NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Charach A, Dashti B, Carson P, et al. Attention Deficit Hyperactivity Disorder: Effectiveness of Treatment in At-Risk Preschoolers; Long-Term Effectiveness in All Ages; and Variability in Prevalence, Diagnosis, and Treatment [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 Oct. (Comparative Effectiveness Reviews, No. 44.)
This publication is provided for historical reference only and the information may be out of date.

Attention Deficit Hyperactivity Disorder: Effectiveness of Treatment in At-Risk Preschoolers; Long-Term Effectiveness in All Ages; and Variability in Prevalence, Diagnosis, and Treatment [Internet].
Show detailsTopic Development
The topic of this report and preliminary key questions (KQs) were developed through a process involving the public, the Scientific Resource Center for the Effective Health Care program of the Agency for Healthcare Research and Quality (AHRQ) (www.effectivehealthcare.ahrq.gov/aboutUS/contract.cfm), and various stakeholder groups. Study, patient, intervention, eligibility criteria, and outcomes, were refined and agreed upon through discussions between the McMaster University Evidence-based Practice Center, the Technical Expert Panel (TEP) members, the AHRQ Task Order Officer (TOO), and comments received from the public posting of the key questions and protocol document.
Analytic Framework
Following consultation with key informants, the AHRQ TOO, and our investigative team, we developed our key research questions. Figure 1 shows a flow diagram indicating the relationship between research questions in this Comparative Effectiveness Review (CER).
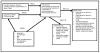
Figure 1
Analytic framework: ADHD in preschoolers and long-term effects of ADHD pharmacotherapy. Abbreviations: ADHD = Attention Deficit Hyperactivity Disorder; KQ = key question
This framework depicts the key questions as described in the PICO table, Table 1, (population, intervention, comparison, and outcomes). The figure illustrates how geography, age, provider type, and sociodemographic characteristics may influence the diagnosis and the treatment of Attention Deficit Hyperactivity Disorder (ADHD), Oppositional Defiant Disorder (ODD), and Conduct Disorder (CD). Treatment results in measurable outcomes, showing improvement or decline in behavior, function or quality of life. Indicators of long-term outcomes are new onset psychiatric disorder, initiation of substance use, gambling, driving infractions, teen parenthood, legal charges, academic attainment, job stability, relationship stability, physical health, and changes in mental health.
Table 1
PICO table for ADHD review.
Methodology for Prevalence and Variations in Management Question
For the prevalence question (KQ3), we searched the literature and screened the resulting citations right up to the full text examination using systematic review methodology, which includes preset inclusion/exclusion criteria screening questions and agreement by two raters for all decisions. All abstracts of the resulting reports were examined and those selected which reported data that directly addressed prevalence, clinical identification, and treatment of ADHD as specified in KQ3. The process of external review identified additional references subsequently incorporated into the final document.
Search Strategy
For KQ1, the databases were searched from their inception date to the 31st of May, 2010. Studies were limited for KQ2 to include any publication from 1997 to the 31st of May, 2010 inclusive because long-term treatment of ADHD has already been reviewed by AHRQ for dates before 1997.10 For KQ3, publications dated back to 1980 were included.
The following databases were searched for KQ1 and KQ2: MEDLINE, Cochrane CENTRAL, EMBASE, PsycInfo, and ERIC (Education Resources Information Center). For KQ3, the Cochrane Library and ERIC Database were not searched because clinical trials were not the target of this review. Strategies used combinations of controlled vocabulary (medical subject headings) and text words. The complete search strings used can be found in Appendix A. Searches were performed on December 1, 2009 and the update performed on May 31, 2010.
Reference lists of eligible studies at full text screening were reviewed. Any potentially relevant citations were cross-checked within our citation database and any references not found within the database were retrieved and screened at full text.
Study Selection
Criteria for Inclusion or Exclusion of Studies in the Review
Target Population
For KQ1, the population includes children less than 6 years of age with a diagnosis of ADHD or DBD (including ODD and CD) by Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases (ICD) criteria. In addition, samples where children showed clinically significant symptoms were included, defined by referral to treatment or high scores on screening measures.
For KQ2, the population includes subjects of greater or equal to age 6 years who have been treated for ADHD or are a control group of ADHD subjects, diagnosed with ADHD by DSM or ICD criteria.
For KQ3, the population includes subjects of any age who have been diagnosed with ADHD or treated for ADHD. Because much of this data would come from cross-sectional, survey, and medical databases using drug treatments and survey symptom checklists to identify ADHD subjects, subjects did not require a DSM or ICD diagnosis for inclusion.
Sample Size
There are no restrictions for study sample size.
Study Design and Publication Types
Inclusion
Full text reports of clinical trials and comparative observational studies were included for KQ1 and KQ2. For KQ3, we also included cross-sectional reports.
Eligible designs include:
- Experimental studies with comparator groups (randomized and quasi-randomized trials)
- Open label extensions following randomized controlled trials (RCTs)
- Observational studies with comparator groups (retrospective and prospective cohort, and case control)
- For KQ3 only, noncomparative cross-sectional studies
Exclusion
Letters, editorials, commentaries, reviews, meta-analysis, abstracts, proceedings, case reports, case series, qualitative studies, and theses were excluded.
Non-English publications were excluded for this review.
Definition of Terms
ADHD, ODD, and CD will be as defined by the version of DSM or ICD current at the time of the study or of the publication.
Further Search Methods
Study authors were contacted via email for missing outcome or design data. Reference lists of included papers were screened for possibly relevant papers that had not already been screened. Grey literature was identified by the AHRQ Scientific Resource Center and included:
- FDA—Medical Reviews and Statistical Reviews
- Health Canada—Drug Monographs
- Authorized Medicines for EU - Scientific Discussions
- ClinicalTrials.gov
- Current Controlled Trials (U.K.)
- Clinical Study Results (PhRMA)
- WHO Clinical Trials (International)
- CSA Conference Papers Index
- Scopus - limited to conference papers
Standardized forms were developed in DistillerSR (Evidence Partners Inc., Ottawa, Ontario, Canada) and Microsoft Excel for the purposes of this systematic review.
Types of Comparators
We identified and included studies with comparative intervention groups. From a design hierarchy perspective, comparative group designs provide stronger evidence for efficacy and effectiveness than non-comparative designs.
The interventions (either alone or in combination) may be compared to any of the following:
- Placebo
- Same pharmacologic agent of different dose or duration
- Other pharmacologic agent
- Behavioral intervention
- Psychosocial intervention
- Academic intervention
- Any combination of pharmacologic, academic, behavioral, or psychosocial intervention
Reports studying any drug for treatment of ADHD were included in this review if the other inclusion criteria were met.
Pharmacological Interventions Reported in This Review
Selective Norepinephrine Reuptake Inhibitor
- Atomoxetine (ATX)
Alpha-2 Agonist
- Guanfacine extended release (GXR)
Non-Medication Interventions Reported in This Review
- Parent behavior training—manualized programs designed to help parents manage child’s problem behavior using rewards and non-punitive consequences
- Psychosocial interventions—include any one of a number of interventions aimed to assist child and family through psychological and social therapies (e.g., psychoeducational, parent counseling and social skills training
- Behavioral interventions—manualized programs designed to help adults (parent, teachers, other) using rewards and non-punitive consequences
- School-based interventions—interventions in which teachers are primary intervenors and where the intervention takes place in a classroom or school setting
Outcomes
No limits have been placed on the effectiveness or adverse event outcomes included in this report. The primary focus for outcome in this report is identification of improvement in child behavior. Numerical or statistical results of any effectiveness or adverse event outcomes are included.
Data Extraction
Relevant fields of information were taken from individual studies by trained data extractors using standardized forms and a reference guide. Key study elements were reviewed by a second person (study investigator) with respect to study outcomes, seminal population characteristics, and characteristics of the intervention. Disagreements were resolved by consensus.
Abstracted data includes study characteristics (e.g., first author, country of research origin, study design, sample size, clinical indications, and study duration or length of followup). Details of the patient population include age, gender, racial composition, socioeconomic status (SES) (e.g., income, education), and comorbidities (e.g., psychiatric and medical disorders). Details of the study intervention include type of intervention (e.g., pharmacological and non-pharmacological) and the comparators, dosage of intervention, duration of followup (from immediately post treatment to long term), and characteristics of treatment providers. Characteristics of the outcomes include the type of instrument or scale, type of effect measure (e.g., endpoint or change score, measure of variance, standard deviation, standard error, etc.), and definition of treatment response.
All forms and guides used in the screening and data extraction process are provided in Appendix B.
Peer Review
Prior to finalization of the report, the AHRQ submitted a draft to seven peer reviewers and their comments were implemented after consideration by the research team. The report was also made available on the AHRQ website for public review; public reviewers’ comments were also implemented after consideration by the research team. In situations where the research team decided not to revise the content of the report based on a reviewer’s comments, a written explanation of the reason(s) for choosing not to revise have been submitted to the AHRQ.
Assessment of Methodological Quality of Individual Studies
We interpret methodological quality to include primarily elements of risk of bias (systematic error) related to the design and conduct of the study. We have selected the Effective Public Health Practice Project, Quality Assessment Tool for Quantitative Studies Risk of Bias (EPHPP) (see Appendix B)13 and used this in KQ1 and 2, where each paper was rated independently by two raters and conflicts resolved by a third. No similar tool for evaluating epidemiological and health service studies was used. The process for preparing this report included peer review by experts in the field of inquiry. For KQ3, we included additional studies recommended for inclusion by the reviewers, all of which had been identified in previous steps through the search methodology.
The tool, which measures internal validity, contains eight sections that include evaluation of the domains of selection bias, study design, confounders, blinding, data collection methods, withdrawals and dropouts, intervention integrity, and analyses. A global rating of “good,” “fair,” or “poor” for each report results from agreement by two raters on the combination of all of these items. Ratings result from a combination of the quality of the study design, execution, and reporting. A “good” paper will have mostly strong ratings in each section with possibly a moderate rating in one or two of the eight sections. A “fair” paper will have mostly moderate ratings for the eight domains, or it will have a split between weak, moderate, and strong ratings. A “poor” paper could have one or two strong domains, but has three or more weak domains in the rating.
Rating the Body of Evidence
We assessed the overall strength of the body of the evidence using the context of the GRADE approach, modified as the Grading System as defined by AHRQ.14,15 Although we included papers that were not RCTs, there are several factors suggested by the GRADE approach that may decrease the overall strength of the evidence (SOE):
- Study limitations (predominately risk of bias)
- Type of study design (experimental versus observational)
- Consistency of results (degree to which study results for an outcome are similar between studies, and variability is easily explained)
- Directness of the evidence (assesses whether interventions can be linked directly to the health outcomes)
- Precision (degree of certainty surrounding an effect estimate for a specific outcome)
The ratings were arrived at through discussion among two or more of the investigators. Only papers rated as “good” were included in these analyses since they represent the best available data at this point in time. See Appendix D.
No limits have been placed on the effectiveness or adverse event outcomes included in this report. Numerical or statistical results of any effectiveness or adverse event outcomes are included. Effect Sizes are reported as Standardized Mean Differences (SMD) whereby the difference in outcome (using continuous measures) between the intervention and comparison groups is divided by the pooled standard deviation to estimate intervention effectiveness. By convention, 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect.11 The SMD is used as a summary statistic in meta-analysis when the studies use different instruments the measure the same outcome. The data are standardized to a uniform scale before they can be combined. The SMD expresses the size of the intervention effect in each study relative to variability observed in that study.12
Data Synthesis
Qualitative Synthesis
For each trial, information on population characteristics (e.g., history of treatment(s), age of first diagnosis, etc.), study outcomes, sample size, settings, funding sources, treatments (type, dose, duration, and provider), methodological limitations, statistical analyses, and any important confounders were summarized in text and summary tables.
Quantitative Synthesis
The decision to pool individual study results was based on clinical judgment with regards to comparability of study populations, treatments, and outcome measures. Aspects considered were: methodological quality (e.g., high-risk of bias vs. low-risk of bias), clinical diversity (e.g., study population gender, disease severity), treatment characteristics (e.g., type of intervention), and outcome characteristics (e.g., long-term followup vs. short-term followup, different measuring scales, different definitions of dichotomous outcomes). The extent of heterogeneity was explored through subgroup and sensitivity analyses.
Subgroup and Sensitivity Analysis
Key patient-specific or intervention-specific factors that may affect the treatment effect were explored. Clinical heterogeneity was assessed by considering any potential differences in participants among the trials (e.g., age, gender, diagnoses, disease severity, definition of response). Methodological heterogeneity was explored by evaluating where studies failed to meet the criteria.
To maximize the similarities among studies that could potentially be combined for meta-analyses, we further stratified where possible studies based on: (1) behavior disorder (ADHD, ODD, CD), and (2) age categories (preschool, child, adolescent, adult). There are several patient characteristics that we further explored for potential subgroup and sensitivity analysis and these include the following: (1) disease severity and ADHD subtype, (2) gender, and (3) comorbidities related to other psychological disorders. Trial specific factors include: (1) duration or dose of intervention, (2) type of treatment provider, and (3) method of defining response.
- Methods - Attention Deficit Hyperactivity DisorderMethods - Attention Deficit Hyperactivity Disorder
Your browsing activity is empty.
Activity recording is turned off.
See more...
