cancer. One report17 suggested that having two or more first-degree relatives with pancreatic cancer is sufficient justification for considering a screening test (or 3 or more blood relatives, one of whom is a first-degree relative). Further, some genetic risk factors (e.g., Peutz-Jeghers syndrome; BRCA2, PALB2, p16 gene mutations; Lynch syndrome) motivate testing when the patient also has had a first-degree relative with pancreatic cancer.17
Imaging Technologies
The following sections describe imaging tests to assist diagnosis and/or staging of pancreatic adenocarcinoma, including multidetector computed tomography (MDCT), endoscopic ultrasound with fine-needle aspiration (EUS-FNA), positron emission tomography–computed tomography (PET/CT), and magnetic resonance imaging (MRI). The various available imaging modalities in the diagnosis and staging of pancreatic adenocarcinoma have different strengths and potential benefits, weaknesses, and potential harms. At present, there does not appear to be universal standard as to which imaging modalities should be used in which cases. This could be, in part, because of the difficulty of diagnosing and managing such an aggressive cancer, as well as limitations in the relevant evidence. It may also be related to clinical utility, local availability, procedural costs, and acceptability to patients.
Multidetector Computed Tomography
An MDCT scan is often the first imaging test in a patient whose symptoms suggest pancreatic adenocarcinoma. It provides three-dimensional multiplanar reconstruction images enabling determination of tumor size, extent, and spread, with a standardized pancreas protocol.18,19 The test does not always differentiate malignant from benign pancreatic lesions, and its ability to detect small tumors or small hepatic/peritoneal metastases is limited. A concern about MDCT is that the procedure exposes the patient to radiation and, therefore, may increase future cancer risk. Also, the quality of the computed tomography (CT) protocol, as well as the experience and expertise of the radiologist reading the CT may influence the accuracy of MDCT for diagnosis and staging of pancreatic adenocarcinoma. The American College of Radiology offers a voluntary accreditation program for CT facilities.20
One notable type of MDCT is MDCT with angiography with or without three-dimensional (3D) reconstruction.21 This technology permits more precise imaging of blood vessels than standard MDCT. This report refers to MDCT without angiography as simply “MDCT.”
Endoscopic Ultrasound with Fine-Needle Aspiration
For EUS-FNA, an ultrasound transducer, which is positioned at the endoscope tip, is directly applied against the duodenal or gastric wall. This minimizes intervening adipose tissue and air that must be traversed by the ultrasound, therefore enhancing the image quality. This allows EUS to access and image the entire pancreas, the related vasculature, lymph nodes, and portions of the liver. The endoscopist can take a small aspiration (FNA) of any suspicious lesions, permitting cytologic evaluation. If the biopsy is adequate, EUS-FNA can distinguish benign from malignant lesions and characterize certain types of lesions (e.g., cystic pancreatic lesions).11 Reported disadvantages of EUS-FNA include the procedure’s invasiveness, dependence on the skill of the endoscopist, and inability to evaluate for distant metastases.19 The relative newness of EUS-FNA could mean large variation in endoscopists’ technical skills. Potential patient harms related to EUS-FNA include perforation and bleeding, pancreatitis, and adverse effects related to sedation. The American College of Radiology has instituted a voluntary general ultrasound accreditation program that offers facilities the opportunity for peer review of their staff qualifications, equipment, and quality control and quality assurance programs.22
We note that some centers may still perform EUS without the ability to take an FNA. Consultations with our Technical Expert Panel indicated that most EUS centers in the United States are equipped with FNA technology. Therefore, we focused on studies that had the potential to perform FNA. In the literature, there is current debate about whether an endoscopist should actually take an FNA of a resectable lesion.23 For our report, we defined EUS-FNA as the procedural ability to take FNA, not the requirement that all lesions must have been sampled by FNA.
Magnetic Resonance Imaging
MRI is an alternative to MDCT as an initial imaging test for patients with a clinical suspicion of pancreatic adenocarcinoma or to evaluate the extent of disease. During an MRI procedure, electromagnetic fields and radiofrequency radiation translate hydrogen nuclei distribution in body tissues into images of anatomic structure. Similar to MDCT, a standardized pancreas protocol is available. MRI may be helpful when characterizing small (less than 1 cm) hepatic lesions, differentiating an inflammatory pancreatic mass from pancreatic adenocarcinoma, or detecting metastases to the liver.19 MRI can also be used as an adjunct to CT to better detect extrahepatic disease.24,25 There is no nationwide compulsory accreditation for MRI facilities. The American College of Radiology administers a voluntary accreditation program.26
Positron Emission Tomography–Computed Tomography
PET is a whole-body scan whose image highlights places where a radioisotope tracer concentrates and is, therefore, particularly useful for detecting distant metastases. The most commonly used radioisotope tracer is fluorodeoxyglucose 18F (FDG). FDG-PET can locate metabolically active sites such as malignant tumors or sites with inflammation and may, therefore, help distinguish malignant tumors from benign pancreatic cysts or other masses not metabolically active. FDG-PET and CT can be combined to add precise anatomic localization (from CT) to functional data (from PET). The two scans are acquired concurrently, and the data from each are merged. The Intersocietal Accreditation Commission (formerly the Intersocietal Commission for the Accreditation of Nuclear Medicine Laboratories [ICANL]) offers voluntary accreditation to PET/CT facilities based on a peer review of their staff qualifications, education, equipment, quality control, and number of clinical procedures.27
Objectives of This Review
This review concerns imaging tests to identify and diagnose suspected pancreatic cancer in symptomatic or asymptomatic high-risk patients, and determine stage and surgical resectability of the disease.4,28 Pancreatic adenocarcinoma is fatal if untreated, so it is critical to choose the right imaging test and initiate therapy in a timely manner. Understanding the accuracy and characteristics of the various imaging tests may help elucidate in which specific circumstances certain imaging test may be more appropriate than others in given clinical situations. A comparative effectiveness review (CER) on this topic can assist medical decisions in several ways:
- First, different imaging tests are used for overlapping purposes. Thus, a critical issue is that when two tests are used for the same purpose (e.g., assessment of vessel involvement) in the same patients, which is more accurate? Answering this question may reduce practice variability and improve patient care.
- Second, the evidence may favor some tests over others, and if so, resources can be devoted to the better tests.
- Third, it is important to clarify the practice of using a second imaging test: under what circumstances to order a second test, and if so, which test to order; and if ordered, what is its influence on diagnosis, staging, survival, and quality of life.
- Fourth, the comparative accuracy of imaging tests depends on the operator’s and reader’s skills and the environment in which the test is performed (e.g., high-volume vs. low-volume centers). Determining the extent to which this is important for various tests can also help better guide clinicians and patients in the workup process.
- Fifth, harms are always a concern, and by estimating the actual rates of various harms of different imaging tests, a CER can help discriminate reasonable fears from unreasonable ones.
Scope and Key Questions
Key Questions
- What is the comparative effectiveness of imaging techniques (e.g., MDCT, MDCT angiography, EUS-FNA, PET/CT, MRI) for diagnosis of pancreatic adenocarcinoma in adults with suspicious symptoms?
- What is the accuracy of each imaging technique for diagnosis and assessment of resectability?
- What is the comparative accuracy of the different imaging techniques for diagnosis and assessment of resectability?
- What is the comparative diagnostic accuracy of using a single imaging technique versus using multiple imaging techniques?
- How is test experience (e.g., operative experience, assessor experience, center’s annual case volume) related to comparative diagnostic accuracy of the different imaging strategies?
- How are patient factors and tumor characteristics related to the comparative diagnostic accuracy of the different imaging strategies?
- What is the comparative clinical management after the different imaging strategies when used for diagnosis?
- What is the comparative impact of the different imaging strategies on long-term survival and quality of life when used for diagnosis?
- What is the comparative effectiveness of imaging techniques (e.g., MDCT, MDCT angiography, EUS-FNA, PET/CT, MRI) for staging of pancreatic adenocarcinoma among adults with a diagnosis of pancreatic adenocarcinoma?
- What is the staging accuracy of each imaging technique (for tumor size, lymph node status, vessel involvement, metastases, stage I–IV, and resectability)?
- What is the comparative staging accuracy among the different imaging techniques?
- What is the comparative staging accuracy of using a single imaging technique versus using multiple imaging techniques?
- How is test experience (e.g., operative experience, assessor experience, center’s annual volume) related to comparative staging accuracy of the different imaging strategies?
- How are patient factors and tumor characteristics related to the comparative staging accuracy of the different imaging strategies?
- What is the comparative clinical management of the different imaging strategies when used for staging?
- What is the comparative impact of the different imaging strategies on long-term survival and quality of life when used for staging?
- What are the rates of harms of imaging techniques (e.g., MDCT, MDCT angiography, EUS-FNA, PET/CT, MRI) when used to diagnose and/or stage pancreatic adenocarcinoma?
- How are patient factors related to the harms of different imaging techniques?
- What are patient perspectives on the tolerance of different imaging techniques and the balance of benefits and harms of different imaging techniques?
- What is the screening accuracy of imaging techniques (e.g., MDCT, MDCTangiography, EUS-FNA, PET/CT, MRI) for detecting precursor lesion(s) of pancreatic cancer or pancreatic adenocarcinoma in high-risk asymptomatic adults (i.e., those at genetic or familial risk of pancreatic adenocarcinoma)?
Patients, Interventions, Comparisons, and Outcomes
Table 1 below summarizes the PICO (population, interventions, comparators, outcomes) for each key question. In the table, population P1 is symptomatic patients being assessed for possible pancreatic adenocarcinoma; population P2 is adults with known pancreatic adenocarcinoma; population P3 is asymptomatic adults at high risk of developing pancreatic adenocarcinoma. Regarding timing, the only issue concerns the outcomes of long-term survival and quality of life; we defined “long-term” as 1 year or more.
Table 1
PICO for each Key Question.
Conceptual Framework
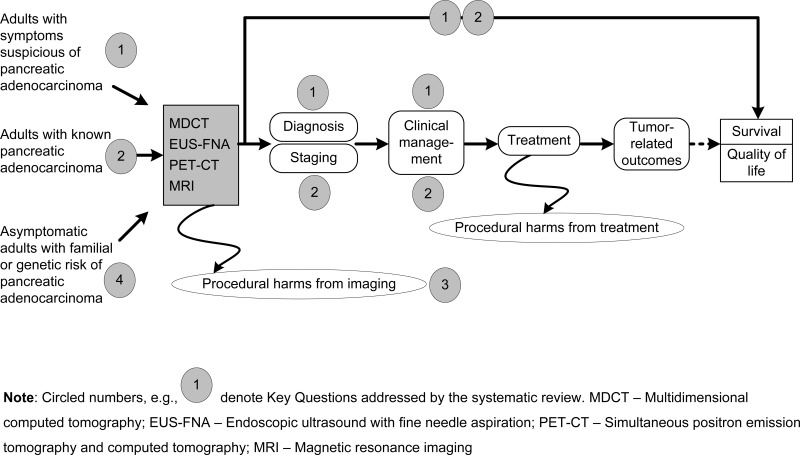
An analytic framework illustrating the connections between the populations of interest, the imaging techniques, and the outcomes is shown in Figure 1 below. Populations that are undergoing or have undergone treatment for pancreatic adenocarcinoma are outside the scope of this report.
The populations of interest enter the diagram at the left, undergo diagnosis (Key Question 1), staging (Key Question 2), and then commence treatment. Some outcomes such as test performance can be measured immediately after performing the tests, but the most important outcomes (such as long-term survival and quality of life) are measured after completion of treatment.
An important factor in selecting an imaging modality is the availability and accessibility of that modality. Although this factor will not be addressed formally in the review via a key question, we plan to collect and provide relevant information about the availability and accessibility of imaging modalities and information about current patterns of care, as available. This information will be presented in the background and discussion sections to help place the evidence review findings in context.
Organization of This Report
In the remaining three chapters of this report, we present the methods for this systematic review, the results for each key question, and a discussion of the findings. Within the Results chapter, we provide the results of the literature searches and selection procedures, then the results for Key Question (KQ) 1.
For the comparative accuracy of imaging tests (KQ1b and KQ2b), each section is divided per comparison (e.g., first we present the evidence on MDCT vs. EUS-FNA, then the evidence on MDCT vs. MRI). Within each of those subsections, we consider different aspects of the clinical process (e.g., for staging, we first consider the evidence on T staging, then evidence on N staging).
The Discussion section, which appears after all Results sections, provides an overview of our findings, and how they relate to what is already known. In that section we also discuss implications for clinical and policy decisionmaking, the applicability of the evidence, limitations of our review as well as limitations of the evidence we reviewed, and any major gaps in existing research.
Publication Details
Copyright
Publisher
Agency for Healthcare Research and Quality (US), Rockville (MD)
NLM Citation
Treadwell J, Mitchell M, Eatmon K, et al. Imaging Tests for the Diagnosis and Staging of Pancreatic Adenocarcinoma [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Sep. (Comparative Effectiveness Review, No. 141.) Introduction.