NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Nearly three decades have passed since the first publications on type 2 diabetes (T2D) in children and adolescents, and it is now well established as a global problem. As the prevalence of obesity continues to increase within the general population, diagnosing T2D in adolescents presents a multifaceted challenge. In this chapter, we shall delve into the epidemiological aspects, as well as the distinct characteristics inherent to various types of diabetes. Cohort studies with long-term follow-ups have illuminated our understanding of the alarming incidence of complications in adolescents diagnosed with T2D. Recent approvals of novel pharmaceutical interventions for teenagers have ushered in a new era of hope. Hopefully, in the forthcoming decade, we anticipate a decline in the prevalence of these complications. However, in the interim, a proactive and assertive approach remains essential for addressing the complications associated with T2D in adolescents. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
SHORT HISTORY
Youth-onset type 2 diabetes (T2D) was initially described in Pima Indian children and adolescents (1). This tribe, also known as “the pathfinders,” has a notable history of obesity and early-onset diabetes. In the mid-nineties, studies from various clinics in the United States reported cases of T2D in children from diverse ethnic backgrounds, primarily non-Hispanic Blacks and Hispanics (2-4). While these reports were initially met with suspicion, it soon became clear that a new disease had emerged among children. Subsequently, similar reports emerged from various parts of the world. In 2000, the SEARCH for Diabetes Study was launched in the US. This prospective nationwide, multi-center study aimed at understanding the epidemiology of both type 1 diabetes (T1D) and T2D in children. A few years later, in 2004, the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study was initiated. The TODAY study was a prospective, randomized treatment trial, designed to explore treatment regimens and the clinical course of T2D in youth. Registries from Germany, Diabetes Prospective Follow-up Registry (DPV) (5), Hong Kong (6), China (7), India (Registry of People with Diabetes with Youth Age at Onset (YDR)) (8) and Israel (9), contributed to the current knowledge. Importantly, while the US is leading in the scope of the T2D epidemic, similar trends have been observed worldwide.
EPIDEMIOLOGY
Epidemiology in the USA
According to the most recent data from the SEARCH study published in 2023, the adjusted incidence of T2DM among children and adolescents nearly doubled, from 9.0 to 17.9 cases per 100,000 persons per year, from 2002-03 to 2017-18 (10).
EPIDEMIOLOGY ACCORDING TO ETHNICITY
The incidence of T2D varies largely based on ethnicity, the highest incidence of T2D, 50.1 per 100,000 children age 10-20 years, was recorded in non-Hispanic Blacks, followed by Pima Indians (46), Hispanics (25.8), and Asian/Pacific Islanders (16.6). Among non-Hispanic Whites, the incidence is only 5.5 per 100,000. An annual increase was observed in all groups, but the highest increase was observed in Asian/Pacific Islanders (8.92%), followed by 7.17% in Hispanics and 5.99% in non-Hispanic Blacks, compared to 1.83% in non-Hispanic Whites.
EPIDEMIOLOGY ACCORDING TO GENDER AND AGE
The current incidence in females is 21.6 per 100,000 and in males 14.2; the temporal increase was similar in both genders. The incidence increases with age. One of the most remarkable findings in the SEARCH study is that among individuals 15-19 years of age, the incidence of T2D in 2017-2018 exceeded that of T1D (19.7 vs. 14.6 per 100,000). This is the first time the incidence of T2D surpassed that of T1D among youth, sounding alarming for other countries, which may have a lower incidence than the USA, but are showing steady increases.
Epidemiology in Europe
Although the overall incidence of T2D in Europe is much lower than in the US, a similar change over time has been reported. In Germany, a three-fold increase in the prevalence of T2D was reported for 10- to 19-year-olds between 2002 and 2020 (3.4 to 10.8 per 100,000) (5). Similar to the US, the estimated standardized prevalence of T2D was 1.4 times higher among girls (12.8) than boys (9.0). In the UK, the number of children registered as having T2D and being treated in pediatric diabetes units has risen by more than 50% in the past five years (11).
Epidemiology in Asia
Data retrieved from the Hong Kong Childhood Diabetes Registry revealed a threefold increase in T2D in children, from 1.27 per 100,000 in 1997-2007 to 3.42 per 100,000 in 2008-2018 (6). Data from China demonstrated an average annual increase of 26.6% in youth aged 10-19 years (12). There was no statistically significant difference in incidence between boys and girls. However, the risk of T2D was 1.49 times higher in urban areas than in rural areas.
RISK FACTORS FOR T2D IN CHILDREN
The increased risk of developing T2D in children is associated with genetics, an obesogenic environment, and obesity, along with the interactions among these factors (Figure 1). This visual representation emphasizes the multifaceted nature of risk factors for T2D in children and adolescents, illustrating the intricate connections between genetics, environmental factors, obesity, and the eventual development of T2D.
Figure 1.
Risk Factors for T2D in Children and Adolescents. Risk factors for T2D in children and adolescents can be visualized as a series of interconnected circles, illustrating the complex interplay of various influences:
Obesity
Severe obesity stands out as one of the most significant risk factors for youth-onset of T2D. Obesity is closely linked to the worsening of insulin resistance, a primary feature of the pathophysiology of T2D, along with progressive β-cell and α-cell dysfunction (13). In a recent meta-analysis comprising data from 30 studies involving 4688 children and adolescents, the prevalence of obesity at the time of T2D diagnosis was found to be 77% (95% CI, 71%-83%) (14). Male participants exhibited higher odds of obesity than their female counterparts (odds ratio, 2.10; 95% CI, 1.3-3.3). These findings were sustained after several sensitivity analyses that excluded studies with uncertain or unspecified T2D diagnostic criteria, studies involving individuals with positive pancreatic autoantibodies, individuals presenting with weight loss, or those with genetically proven monogenic diabetes. While acknowledging the limitations of using BMI-based measures as a surrogate for obesity and considering the retrospective nature of the included studies, these data suggest that approximately 20-25% of adolescents with diabetes but without obesity may still have T2D. Furthermore, the definition of obesity needs to consider body composition variations among certain populations, such as East Asian and South Asian populations.
Family History of T2D
Most adolescents with T2D have a family member with the disease. In the TODAY study, 59.6% of adolescents with T2D had a first-degree family member with a history of diabetes, and 89% had a grandparent affected with diabetes (15). This increased risk of T2D associated with family history reflects genetic influences, the impact of the environment, and the intrauterine environment.
Maternal Obesity and Maternal T2D During Pregnancy
Exposure to maternal obesity has been linked to an increased risk of childhood obesity in offspring (16) and 2.8-fold higher odds of T2D. Exposure to maternal gestational diabetes was associated with 5.7-fold higher odds of T2D (17). Moreover, individuals exposed to maternal diabetes during pregnancy were diagnosed with diabetes at a younger age and exhibited worse β-cell function. Exposure in utero to maternal diabetes and obesity accounted for 47% of the T2D risk in this population (17,18), and, importantly, it sets up a vicious cycle for future generations. Suggested mechanisms affecting the developmental programming of offspring towards T2D include epigenetic modifications, alterations in stem cell differentiation, variation in the metabolome and microbiome, and immune dysregulation (19). Of note, assisted reproductive technology was not found to be a risk factor for early-onset T2D (20).
Pregnancies complicated by pre-existing diabetes are associated with extreme birth weights. In a large study, small for gestational age (SGA) babies, defined as below the 10th percentile for birth weight, were reported in 14.1% of pregnancies of women with T2D, while 26.2% had newborns with a large birth weight, defined as above the 90th percentile (21). Both SGA and high birth weight are associated with an increased risk of a history of early-onset T2D among adults. Of note, a lower birthweight was associated with an increased risk of developing T2D independently of adult BMI and the genetic risk of T2D (22).
Pregnancies complicated by pre-existing diabetes are associated with preterm delivery and birth weight extremes. In a national cohort study involving over 4 million singletons born in Sweden from 1973 to 2014, preterm birth (occurring before 37 weeks) was associated with a 1.3-fold increased risk of developing T2D before age 18 years (23). Notably, the association between preterm birth and T2D was significantly stronger among females (23).
Mental Health and Treatment
The association between psychiatric disorders in adolescents and T2D is bidirectional, complex, and not well studied in the pediatric population (24). On the one hand, youth with chronic illnesses such as obesity are more likely to develop depression, depressive symptoms, and anxiety than those without chronic illnesses. On the other hand, youth who have mental health morbidities are at increased risk for isolation, sedentary lifestyle, weight gain, and the development of T2D. Furthermore, some psychotropic medications, particularly atypical antipsychotics, are associated with weight gain and increased risk for T2D in adults. These associations have not been systematically studied in youth, but clinical experience suggests a contribution.
Immigration
Globally, immigration is on the rise. Studies conducted among adults have indicated that immigrants, when compared to non-immigrants, exhibit higher rates of obesity, insulin-resistance, and hypertension. In a recent review (25) focusing on European countries, non-European migrant children face a greater risk of being overweight or obese compared to their native counterparts; the prevalence of obesity in migrant and native children ranged from 1.2 to 15.4% and from 0.6 to 11.6%, respectively. The increased rates of overweight and obesity among migrating children and adolescents render them more susceptible to developing T2D and other metabolic abnormalities. An Israeli study found that among adolescents of Ethiopian origin, the prevalence of overweight and obesity increased two-and-a-half fold and fourfold in males and females, respectively, during the study period, compared to a 1.5-fold increase among native Israeli-born males and females (26).
Socioeconomic Status
A discernible disparity exists in the socioeconomic status (SES) of children and adolescents with T2D. In developed countries, such as the United States, low SES is a recognized risk factor for the development of obesity and T2D. This is attributed to factors such as limited access to healthy foods and reduced physical inactivity. However, in developing countries, higher SES groups tend to have greater levels of physical inactivity and consume higher quantities of fat, salt, and processed foods compared to their lower SES counterparts (Figure 2). When data from 384 youth with T2D who completed a baseline research visit as part of the SEARCH study and 227 youth with T2D from the Registry of People with Diabetes with Youth Age at Onset (YDR) in India who completed a baseline visit were compared, only 23.6% of SEARCH youth belonged to a high SES group, in contrast to 88.5% of YDR youths (P < .001) (8).
Data from 747 children and youths with T2D under 19 years of age, collected between 2009 and 2016 (from the population-based National Pediatric Diabetes Audit, covering over >95% of diabetes cases in England and Wales), revealed that under half of those with T2D reside in the most disadvantaged areas of England and Wales, and 41% fall into the most disadvantaged SES quintile (27). In contrast, in China, in Zhejiang, one of the most economically prosperous coastal provinces marked by industrialization and urbanization, changes in diet and decreased physical activity have led to a higher incidence of pediatric obesity. The mean annual incidence was 2.32 per 100,000 person-years in urban area compared with 1.44 in urban and rural area (12).
COVID-19 Pandemic
In a study conducted in the United States, the incidence of T2DM at 24 diabetes centers during the first year of the COVID-19 pandemic was examined (28). The average number of new diagnoses per year in the two years before the pandemic was 825 but rose to 1463 during the first pandemic year. This increase of 77% is significantly higher than the 5% expected annual increase in incidence observed in the two previous years. Similarly, a retrospective cross-sectional review of youths (age ≤ 21) diagnosed with T2D during the COVID-19 pandemic (from May 1, 2020, to April 30, 2021) and the five years preceding it (from May 1, 2015, to April 30, 2020) at a tertiary diabetes center revealed an increase of 293% (29).
Similar trends were reported from Germany (30). Data on T2D in adolescents during 2 years of the COVID-19 pandemic (2020–2021) were compared with the control period 2011–2019 in children aged 6 to <18 years were obtained from the DPV (German Diabetes Prospective Follow-up) Registry. The incidence of youth-onset T2D increased from 0.75 per 100,000 patient-years in 2011 to 1.25 per 100,000 in 2019, an annual increase of 6.8%. However, in 2021, the observed incidence was 1.95, significantly higher than expected. Of note, the observed incidence was significantly higher in boys (2.16), leading to a reversal of the sex ratio of pediatric T2D incidence (30).
The surge in T2D incidence during the pandemic can be attributed to several factors. First, there was an increase in obesity among young people during the COVID-19 pandemic, accompanied by increased consumption of processed foods and reduced physical activity, both of which contribute to the risk of developing T2D (31). In addition, there was increased psychosocial stress, which is emerging as an important contributor to the risk of T2D. Furthermore, viral mediated non-autoimmune β-cell destruction, resulting in reduced β-cell function in predisposed adolescents, has been suggested as a contributing factor (28).
Genetics
The genetics of T2D in children and adolescents have largely remained unexplored. ProDiGY is a multiethnic collaboration encompassing three studies (TODAY, SEARCH, and T2D-GENES) involving 3,006 youth subjects with T2D, diagnosed at a mean age of 15.1±2.9 years, along with 6,061 diabetes-free adult control subjects. Association analyses were conducted on approximately 10 million imputed variants, employing a generalized linear mixed model incorporating a genetic relationship matrix to account for population structure. The analysis was further adjusted for sex. This comprehensive study identified seven genome-wide significant loci that included a novel locus in PHF2, along with TCF7L2, MC4R, CDC123, KCNQ1, IGF2BP2, and SLC16A11. A secondary analysis involving 856 diabetes-free youth subjects uncovered an additional locus in CPEB2, and consistent directional effects for diabetes risk were observed (32).
CLINICAL PRESENTATION
Diabetes Related Signs
The classical signs at the onset of diabetes - polydipsia and polyuria - are observed in about two-thirds of youth at the diagnosis of T2D. Only about one-third are diagnosed through routine screening of asymptomatic youth with obesity. Furthermore, the prevalence of diabetic ketoacidosis (DKA) among youth with T2D in the SEARCH study and the YDR in India was 5.5% and 6.6%, respectively. Importantly, the incidence of DKA at the onset of T2D was higher during the SARS-CoV-2 pandemic (33). Hyperglycemic hyperosmolar state is present at diagnosis in 2% of youth with T2D (34).
Seasonal Diagnosis of T2D
There is significant seasonal variation in the onset of T2D in children and young people in the United States; diagnoses increased in August. Possible explanations for an August peak in diagnoses include weight gain during the summer vacation and an increase in physical exams for school athletic programs that may detect asymptomatic hyperglycemia. The greater proportion of diagnoses made during routine health visits rather than following symptoms is consistent with this latter possibility. Similar seasonal variations in incidence are beginning to emerge in other countries.
Age of Onset of T2D
The incidence of T2D rises gradually during puberty, primarily due to the physiological insulin resistance characteristic of this period. However, illustrating the heterogeneity of T2D in children and adolescents, there was a significant difference between the mean age of onset of youth in the United States (Pediatric Diabetes Consortium, PDC) databases who were diagnosed at the mean age of 12 years compared to the mean age in Germany of 13 years (35).
PREPUBERTAL T2D
With the worldwide epidemic of childhood obesity on the rise, there are increasing reports of T2D occurring in prepubertal children. Initially, there were case reports on prepubertal children with T2D, including cases from Nigeria (36), and a 5-year-old Indigenous girl from Australia (37). Lately, several reports have described the onset of T2D in prepubertal children. A total of 42 children ≤ 10 years with T2DM were reported from Alabama (38), 12 from Australia (39), 35 prepubertal children from Houston, Texas (40), from San Antonio, Texas (41) and India (42). The common denominator of all reports of prepubertal children is extreme obesity, with a disproportionate impact on females and ethnic minorities as shown in Table 1. Additionally, there is a high prevalence of comorbidities.
Table 1.
Characteristics of Prepubertal Children Diagnosed with T2D
| San Antonio, Texas N=20 | Alabama, US N=42 | Australia N=12 | Houston, Texas N=35 | Chennai, Tamil Nadu India N=4 | |
|---|---|---|---|---|---|
| Age, years Range | 8.1 4-9 | 7-10 | 10.6± 2.5 | ||
| 5-8 | 10 | 8 | |||
| 9-10 | 10 | 34 | |||
| Ethnicity | Hispanic 80% | African American 88(%) Caucasian 12% | Aboriginal Australians 92 Maori 8 | Hispanic 71 Black 23 Non Hispanic White 3% | India |
| Females (%) | 75 | 88 | 75 | 51 | 100 |
| BMI Z score | 2.72 (1.7-5.0) | 2.5 ± 0.4 | 2.38± 0.64 | 2.4±0.4 | >97%ile |
| Hypertension (%) | NA | 21 | 25 | 14 | |
| Dyslipidemia | NA | 58 | 58% | 100 | |
| NAFLD | NA | 20 | |||
| Microalbuminuria | NA | 16.6 | 5 |
These reports suggest that there are unique features and pathophysiologic drivers distinct from the influence of pubertal hormones, which are often implicated in the mechanism underlying T2D in older children and adolescents. Furthermore, the emergence of T2D in prepubertal children and the high prevalence of comorbidities among them serve as a warning for an impending public health challenge.
Sex
In the Western world, youth-onset T2D is almost twice as common in girls as in boys, whereas Asian countries report no differences in incidence by sex. For T2D, SEARCH had a higher proportion of females, while studies from China show no difference between females and males, and there are reports of increased prevalence in males in the Middle East; in the UAE (43), the female to male ratio was 0.5:1, in Iraq (44) 0.6:1, and Kuwait (45) 0.8:1 (Figure 2). This may reflect the higher prevalence of obesity in males in these countries (46). Indeed, in the UAE, among children aged 11 to 14 years, the prevalence of obesity was reported as 24.3% and extreme obesity (BMI ≥99th percentile) as 5.7%; the rate of extreme obesity was 9.6-fold higher in boys than girls (47). In Kuwait, 41.4% of boys were classified as obese, compared to 28.9% of girls of the same age (46).
DIAGNOSIS
Early diagnosis of T2D is likely to bring several benefits, enabling prompt multifactorial treatment and management of cardiovascular risk factors. Considering the increasing prevalence of T2D among children and adolescents, clinical symptoms have become less distinct, and age, sex, and weight are no longer definitive criteria for a clear diagnosis. Therefore, the most important diagnostic feature is the presence or absence of pancreatic autoantibodies in any child diagnosed with hyperglycemia; positive antibodies indicate T1D even if the child is overweight or has a family history of T2D. If the antibodies are negative, a genetic test for monogenic diabetes (previously referred to as Maturity-Onset Diabetes of the Young (MODY) should be considered if available.
TREATMENT
Initial treatment for T2D includes a focus on lifestyle changes, including healthy diet, increasing regular physical activity, achieving weight loss, and receiving emotional support. While lifestyle change is recommended as a practical approach, there is limited evidence that lifestyle intervention alone has a sustained impact on glycemic control in youth with T2D (48).
The Impact of Lifestyle Changes
The impact of lifestyle intervention, as determined by changes in diet and cardiovascular fitness, on glycemic control in youth with T2D was assessed in the TODAY clinical trial cohort spanning 15 centers across the United States. The study included 699 youth aged 10 to 17 years with T2D for less than 2 years. Those participants randomly assigned to the lifestyle intervention participated in a family-based behavioral program aimed at promoting weight loss. Each family was assigned a dedicated leader responsible for guiding them through their journey of physical activity and nutrition improvement. The intervention was structured across three stages, initially there were weekly meetings for 6 to 8 months, focusing on physical activity, setting individual calorie intake goals, self-monitoring, and problem-solving. In the subsequent stage, biweekly meetings were held for 12 to 16 months. Lastly, meetings were held monthly for 24 to 28 months, concentrating on maintaining a healthy lifestyle. Dietary data were collected through an interviewer-administered food frequency questionnaire, and cardiovascular fitness was assessed using a submaximal cycle ergometer test at baseline, 6 months, and 24 months (49). At 6 months, approximately 25% of females and 33% of males improved cardiovascular fitness. The authors concluded that a minority of youth improved fitness and/or diet over time, although those who did showed a beneficial impact on glycemic outcomes. However, the positive lifestyle behavior changes did not persist after 24 months, providing a sobering perspective on the challenges of implementing lasting lifestyle changes in this population.
Pharmacotherapy
The beginning of the third decade of the 21st century will be marked by the introduction of novel medications for children with T2D. There are several groups of medications for T2D, biguanides, sulfonylureas, GLP1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase inhibitors, and combinations of the dual incretin analogs and the new triple G agents (dual incretin and glucagon). However, only biguanides, GLP1 receptor agonists, and SGLT2 inhibitors are currently FDA-approved for children. Doses and modes of administration are presented in Table 2.
Table 2.
FDA-Approved Medications for Treating Type 2 Diabetes in Children Aged 10 Years and Older
| Biguanide | Metformin | Glucophage | p.o daily | 850, 1000. up to 3000 day |
 |
| GLP receptor agonist | Liraglutide | Victoza | s.c. daily | 0.6mg 1.2mg 1.8mg |
 |
| Exenatide | Bydureon | s.c weekly | 2 mg |
 | |
| Dulaglutide | Trulicity | s.c weekly | 0.75mg/0.5mL 1.5mg/0.5mL 3mg/0.5mL 4.5mg/0.5mL | ||
| SGLT2 inhibitor | Empagliflozin | Jardiance | p.o daily | 10 mg 25 mg | |
| Dapagliflozin | Forxiga | p.o daily | 5mg 10 mg |
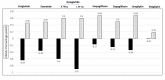
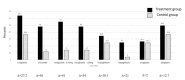
Five randomized control studies assessed the impact of these drugs in children with T2D. The inclusion criteria were consistent across all studies and included an age above 10 years, a BMI above the 85th percentile, and minor variations in HbA1c levels (between 7.0 and 11.0%, or 6.5% and 10%). The characteristics of the participants and outcomes are detailed in Table 3. Figure 3 depicts HbA1c results vs. placebo for each medication, and Figure 4 depicts the percent of individuals with HbA1c less than 7% at the end of each study.
Table 3.
Effects of Various Medications on Glycemic and Anthropometric Parameters in Adolescents with Type 2 Diabetes After 26 Weeks of Treatment
| Liraglutide | Exenatide | Dulaglutide 0.75 mg | Dulaglutide 1.5 mg | Empagliflozin | Dapagliflozin | Linagliptin | Sitagliptin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tx | Pl | Tx | Pl | Tx | PL | Tx | PL | Tx | PL | Tx | PL | Tx | Pl | Tx | Pl | |
| Number | 66 | 68 | 59 | 24 | 52 | 51 | 52 | 51 | 52 | 5 | 39 | 33 | 53 | 53 | 95 | 95 |
| Age (years) | 14.6 | 14.6 | 15 | 16 | 14.7 | 14.2 | 14.7 | 14.2 | 14.6 | 14.4 | 16.1 | 14.4 | 14.6 | 14.4 | 14.3 | 13.7 |
| Baseline HbA1c )%( | 7.9 | 7.7 | 8.1 | 8.1 | 7.9 | 8.1 | 8.2 | 8.1 | 8.0 | 8.05 | 7.95 | 7.85 | 8.05 | 8.05 | 7.4 | 7.6 |
| HbA1c change | -0.64 | 0.46 | -0.36 | 0.49 | -0.6 | 0.6 | -0.9 | 0.6 | -0.17 | 0.68 | -0.25 | 0.5 | 0.33 | 0.68 | -0.01 | 0.18 |
| HbA1c ETD | 1.06 | 0.85 | 1.2 | 1.5 | 0.84 | 0.75 | 0.35 | 0.19 | ||||||||
| % HbA1c <7% | 63.7 | 36.5 | 48 | 35.1 | 55 | 14 | 48 | 14 | 34.6 | 24.5 | 25 | 4 | 26.5 | 24.5 | 49.5 | 36.8 |
| Baseline BMI (kg/m2) | 34.6 | 33.3 | 36.9 | 35.4 | 33.6 | 34.3 | 34.3 | 34.3 | 35.5 | 36.1 | 31.3 | 33.6 | 36.5 | 36.1 | 0.0 | -0.7 |
| BMI ETD | -0.25 | -0.21 | ND | ND | -0.2 | 0 | -0.1 | 0 | ND | ND | -0.08 | -0.11 | ND | ND | -0.7 | |
| Weight change (kg) | -2.3 | -0.99 | -0.59 | 0.63 | 0.3 | 0.1 | 0.2 | 0.1 | -0.79 | -0.04 | ND | ND | 1.42 | -0.04 | -1.1 | 0.06 |
| Weight ETD | -1.3 | -1.2 | 0.2 | 0.1 | -0.75 | 1.46 | 1.04 | |||||||||
| Systolic BP ETD | 0.03 | -2.9 | 3.8 | 2.5 | -1.42 | 1.9 | 0.91 | |||||||||
|
Diastolic BP
ETD | -1.08 | ND | -0.8 | -2.6 | 0.02 | -0.5 | 1.5 | |||||||||
Pl=placebo, Tx= treatment, ETD = estimated treatment difference, ND= no data, placebo group for Empagliflozin and Linagliptin was the same.
METFORMIN
Metformin is a biguanide antihyperglycemic used in conjunction with diet and exercise to control glycemia. Metformin is considered the preferred first-line agent for treating T2D in pediatric patients 10 or older (50). Its onset of action is around 1.5 hours, and its total duration of action is 16-20 hours. Metformin exerts its glucose-lowering effect through various mechanisms, including inhibiting hepatic gluconeogenesis, increasing glucose uptake in skeletal muscles, reducing adipogenesis, and activating brown adipose tissue, leading to enhanced thermogenesis. Additionally, metformin decreases the absorption of glucose from the intestine and downregulates inflammation.
Although it is the first line of treatment for children and adolescents with T2D, prospective, randomized, controlled, long-term studies are limited. In a randomized control study involving 82 children and adolescents, 42 received metformin at doses up to 2000 mg/day, and 40 received placebo (51). After 16 weeks, mean HbA1c levels were lower in the metformin group compared to the placebo (7.5% and 8.6%, respectively). The metformin group also experienced a mean weight decrease of -1.5 kg compared with a mean increase of 0.9 kg in the placebo group. The mean BMI changed by -0.5 units in the treatment group vs. -0.4 units in the placebo group. In another study, metformin (500–1000 mg twice daily) was compared to glimepiride, a sulfonylurea (1–8 mg once daily), for 24 weeks (52). Both metformin (-0.71%) and glimepiride (-0.54%) groups showed significant reductions from baseline HbA1c levels. A total of 48.1% of metformin-treated and 42.4% of glimepride-treated participants achieved HbA1c levels below 7.0% at week 24. While the incidence of hypoglycemia was similar in both groups, metformin resulted in less weight gain compared to glimepiride. In a large observational study (53), 927 youth aged 13.7±2.0 years old, who had been diagnosed with T2D for a median of 2 months and had a baseline HbA1c of 7.7±2.2%, were treated with metformin. After a median of 71 days, the mean HbA1c change was -1.33, and the mean weight change -0.43 kg (53,54).
Metformin-related side effects are gastrointestinal, including diarrhea, abdominal pain, bloating, nausea, and decreased appetite, which occur in about 50% of users. Gastrointestinal side effects are usually transient and improve over time. Another potential side effect of metformin is vitamin B12 deficiency, and regular monitoring is indicated, though no cases of vitamin B12 deficiency were identified in the TODAY study. Lactic acidosis is a concerning side effect associated with metformin use in adults. It occurs in the presence of hypoperfusion or hypoxemia; however, it has rarely been reported in children and adolescents.
GLUCAGON-LIKE-PEPTIDE 1 (GLP1)
Glucagon-like peptide-1 (GLP-1) is a peptide hormone primarily produced by intestinal enteroendocrine cells at low basal levels that rapidly increases within minutes of food consumption. GLP-1 plays a vital role in regulating meal-related glycemic excursions by enhancing insulin secretion and inhibiting glucagon secretion. Additionally, GLP-1 slows gastric emptying and reduces food intake through a central effect on appetite, which contributes to weight loss. However, GLP-1 has a short half-life of approximately 2 minutes, as it is rapidly degraded by dipeptidyl peptidase-4 (DPP-4). To enhance GLP-1 activity, medications such as DPP-4 inhibitors and GLP-1 receptor agonists have been developed.
The story of the development of GLP1 agonists is truly fascinating. It begins with the venomous Gila monster (Heloderma suspectum), native to New Mexico and Arizona. H. suspectum, a long-lived and reclusive species, spends a significant portion of its life underground. Although its venomous bite causes pain and weakness, it is rarely fatal to adult humans. In 1990, endocrinologist Dr. John Eng, while analyzing the venom to identify new hormones, identified a peptide named exendin-4. He discovered that exendin-4 had a remarkable ability to stimulate the synthesis and release of insulin from β-cells in the pancreas. Interestingly, exendin-4 closely resembled GLP-1. However, while GLP-1 remains active for about two minutes, the effect of exendin-4 persists for several hours. Preclinical studies demonstrated that a single daily injection of exendin-4 normalized blood glucose concentrations in mice with diabetes. Following extensive clinical testing, exenatide, an analog of exendin-4, was found to be safe and effective, leading to its FDA approval for adults in 2005.
Of note, in a 2-year rat carcinogenicity study involving prolonged-release exenatide, an increased incidence of thyroid adenoma and C-cell carcinoma was observed at doses ≥2-fold the human systemic exposure. The clinical relevance of these adverse findings is currently unknown. However, GLP-1 analogs are contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or in patients with multiple endocrine neoplasia type 2 (MEN 2), as well as in patients with a serious hypersensitivity reaction. Patients should be counseled about the potential risk of MTC associated with the GLP1 receptor agonists, and it is important to inform them about the symptoms that may indicate thyroid tumors, such as a neck mass, dysphagia, dyspnea, and persistent hoarseness. Routine monitoring of serum calcitonin levels or use of thyroid ultrasound for the detection of MTC is of uncertain value.
Side effects include serious hypersensitivity reactions, including anaphylactic reactions and angioedema. Additionally, acute pancreatitis, including fatal and nonfatal hemorrhagic or necrotizing pancreatitis, and the acute onset of gallbladder disease, such as cholelithiasis or cholecystitis, have been reported in GLP-1 receptor agonists trials and post-marketing surveys (55).
Liraglutide-Victoza®
Children aged 10 to less than 17 years with T2D were randomly assigned 1:1 to receive subcutaneous liraglutide (up to 1.8 mg per day) or placebo for a 26-week double-blind period, followed by a 26-week open-label extension (56). At the 26-week visit, the mean HbA1c had decreased by 0.64 percentage points with liraglutide and increased by 0.42 percentage points with placebo, resulting in an estimated treatment difference of -1.06 percentage points. By 52 weeks, the difference had increased to -1.30 percentage points (Table 3). In the liraglutide group, 63.7% of patients achieved HbA1c levels of less than 7.0%, compared with 36.5% in the placebo group. On the other hand, there was no statistical benefit of liraglutide over placebo in lowering BMI or blood pressure (Table 3). The overall rate of adverse events, including gastrointestinal adverse events, was higher among patients receiving liraglutide. The U.S. Food and Drug Administration approved liraglutide injection for the treatment of pediatric patients 10 years of age or older with T2D. Liraglutide was the first non-insulin drug approved to treat T2D in pediatric patients since metformin was approved for pediatric use in 2000.
Mode of administration: The recommended initial dose is 0.6 mg subcutaneously once daily for the first week, followed by an increase to 1.2 mg once daily. It’s important to note that the initial dose of 0.6 mg once daily is primarily intended to mitigate gastrointestinal adverse effects and does not provide glycemic control. If adequate glycemic control is not achieved, the dose can be further increased to 1.8 mg once daily.
Exenatide-Bydureon
The effectiveness of once-weekly exenatide 2 mg (Bydureon AstraZeneca) in youth with suboptimally controlled T2D was evaluated (57). At 24 weeks, exenatide demonstrated superiority over the placebo in reducing HbA1c levels (a decrease of -0.36% with exenatide compared to +0.49% with placebo), resulting in a significant between-group difference of 0.85 percentage points. Notably, no major hypoglycemic events were reported. and the most frequently reported adverse events included gastrointestinal symptoms such as nausea, diarrhea, and vomiting, along with injection site reactions such as pruritus, erythema, and nodules. Clinically meaningful differences were not observed in change from baseline in BMI Z score, body weight, or waist circumference, nor were there significant differences in blood pressure. The prolonged-release suspension of exenatide administered via injection with a pre-filled pen has received approval from the European Union and the FDA for the treatment of T2DM in children and adolescents aged 10 years and older.
Mode of administration: Bydureon (2 mg per dose) should be administered once every 7 days. The dose can be administered at any time of day, with or without meals. Bydureon is not recommended as first-line therapy for patients with inadequate glycemic control on diet and exercise because of the uncertain relevance of the rat thyroid C-cell tumor findings to humans.
Dulaglutide-Trulicity
Dulaglutide (Trulicity) is a GLP-1 receptor agonist administered via subcutaneous injections once a week. In a randomized, double-blind, phase 3 trial involving youth with inadequately controlled T2DM, the efficacy and safety of dulaglutide treatment in reducing HbA1c levels at 26 weeks were assessed (58). Remarkably, there was 99% adherence to treatment in this trial. Treatment resulted in a significant reduction in HbA1c relative to placebo; at week 26, HbA1c decreased by 0.8 percentage points but increased by 0.6 percentage points in the placebo group (1.4 percentage point difference). Gastrointestinal symptoms were among the most common adverse events, but they were primarily mild and were most likely to occur soon after the initiation of therapy. There were no clinically meaningful differences in the incidence or annual rate of hypoglycemia between the dulaglutide groups and the placebo group. Body weight did not decrease significantly following treatment.
Mode of administration: The recommended initiating dose of Trulicity is 0.75 mg once weekly, administered subcutaneously. The dose may be increased to 1.5 mg once a week for additional glycemic control. Trulicity can be administered any time of day, with or without meals, and should be injected subcutaneously in the abdomen, thigh, or upper arm. The day of weekly administration can be changed, if necessary, as long as the last dose was administered 3 days (72 hours) or more before. Trulicity and insulin should not be mixed in the same syringe and must be administered as two separate injections at two different injection sites.
SODIUM-GLUCOSE TRANSPORT PROTEIN 2 - SGLT2 INHIBITORS
Sodium-glucose transport protein 2 (SGLT2) inhibitors belong to a class of medications that modulate sodium-glucose transport proteins in the nephron. These inhibitors primarily target the SGLT2 proteins, expressed in the renal proximal convoluted tubules, to reduce the reabsorption of filtered glucose and sodium (59). Apart from their role in controlling glucose concentrations, SGLT2 inhibitors have demonstrated substantial cardiovascular benefits, particularly reducing heart failure events, for adults with T2D. In adults, the FDA-approved indications for SGLT2 inhibitors include the reduction of major adverse cardiovascular events in individuals with T2D and established cardiovascular disease, as well as the reduction of the risk of eGFR decline in patients with chronic kidney disease at risk of progression. The most common reported adverse events associated with SGLT2 inhibitors include female genital mycotic infections, urinary tract infections (including urosepsis and pyelonephritis), as well as nausea and constipation. Most importantly, SGLT2 inhibitors are associated with an almost three-fold increased risk for DKA in adults. The risk for DKA is highest for canagliflozin, followed by empagliflozin and dapagliflozin. Furthermore, individuals may develop euglycemic DKA, i.e., a triad of increased anion gap acidosis, ketosis, and a serum glucose level below 250 mg/dL. The association between SGLT-2 inhibitors and euglycemic DKA appears to be secondary to their noninsulin-dependent glucose clearance, compensatory hyperglucagonemia, and volume depletion. Currently, euglycemic DKA has not been reported among youth with T2D taking SLGT2 inhibitors.
Empagliflozin - Jardiance®
A total of 105 children and adolescents were randomly assigned 1:1 to receive empagliflozin 10 mg or placebo (60). The adjusted mean change in HbA1c from baseline at week 26 was significantly greater in the empagliflozin group (–0·84%). Changes in body weight and blood pressure did not reach statistical significance. Adverse events occurred in 64% of participants in the placebo group and 77% in the empagliflozin group up to week 26, including severe adverse events in 4% of participants in the placebo group and 2% in the empagliflozin group. Mild and moderate hypoglycemia were the most frequently reported adverse events, with higher rates for those on active drug treatment compared with placebo; no severe hypoglycemic events were reported, and there were no episodes of DKA or necrotizing fasciitis associated with empagliflozin treatment. An increased incidence of bone fractures has been reported in adults, leading the American Diabetes Association to recommend avoiding SGLT-2 inhibitors in patients with a risk factor for fractures. Furthermore, SGLT2 inhibitors are not recommended for glycemic lowering in patients with T2D with an eGFR less than 30 mL/min/1.73 m2.
Mode of administration: Empagliflozin is an oral medication dosed at either 10 mg daily or 25 mg daily. The recommended dose is 10 mg once daily in the morning, taken with or without food. If tolerated initially, dosing may increase up to 25 mg.
Dapagliflozin - Farxiga®
Participants aged 10–24 years with T2D and HbA1c concentrations ranging from 6.5 to 11% were given oral dapagliflozin 10 mg or placebo during a 24-week double-blind period (61); 39 participants were assigned to dapagliflozin and 33 were assigned to placebo. After 24 weeks, the mean change in HbA1c concentration was −0·25% for dapagliflozin and 0·50% for the placebo group; the between-group difference was −0·75%, which did not reach statistical significance. However, in a sub-analysis, excluding participants with poor compliance, the HbA1c change was -0.51%, while it was 0.62% for the placebo group, resulting in a between group significant difference of 1.13%.
Adverse events occurred in 69% of participants assigned to dapagliflozin and 58% of those assigned to placebo over a 24-week period. Over 52 weeks, adverse events occurred in 74% of participants who received dapagliflozin. Hypoglycemia was noted in 28% of participants assigned to dapagliflozin and in 18% of those assigned to placebo, none were considered serious adverse events. Notably, there were no reported episodes of DKA.
Mode of administration: To improve glycemic control the recommended starting dose is 5 mg once daily, taken in the morning. Increase dose to 10 mg once daily in patients tolerating 5 mg who require additional glycemic control. Dapagliflozin is not recommended for use in patients receiving loop diuretics or who are volume depleted, e.g., due to acute illness (such as gastrointestinal illness).
DIPEPTIDYL PEPTIDASE 4 (DPP 4) INHIBITORS
DPP-4 is a ubiquitous enzyme that degrades GLP-1 and GIP (gastric inhibitory peptide), resulting in a short half-life. By inhibiting the DPP-4 enzyme, DPP-4 inhibitors increase the levels of GLP-1 and GIP. This, in turn, enhances beta-cell insulin secretion, thus reducing postprandial and fasting hyperglycemia (62).
Linagliptin (Tradjenta)
105 children and adolescents were randomly assigned 1:1 to oral linagliptin (5mg daily) or placebo (60). The adjusted mean HbA1c change from baseline at week 26 was 0.33% for linagliptin and 0·68% for the placebo group; the difference was not statistically or clinically significant. None of the adverse events of special interest occurred with DPP-4 inhibitors, such as hypersensitivity reactions, angioedema, angioedema-like events and anaphylaxis, skin lesions, pancreatitis, or hepatic injury, were present. Linagliptin is not approved for adolescents with T2D (60).
Sitagliptin (Januvia)
A total of 190 children and adolescents were randomly assigned 1:1 to oral sitagliptin 100 mg daily or placebo (63). The adjusted mean change in HbA1c from baseline at week 20 was -0.01% for sitagliptin and 0·18% for the placebo group, resulting in a between-group difference of 0.19%, which was not significant. There was no significant difference in the percentage of participants with HbA1c below 7%, with 49.5% in the sitagliptin group and 36.8% in the placebo group. Notably, after 54 weeks, the HbA1c in the sitagliptin group decreased from 7.4% at baseline to 7.1%. Sitagliptin is not approved for adolescents with T2D (50).
ANTI-OBESITY PHARMACOTHERAPY FOR TREATMENT OF PEDIATRIC TYPE 2 DIABETES
As obesity is a significant risk factor for the development of T2D, the American Diabetes Association (ADA) recommends the use of anti-obesity medications as adjuvant therapy for adults with both T2D and overweight/obesity. In adults, adding anti-obesity medications to a diabetes regimen can improve glycemic control, reduce weight, and decrease the use of anti-diabetes medication (64). Semaglutide and tirzepatide have shown promise in reducing weight in adults with obesity and T2DM compared to liraglutide (65). Tirzepatide is a novel dual glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist. A study to evaluate tirzepatide in pediatric and adolescent participants with T2D, who are inadequately controlled with metformin, or basal Insulin, or both (SURPASS-PEDS) is currently ongoing.
Liraglutide 3.0mg/day
A higher dose of liraglutide of 3.0 mg/day (Saxenda®) is FDA-approved for obesity in youth aged 12 years and older who weigh ≥ 60kg or have an initial BMI ≥ 30kg/m2. The Satiety and Clinical Adipose-Liraglutide Evidence Trial in Adolescents with and without T2D (SCALE-Teens) was a double-blind, placebo-controlled RCT that randomized 251 adolescents to either liraglutide 3.0 mg/day or placebo. However, only two adolescents with T2D were enrolled. Consequently, no conclusive findings can be drawn regarding the impact of high-dose liraglutide on reducing BMI and improving HbA1c in young individuals with T2D. Since almost all adolescents with T2DM have overweight or obesity and could potentially meet the prescribing criteria, it is reasonable to speculate that the results might resemble those observed in adult trials for T2D, potentially leading to reductions in weight and BMI as well as improvements in hyperglycemia.
Semaglutide 2.4mg Weekly
Semaglutide in its once-weekly injectable GLP-1RA form at doses up to 2.4 mg weekly (Wegovy®) has shown significant weight loss in obese children and has now been approved for treatment of obesity in adolescents aged 12 years and older (66). The mean change in BMI from baseline to week 68 was −16.1% with semaglutide and 0.6% with placebo. Of note, HbA1c decreased 0.4 percentage points in the treatment group compared with 0.1 in the placebo. However, the impact on youth with T2D has not yet been studied.
Semaglutide injection up to 2.0 mg (Ozempic®) and oral semaglutide up to 14 mg (Rybelsus®) are approved for adults with T2D, but studies in youth with T2D have not yet been reported.
SUMMARY: WHICH TREATMENT DO I RECOMMEND TO MY PATIENT?
The answer to this pivotal question hinges on the art of personalized medicine. First, it is crucial to recognize that 50% of youth with T2D can maintain good glycemic control on metformin monotherapy, making this agent still an excellent choice for initial therapy. However, several measures can predict the likelihood of glycemic deterioration, including decreased insulin secretion, impaired insulin processing (elevated proinsulin/insulin ratio), and HbA1c concentration after a few months on monotherapy. Longitudinally, a rising proinsulin and an increase in HbA1c of more than 0.5 percentage points over any 6-month period are predictive of loss of glycemic control on monotherapy (67).
If these parameters suggest a risk for deterioration, the next step is to consider the therapeutic objective(s). Is it primarily elevated HbA1c? If so, the best approach may involve selecting a medication known for its efficacy in reducing HbA1c. Is obesity the central concern for the patient? In such cases, exploring pharmaceutical interventions tailored for weight management may be prudent. Does the individual have concerns about needle-based treatments? In such instances, favoring orally administered medications could offer a more suitable solution. If there is an elevated risk of cardiac or renal complications, it would be wise to consider options from the SGLT2 inhibitor group or GLP1 agonist group. In essence, treatment needs to be tailored to the unique needs and circumstances of each individual, ensuring that treatment decisions align with their specific health challenges and objectives.
BARIATRIC SURGERY
Weight reduction plays a crucial role in the management of T2D because weight loss is associated with improved insulin resistance and glycemic status. Considering the limited efficacy of lifestyle and pharmacologic interventions in treating severe obesity and obesity-related T2D, along with the remarkable weight reduction and remission of T2D observed in youth who undergo bariatric surgery the ADA recommends considering bariatric surgery for adolescents with T2D who have a BMI >35 kg/m2 (68). The two most common bariatric surgeries performed in adolescents with T2D are laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass (LRYGB).
Impact of Bariatric Surgery on Glycemic Control
Studies have demonstrated improvement in HbA1c across all age groups. In one study, sixty-four pediatric patients diagnosed with morbid obesity and T2D underwent LSG surgery. The patients’ ages ranged from 5 to 14 years old. Their BMI decreased from 44.6±9.3 to 34.8 ± 9.6 kg/m2.Their HbA1c decreased from 6.0±0.8% prior to LSG surgery to 5.4±0.4%, 12 months post-surgery, a change of 10.9% (p = 0.001) (69). There was no significant difference in post-operative HbA1c between the age groups.
Impact of Bariatric Surgery on T2D Remission
Bariatric surgery in adolescents with T2D has a better outcome compared to medical treatment with metformin alone or in combination with rosiglitazone or intensive lifestyle intervention with insulin therapy given for glycemic progression (70). In a multicenter, nonrandomized, retrospective study of 202 obese adolescents with T2D (before the approval of new drugs for adolescents with T2D), 109 adolescents underwent surgery, and 93 adolescents received nonsurgical treatment (71(. In the surgery group, the remission rate for diabetes was 76%, compared to 6.5% in the medical treatment group. The remission was sustained for the two years of follow-up. Of note, LRYGB had better effects on weight loss and glycemic control than LSG.
Impact of Bariatric Surgery on Kidney Outcomes
In a three-year longitudinal study, kidney outcomes in youth following bariatric surgery were assessed. Improvement in albuminuria and GFR was observed in those with pre-operative kidney impairment. Among participants with a preoperative eGFR < 90 ml/min/1/73m2, eGFR improved from a mean of 76 ml/min/1.73m2 to 102 ml/min/1.73m2. Similarly, in those with an elevated albumin-to-creatinine ratio at baseline (ACR ≥30mg/g), the median ACR decreased from 74 mg/g to 17 mg/g. Estimated GFR and albuminuria remained stable in those without any evidence of pre-operative impairment. The effect of bariatric surgery on diabetic kidney disease was also investigated in adolescents with severe obesity and T2D five years after surgery compared to adolescents receiving medical management in the TODAY study (72). Elevated albuminuria was present in 21% of those receiving medical management at baseline, and it increased to 43% at 5 years. Conversely, albuminuria decreased from 27% prior to surgery to 5% at the 5-year follow-up. In adjusted analyses, the medical group had 27-fold higher odds of diabetic kidney disease at 5 years compared to Teen-LABS participants (72-73).
Impact of Bariatric Surgery on CVD Events
In another analysis, the risk for CVD events in two cohorts of adolescents with T2D and severe obesity undergoing medical or surgical treatment of T2D was assessed. Thirty adolescents who underwent bariatric surgery were matched to 63 who had received metformin alone or in combination with rosiglitazone or an intensive lifestyle intervention, with insulin therapy given for glycemic progression. The BMI of those who underwent bariatric surgery was 54.4±9.5 kg/m2, and that of the medical group was BMI 40.5±4.9 kg/m2. Although the baseline likelihood of CVD events was higher in the group that underwent bariatric surgery, one year after the surgery, the event risk was significantly lower and sustained at 5-year follow-up, whereas medical therapy was associated with an increase in risk among adolescents with T2D and severe obesity (74).
Adverse Effects of Bariatric Surgery
Bariatric surgery in adolescents can lead to various potential complications beyond the first postoperative month. The most commonly reported include gastroesophageal reflux, incisional hernia, treatment failure requiring operative revision, and nutritional deficits (50). Less frequent complications include stomach cancer, liver necrosis, gallbladder disorders, pancreatic disorders, acute kidney failure, neuromuscular complications, skin complications, and rarely mortality (51).
Summary
In summary, bariatric surgery currently outperforms medical management of T2D in adolescents and is the most efficacious treatment for youth-onset T2D, albeit with significant risks and economic implications. However, given the rapidly emerging medical therapies for weight reduction in adults and youth, many of which (e.g., dual-incretin analogs, triple-G agonists (75)) are now achieving degrees of weight loss approaching those seen following surgery, the individual roles of surgical and medical treatment for youth-onset T2D will need continued reassessment in the coming years.
COMPLICATIONS AND COMORBIDITIES AMONG ADOLESCENTS WITH T2D
Large cohort studies investigating the incidence of complications in children and teenagers with T2D unequivocally demonstrated a higher incidence of complications, both compared to the incidence of complications in T2D in adults and in T1D in children. In a multicenter observational study conducted from 2011 to 2020, the cumulative incidence of diabetic complications was assessed in 500 adolescents who had participated in the TODAY study (76). After only 13 years from diagnosis, higher complication rates were observed compared to those reported for pediatric patients with T1DM or for adults with T2D. The first striking finding from this report is the participants’ poor glycemic control despite participation in a well-resourced clinical trial. While glycemic control in adolescents with T1DM tends to improve with age, a gradual deterioration occurred in adolescents with T2D throughout the follow-up period. Approximately 45.0% had HbA1c of at least 10%, and an additional 20% were in the range of 8-10%. Their BMI remained consistently in the range of 35.0 to 37.5 kg/m2. It is important to note that poor glycemic control and obesity are associated with early complications. The complications associated with T2D in children affect various systems (Figure 5).

Figure 5.
Comprehensive Overview of Type 2 Diabetes (T2D) Complications in Adolescents. A comprehensive visual representation of the diverse range of complications affecting major physiological systems that can arise in adolescents with T2D.
On average, thirteen years after the initial diagnosis of T2D, incidence rates for hypertension, dyslipidemia, diabetic kidney disease, and neuropathy were 67.5%, 51.6%, 54.8%, and 32.4%, respectively. Additionally, retinopathy was present in 51% of the participants. One-fifth of the cohort (21.3%) had two complications, and 7.1% had three. There were seventeen serious cardiovascular events, including myocardial infarctions, six cases of congestive heart failure, three cases of coronary artery disease, and four stroke events. Additionally, six deaths were reported. This rapid development of complications was associated with severe insulin resistance and poor socioeconomic circumstances.
Hypertension
Hypertension, defined as blood pressure ≥ 95th percentile for age, sex, and height or systolic BP ≥ 130/80 mm Hg, is often present at the time of diagnosis in adolescents with T2D. In a study of 391 children from Hong Kong under 18 years of age, 22.5% had hypertension at diagnosis. In the TODAY study, 11.6% were hypertensive at the time of diagnosis (77) and the cumulative incidence of hypertension was 67.5% at a mean age of 26.4±2.8 years and 13.3±1.8 years from diagnosis (78). A systematic review and meta-analysis of 31 international studies on 4363 children with T2D aged 6.5 to 21 years at diagnosis found a pooled prevalence of hypertension of 25.33% (95% CI, 19.57%-31.53%) (79). Male participants had a higher hypertension risk than female participants (odds ratio 1.42 [95% CI, 1.10-1.83]).
Initial treatment involves lifestyle modification, aiming to not only enhance glycemic control but also incorporate a diet that is low in sodium and abundant in fruits, vegetables, whole grains, low-fat dairy products, and lean proteins. The Dietary Approaches to Stop Hypertension (DASH diet) is highly recommended for its effectiveness in reducing high blood pressure and supporting weight loss. In addition to lifestyle modification, the ADA recommends starting Angiotensin-Converting Enzyme inhibitors (ACEis) or Angiotensin Receptor Blockers (ARBs). Due to the potential teratogenic effects, the use of reliable contraception should be encouraged in individuals of childbearing age (68). There are no agents specifically approved for use in youth with T2D, however, a once-daily medication is recommended for better compliance (80,81).
Nephropathy
The primary microvascular diabetic complication in adolescents with T2D is diabetic kidney disease (DKD), which develops in 25–40% of T2D patients and is associated with rapid progression and poor prognosis. The severity and risk of rapid deterioration in young people with T2D are reflected in the fact that microalbuminuria is reported at diagnosis among adolescents with T2D. In a recent systematic review and meta-analysis (79) that included 14 studies of 2250 children and adolescents with T2D, the prevalence of albuminuria was 22.17% (95%CI, 17.34%-27.38%), and the pooled prevalence of macroalbuminuria among 730 children and adolescents was 3.85% (79). In the SEARCH study (82) after a duration of 8 years, the prevalence was 19.9% among adolescents with T2D compared with 5.8% in those with T1D.
The accelerated development of kidney damage is attributed to inadequate glycemic control and the presence of various risk factors, including obesity, dyslipidemia, insulin resistance, hypertension, elevated serum uric acid, female sex, and the presence of chronic inflammation (83).
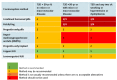
While albuminuria is the earliest clinical sign of diabetic kidney disease (DKD), the natural history of DKD begins with hyperfiltration, characterized by an increase in glomerular filtration rate (GFR) >120 mL/min/1.73 m2 as a consequence of obesity and impaired glucose tolerance (84). This increase predicts deterioration before other clinical signs appear. The second stage in the evolution of kidney dysfunction, still without clinical manifestations, is a mild reduction in GFR (60-89 mL/min/1.73 m2) (Figure 6). Structural changes of the kidney are typical at this stage, yet they are often reversible, making this a critical time for risk factor reduction (84).

Figure 6.
Heat map depicting diabetes kidney disease (DKD) staging by GFR and albuminuria and suggested treatments. Adapted from Naaman SC, Bakris GLJDC. Diabetic Nephropathy: Update on Pillars of Therapy Slowing Progression. 2023;46:1574-86.
Treatment in these early stages involves achieving adequate glycemic control along with lifestyle modification, including increasing physical activity, improving sleep quality, and quitting smoking. Additionally, a healthy diet with lower salt and potassium intake, along with a low protein intake of 0.8 g/kg/day, is recommended (68). Pharmacological treatment includes control of blood pressure and managing dyslipidemia. Theoretically, medications that improve glycemic control would also provide benefits for preventing and/or treating DKD. GLP1 receptor agonists are reported to have a renal protective effect in adults with T2D. A recent meta-analysis of approximately 60,000 adults with T2DM treated with GLP-1 receptor agonist found a significant reduction in the composite kidney outcome (development of macroalbuminuria, doubling of serum creatinine, 40% or greater decline in eGFR, kidney replacement therapy, or death from kidney disease) when compared to placebo, as well as a trend towards a reduction in worsening kidney function (84). Similarly, the hemodynamic and natriuretic effects of SGLT2 inhibition are also postulated as protective mechanisms for DKD (85). However, studies in adolescents with T2D did not show an effect of systolic or diastolic blood pressure (Table 3). Of note, these studies involved relatively small groups of youth, and were short-term. Larger, long-term studies are needed, as well as additional therapeutic options for reducing the risk of DKD, such as mineralocorticoid receptor antagonists (MRAs), and endothelin receptor antagonists (ERAs).
Dyslipidemia
Screening for dyslipidemia should be conducted once optimal glycemic control has been achieved or within 3 months after T2D diagnosis. Initial cholesterol screening can be performed non-fasting. The recommended target values are triglycerides <150 mg/dL (1.7 mmol/L), HDL cholesterol >35 mg/dL (0.91 mmol/L), and LDL cholesterol <100 mg/dL (2.6 mmol/L). If these levels are outside the normal range, medical nutritional therapy (MNT) should be initiated, including restricting caloric intake from fat to 20–30% and daily cholesterol intake to <200 mg/day, avoiding trans fatty acids, limiting saturated fats to <7%, and controlling mono and poly unsaturated fats to 10-15%. If, after 6 months of dietary intervention, the LDL-C remains >130 mg/dL, statin therapy should be initiated, with a goal of achieving LDL-C <100 mg/dL. Currently, there are 7 approved statins for use in children and adolescents: Lovastatin, Simvastatin, Atorvastatin, and Fluvastatin [children ≥10 years old], Pitavastatin and Pravastatin [> 8 years], and Rosuvastatin [>6 years old]. It is important to note that due to potential teratogenic effects, individuals of childbearing age should receive reproductive counseling, and statins should be avoided in individuals of childbearing age who are not using reliable contraception. If triglyceride levels are greater than 400 mg/dL (4.7 mmol/L) in a fasting state or exceed 1,000 mg/dL (11.6 mmol/L) in a non-fasting state, it is imperative to optimize glycemic control and initiate fibrate therapy. This is essential for reducing the risk of pancreatitis.
In a study assessing whether clinicians are sufficiently aggressive in treating diabetes-related hyperlipidemia in youth, among 278 youth with T2D <10 years, 57% had LDL-C exceeding 100 mg/dL, 24% had LDL-C at or above 130 mg/dL, and 9% had LDL-C surpassing 160 mg/dL (86). Additionally, 29% had hypertriglyceridemia, and 44% had HDL-C levels below 40 mg/dL. However, only 5% of these youth were prescribed lipid-lowering medications.
Polycystic Ovarian Syndrome (PCOS)
There is a strong association between T2D in adolescent girls and polycystic ovary syndrome (PCOS). A systematic review and meta-analysis comprising 6 studies that involved 470 girls diagnosed with T2D, with an age range at diagnosis between 12.9 and 16.1 years, revealed that the prevalence of PCOS was estimated at 19.58% (87). PCOS is associated with a range of metabolic diseases, including hypertension and dyslipidemia. Furthermore, it is associated with a higher prevalence of cardiovascular risk factors, such as higher carotid intima thickness, β stiffness index, and reduced arterial compliance. Additionally, adolescent girls with PCOS have an increased likelihood of experiencing psychiatric comorbidities, such as anxiety and depression, which can negatively impact their health-related quality of life. A menstrual history should be taken on every girl with T2D at the diagnosis and at every follow-up visit. Metformin, in addition to lifestyle modification, is likely to improve menstrual cyclicity and hyperandrogenism in female individuals with T2D.
Pregnancy and Contraception
In the TODAY study, the maternal and offspring outcomes of young women with youth-onset T2D who experienced one or more pregnancies were evaluated (88). Over a span of up to 15 years, a total of 260 pregnancies were reported by 141 women. Their average age was 21.5±3.2 years, a mean BMI of 35.6±7.2 kg/m2, and an average diabetes duration of 8.1±3.2 years (88). Complications during pregnancy were identified in 65% of the women. Elevated HbA1c levels of ≥8% were observed in 31.9% of the pregnancies, and chronic hypertension complicated 35% of them. Pregnancy loss was observed in 25.3% and preterm birth occurred in 32.6% of pregnancies. Among the offspring, 7.8% of the babies were classified as small for gestational age, 26.8% as large for gestational age, and 17.9% fell into the macrosomic range. Other complications and congenital anomalies were noted in 10% of infants, including anencephaly, renal anomalies, and complications related to prematurity. The rate of known miscarriage for the entire study was 12.3%. The rate of known stillbirths in the cohort was 3%, which is more than triple the reported national rates.
The impact of T2D during pregnancy extends beyond the immediate prenatal and birth periods, exerting long-term effects on offsprings. Data pertaining to the diagnosis of diabetes in biological mothers were available for 621 participants in the TODAY study, of whom 301 had never been diagnosed with diabetes, 218 were diagnosed either before or during pregnancy, and 102 were diagnosed after pregnancy (89). For biological fathers, the data were available for 519 participants, with 352 having no history of diabetes and 167 having paternal diabetes. Notably, it was found that maternal diabetes, but not paternal diabetes, was associated with lower beta-cell function and deterioration of glycemic control over time. These effects remained significant even after accounting for variables such as age, sex, race/ethnicity, and household income (89).
Contraception is indicated in adolescent girls with T2D for several compelling reasons. Firstly, it is a recommended treatment for managing the symptoms of PCOS. Secondly, these young women are at increased risk of unintended pregnancy, which can lead to poor outcomes. Lastly, it is imperative for those treated with potentially teratogenic medications such as ACEis, ARBs, and statins. Unfortunately, despite the evident need, preconception counseling was reported only in 16.3% of women prior to their first pregnancy and only 14.9% used any method of contraception prior to the first pregnancy (88). This gap in reproductive health care highlights the importance of healthcare providers and support staff addressing this issue promptly and effectively.
Although in theory, adolescent girls with T2D without severe obesity, micro- or macrovascular disease, or other cardiovascular risk factors can consider a wide range of contraceptive methods, the practical reality often differs. A significant proportion of these individuals are afflicted by severe obesity, and a considerable number also exhibit elevated blood pressure. Consequently, when morbid obesity, severe hypertension, micro- or macrovascular disease, or multiple cardiovascular risk factors are present, it is advisable to prioritize nonhormonal or progestin-only contraceptive methods (Figure 7) (90).
Diabetic Retinopathy
Diabetic retinopathy (DR) is one of the most important causes of visual loss worldwide. DR is often asymptomatic until the very late stages. Given the potential for a rapid rate of progression and the effectiveness of new therapies in slowing deterioration, it is imperative to routinely screen individuals with diabetes for the early detection of retinal changes. It is important to note that direct fundoscopy may be less sensitive than 7-field stereoscopic fundus photography for detecting retinopathy (91). Diabetic retinopathy (DR) is divided into two major forms: non-proliferative and proliferative, according to the presence of abnormal new blood vessels emanating from the retina. Non-proliferative DR consists of microvascular abnormalities (including microaneurysms, occluded vessels, and dilated or tortuous vessels) primarily in the macula and posterior retina, intraretinal hemorrhages, hard exudates; and the variable presence of nerve-fiber layer infarcts (cotton wool spots). Visual loss is mainly due to the development of macular edema. Proliferative DR is marked by the presence of neovascularization arising from the disc and/or retinal vessels, resulting in preretinal and vitreous hemorrhage, subsequent fibrosis, and traction retinal detachment resulting in visual loss.
In a meta-analysis that included 27 studies involving 5924 children and adolescents (91) with T2D duration between 6.5-21.0 years, 1.11% had DR less than 2.5 years after T2D diagnosis. The prevalence of DR increased over time, with rates of 9.04% at 2.5 to 5.0 years after T2D diagnosis, and 28.14% more than 5 years after T2D diagnosis. The global prevalence of diabetic retinopathy in pediatric T2D was found to be 6.99%. Optical coherence tomography (OCT) performed in adolescents with T2D in the TODAY study revealed that changes in retinal thickness correlated with HbA1c, fasting glucose, and blood pressure, illustrating the importance of intensive control of hyperglycemia, hypertension, and hyperlipidemia.(92)
Among the systemic strategies for the prevention and treatment of diabetic retinopathy, two randomized clinical trials, "The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study and the Action to Control Cardiovascular Risk in Diabetes (ACCORD)-Eye Study, documented a reduction in the risk of non-proliferative DR progressing in individuals using fenofibrate (93). The protective effects of fenofibrate have been attributed to its antioxidative, anti-inflammatory, anti-apoptotic, and anti-angiogenic properties. Other treatments include pan-retinal photocoagulation, intravitreal anti-vascular endothelial growth factor (VEGF) therapy, integrin antagonists, and anti-inflammatory agents. Corticosteroids remain important second-line therapies for patients with macular edema.
Diabetic Neuropathy
DIABETIC PERIPHERAL NEUROPATHY
Diabetic peripheral neuropathy (DPN) is a cause of significant disability and poor quality of life. Signs and symptoms include sensory loss, paresthesia, and pain. However, subclinical signs of DPN may precede the development of frank neuropathic symptoms, and systematic screening is required to identify DPN in its earliest stages. Studies from USA (78,94), Canada (95), and India (96) reported the prevalence of DPN in adolescents with T2D assessed by examination for foot abnormalities, distal vibration perception, and ankle reflexes (97). In the SEARCH study, the prevalence of peripheral neuropathy assessed after 8 years in 258 adolescents with T2D was 22%, compared with 7% in T1DM (98). Data from the TODAY Study Group revealed that at baseline, 1.0% of the participants had nerve disease, and the cumulative incidence at 15 years was 32.4% (78). The risk factors associated with peripheral neuropathy in youth are longer duration of diabetes, older age, male sex, and smoking.
Calcium channel a2δ ligands (gabapentin, pregabalin= Lyrica), serotonin- norepinephrine reuptake inhibitors, and tricyclic antidepressants are the most widely used medications for painful DN in adults. These medications are used to treat various presentations of neuropathic pain in children, but there have been no clinical studies devoted to painful DPN and no agents are licensed specifically for painful DN in childhood and youth (99). “Fortunately, painful DPN is relatively rare in children and youth.
AUTONOMIC NEUROPATHY
Diabetic autonomic neuropathy (DAN) is a common form of neuropathy in individuals with diabetes mellitus characterized by dysfunction due to impairment of peripheral autonomic nerves, with a wide spectrum of manifestations (100). DAN that involves autonomic fibers of the enteric nervous system may cause upper and lower gastrointestinal symptoms, including gastroesophageal reflux disease, gastroparesis, and constipation. Additional manifestations include bladder and sexual dysfunction. DAN also involves the cardiovascular system. Cardiovascular autonomic neuropathy (CAN), secondary to the pathology of the autonomic nerve fibers that innervate the heart and blood vessels, results in resting tachycardia, exercise intolerance, orthostatic hypotension, syncope, and silent myocardial infarction and ischemia (101). A reduction in heart rate variability is an early sign of CAN and was reported in 47% of youth with T2D after a mean disease duration of only 1.7 years (102). In the SEARCH study, the prevalence of CAN assessed after duration of 8 years in 252 adolescents with T2D was 17% compared with 12% in T1DM (103). CAN was associated with elevated triglycerides and increased urinary albumin excretion.
Cardiovascular Complications
T2D is a major risk factor for cardiovascular disease (CVD), including myocardial infarction and stroke. Markers for early CVD include increased arterial stiffness, carotid intima-media thickness, an alteration in the vascular endothelium, and cardiac autonomic neuropathy. Arterial stiffness prevalence was significantly higher among youth with T2D than those with T1D (104). Arterial stiffness (measured by pulse wave velocity from carotid-femoral, femoral-foot, and carotid-radial), was measured in 388 youth with T2D at a mean age of 21 years and a diabetes duration of 7.7±1.5 years (105). Higher arterial stiffness was associated with an adverse change in left ventricular diastolic function. Similarly, cardiac parameters were assessed in 177 individuals with T2D, with a mean age of 24.6±4.2 years and a disease duration of 10.3±3.5 years (106). Of these, 72% were females, and their HbA1c levels averaged 8.9±1.9. Their parameters were compared to those of individuals with T1D. The prevalence of increased atrial stiffness was significantly higher in those with T2D, at 75.7%, compared to only 23.4% in individuals with T1D. In addition, the prevalence of cardiac autonomic neuropathy was significantly higher in those with T2D, at 16.9%, compared to 9.0% in individuals with T1D. Participants with T2D exhibited a higher left ventricular mass index, lower systolic function, and lower diastolic function compared to those with T1D.
Adolescents with T2DM have increased carotid intima-media thickness (cIMT) compared to obese and normal weight groups; the group difference was detected early at 14 years of age (107).
Vascular endothelial function was lower in adolescents with T2D compared to normal weight and compared to adolescents with T1D (108). These changes are of importance as the rate of all adjudicated heart, vascular, and cerebrovascular events was 3.73 per 1000 person-years (78). There were 17 serious cardiovascular events, myocardial infarction [4 events], congestive heart failure [6 events], coronary artery disease [3 events], and stroke [4 events].
Decreased Bone Mineral Density (BMD)
In a study involving 17 adolescents newly diagnosed with T2D and 59 age, sex, and BMI-matched controls, bone mineral density (BMD) was assessed at multiple sites while accounting for potential confounding factors such as age, sex, Tanner stage, and BMI (109). The results revealed that BMD Z-scores for the femoral neck and bone mineral apparent density Z-scores of the lumbar spine were notably lower in individuals with T2D compared to their healthy counterparts. In another cross-sectional study of BMD in 180 youths aged 10 to 23 years, the participants were categorized into three groups: those with T2D were compared to those with obesity (n=226) and those with a healthy weight (BMI <85th percentile; n=238) (110). An age-dependent pattern emerged, wherein the BMD Z-score in children was higher in the T2D group compared to the obese group. However, in adolescents and young adults, the BMD Z-scores were lower in the T2D group when compared to the obese group. These findings suggest that T2D may have a detrimental impact on bone density, particularly around the age when individuals reach peak bone mass. Considering the elevated fracture risk observed in adults with T2D, the decrease in bone density at a young age raises concerns about potential long-term morbidity and fracture risk in adulthood.
Mental Health Comorbidity
DEPRESSION
As part of routine diabetes care, a total of 197 adolescents and youth diagnosed with T2D, with a mean age of 16.9 years and 57% male, completed the Patient Health Questionnaire (PHQ) (111). 19.3% reported elevated depressive symptoms (PHQ score ≥10). Furthermore, in a sample of 53, 18.9% acknowledged thoughts of self-harm. Despite the prevalence of depressive symptoms, only 50.0% of those with depressive symptoms had a documented referral for mental health treatment in the electronic health record after the positive screening outcome. Older age, shorter diabetes duration, a higher HbA1c level, more blood glucose checks per day, and being prescribed oral medications were significantly associated with more depressive symptoms. Results from the TODAY study revealed that older teen girls had the highest rates of clinically significant depressive symptoms (112). Increased number of stressful life events were associated with elevated depressive symptoms. Depressive symptoms were correlated with low adherence to diabetes treatment, lower psychosocial functioning, and impaired health-related quality of life (QOL). In another study comparing PHQ between adolescents with T2D and T1D, the median PHQ-9 score in females with T2D was significantly greater than in females with T1D, but did not differ between males with T2D and T1D (113). The association between depression and T2D has been reviewed elsewhere (114).
DISORDERED EATING BEHAVIORS
In the TODAY study, out of 678 adolescents with T2D at a mean age of 14.0 years, 6% had clinical and 20% had subclinical levels of binge eating (115). Moreover, 50.3% of adolescents with T2D who are receiving insulin therapy had disordered eating behaviors (DEB) compared with 21.2% of those with T1DM (116). Disordered eating behavior is associated with poorer clinical outcomes and psychosocial well-being.
Mortality
In the SEARCH study, over a median follow-up of 5.3 years, the mortality rate among individuals with T1D was 70.6 deaths per 100,000 patient-years, compared with 185.6 deaths per 100,000 patients-years for those with T2D (117).. When compared to the general populations of US states, it was observed that the mortality rate for individuals with T2D was significantly higher than expected while this was not the case for individuals with T1DM. Females had higher mortality rates than males. Among adolescents with T2D, the leading underlying cause of death was attributed to transport/motor vehicle accidents followed by accidental poisoning, and intentional self-harm. According to a life expectancy model, it was predicted that youth with T2DM lose approximately 15 years (118).
CONCLUSION
A summary of the key points is presented in Table 4.
Table 4.
Key Points
| • The prevalence of type 2 diabetes (T2D) varies across different countries and regions globally, but there is a consistent upward trend in its occurrence. Large-scale national studies in countries like Sweden, Germany, and India have reported similar increasing trends. • Contrary to common perception, not all adolescents with T2D are severely obese. Only 70-80% fall into this category. • Socioeconomic status plays a significant role in the prevalence of T2D, with lower socioeconomic status being associated with higher incidence in Western countries, while the opposite is observed in low-income countries. • Although adolescents are at higher risk of developing T2D, there are growing reports of T2D cases in children younger than 10 years old. • The gender distribution of T2D varies, with a higher proportion of cases in females in Western countries but a higher proportion in males in the Middle East and China. • Promisingly, there are new medications designed for weight loss, which hold the potential to reduce the high rates of complications associated with T2D in children. • There are low prescription rates for statins and contraception in the management of T2D in adolescents, highlighting potential gaps in medical care. • Many complications of T2D can potentially be reversed if detected and treated aggressively in their early stages. |
ACKNOWLEGEMENTS
The authors extend their heartfelt gratitude to the gifted illustrator, Mrs Noah Levek, for her exceptional eye-opening illustrations.
REFERENCES
- 1.
- Knowler WC, Pettitt DJ, Savage PJ, Bennett PH. Diabetes incidence in Pima indians: contributions of obesity and parental diabetes. Am J Epidemiol 1981; 113:144-156 [PubMed: 7468572]
- 2.
- Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr 1996; 128:608-615 [PubMed: 8627431]
- 3.
- Glaser NS, Jones KL. Non-insulin dependent diabetes mellitus in Mexican-American children. West J Med 1998; 168:11-16 [PMC free article: PMC1304744] [PubMed: 9448482]
- 4.
- Neufeld ND, Raffel LJ, Landon C, Chen YD, Vadheim CM. Early presentation of type 2 diabetes in Mexican-American youth. Diabetes Care 1998; 21:80-86 [PubMed: 9538974]
- 5.
- Stahl-Pehe A, Kamrath C, Prinz N, Kapellen T, Menzel U, Kordonouri O, Schwab KO, Bechtold-Dalla Pozza S, Rosenbauer J, Holl RW. Prevalence of type 1 and type 2 diabetes in children and adolescents in Germany from 2002 to 2020: A study based on electronic health record data from the DPV registry. J Diabetes 2022; 14:840-850 [PMC free article: PMC9789390] [PubMed: 36515004]
- 6.
- Tung JY, Kwan EY, But BW, Wong WH, Fu AC, Pang G, Tsang JW, Yau HC, Belaramani K, Wong LM, Wong SM, Lo P, Ng KL, Yeung WK, Chan KT, Chan AM, Wong SW, Tay MK, Chung J, Lee CY, Lam YY, Cheung PT. Incidence and clinical characteristics of pediatric-onset type 2 diabetes in Hong Kong: The Hong Kong childhood diabetes registry 2008 to 2017. Pediatr Diabetes 2022; 23:556-561 [PubMed: 33978300]
- 7.
- Urakami T, Miyata M, Yoshida K, Mine Y, Kuwabara R, Aoki M, Suzuki J. Changes in annual incidence of school children with type 2 diabetes in the Tokyo Metropolitan Area during 1975-2015. Pediatr Diabetes 2018; 19:1385-1392 [PubMed: 30101568]
- 8.
- Hockett CW, Praveen PA, Ong TC, Amutha A, Isom SP, Jensen ET, D'Agostino RB, Jr., Hamman RF, Mayer-Davis EJ, Lawrence JM, Pihoker C, Kahn MG, Mohan V, Tandon N, Dabelea D. Clinical profile at diagnosis with youth-onset type 1 and type 2 diabetes in two pediatric diabetes registries: SEARCH (United States) and YDR (India). Pediatr Diabetes 2021; 22:22-30 [PMC free article: PMC7785282] [PubMed: 31953884]
- 9.
- Zuckerman Levin N, Cohen M, Phillip M, Tenenbaum A, Koren I, Tenenbaum-Rakover Y, Admoni O, Hershkovitz E, Haim A, Mazor Aronovitch K, Zangen D, Strich D, Brener A, Yeshayahu Y, Schon Y, Rachmiel M, Ben-Ari T, Levy-Khademi F, Tibi R, Weiss R, Lebenthal Y, Pinhas-Hamiel O, Shehadeh N. Youth-onset type 2 diabetes in Israel: A national cohort. Pediatr Diabetes 2022; 23:649-659 [PubMed: 35521999]
- 10.
- Wagenknecht LE, Lawrence JM, Isom S, Jensen ET, Dabelea D, Liese AD, Dolan LM, Shah AS, Bellatorre A, Sauder K, Marcovina S, Reynolds K, Pihoker C, Imperatore G, Divers J, study SfDiY. Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002-18: results from the population-based SEARCH for Diabetes in Youth study. The lancet Diabetes & endocrinology 2023; 11:242-250 [PMC free article: PMC10091237] [PubMed: 36868256]
- 11.
- Wise J. Type 2 diabetes: Charity warns of "perfect storm" putting more children at risk. BMJ (Clinical research ed) 2022; 377:o1451 [PubMed: 35705201]
- 12.
- Wu H, Zhong J, Yu M, Wang H, Gong W, Pan J, Fei F, Wang M, Yang L, Hu R. Incidence and time trends of type 2 diabetes mellitus in youth aged 5-19 years: a population-based registry in Zhejiang, China, 2007 to 2013. BMC pediatrics 2017; 17:85 [PMC free article: PMC5361693] [PubMed: 28330444]
- 13.
- Biondi G, Marrano N, Borrelli A, Rella M, Palma G, Calderoni I, Siciliano E, Lops P, Giorgino F, Natalicchio A. Adipose Tissue Secretion Pattern Influences β-Cell Wellness in the Transition from Obesity to Type 2 Diabetes. 2022; 23:5522 [PMC free article: PMC9143684] [PubMed: 35628332]
- 14.
- Cioana M, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, Rivas A, Banfield L, Toor PP, Zhou F, Guven A, Alfaraidi H, Alotaibi A, Thabane L, Samaan MC. The Prevalence of Obesity Among Children With Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Network Open 2022; 5:e2247186-e2247186 [PMC free article: PMC9856349] [PubMed: 36520430]
- 15.
- Copeland KC, Zeitler P, Geffner M, Guandalini C, Higgins J, Hirst K, Kaufman FR, Linder B, Marcovina S, McGuigan P, Pyle L, Tamborlane W, Willi S. Characteristics of Adolescents and Youth with Recent-Onset Type 2 Diabetes: The TODAY Cohort at Baseline. The Journal of Clinical Endocrinology & Metabolism 2011; 96:159-167 [PMC free article: PMC3038479] [PubMed: 20962021]
- 16.
- Lowe WL, Jr., Lowe LP, Kuang A, Catalano PM, Nodzenski M, Talbot O, Tam WH, Sacks DA, McCance D, Linder B, Lebenthal Y, Lawrence JM, Lashley M, Josefson JL, Hamilton J, Deerochanawong C, Clayton P, Brickman WJ, Dyer AR, Scholtens DM, Metzger BE. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia 2019; 62:598-610 [PMC free article: PMC6421132] [PubMed: 30648193]
- 17.
- Dabelea D, Mayer-Davis EJ, Lamichhane AP, D'Agostino RB, Jr., Liese AD, Vehik KS, Narayan KM, Zeitler P, Hamman RF. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care 2008; 31:1422-1426 [PMC free article: PMC2453655] [PubMed: 18375420]
- 18.
- Shah AS, Nadeau KJ, Dabelea D, Redondo MJ. Spectrum of Phenotypes and Causes of Type 2 Diabetes in Children. Annual review of medicine 2022; 73:501-515 [PMC free article: PMC9022328] [PubMed: 35084995]
- 19.
- Rughani A, Friedman JE, Tryggestad JB. Type 2 Diabetes in Youth: the Role of Early Life Exposures. Current diabetes reports 2020; 20:45 [PubMed: 32767148]
- 20.
- Norrman E, Petzold M, Gissler M, Spangmose AL, Opdahl S, Henningsen AK, Pinborg A, Tiitinen A, Rosengren A, Romundstad LB, Wennerholm UB, Bergh C. Cardiovascular disease, obesity, and type 2 diabetes in children born after assisted reproductive technology: A population-based cohort study. PLoS medicine 2021; 18:e1003723 [PMC free article: PMC8423242] [PubMed: 34491995]
- 21.
- Murphy HR, Howgate C, O'Keefe J, Myers J, Morgan M, Coleman MA, Jolly M, Valabhji J, Scott EM, Knighton P, Young B, Lewis-Barned N. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: a 5-year national population-based cohort study. The lancet Diabetes & endocrinology 2021; 9:153-164 [PubMed: 33516295]
- 22.
- Wibaek R, Andersen GS, Linneberg A, Hansen T, Grarup N, Thuesen ACB, Jensen RT, Wells JCK, Pilgaard KA, Brøns C, Vistisen D, Vaag AA. Low birthweight is associated with a higher incidence of type 2 diabetes over two decades independent of adult BMI and genetic predisposition. Diabetologia 2023; [PMC free article: PMC10390608] [PubMed: 37303008]
- 23.
- Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia 2020; 63:508-518 [PMC free article: PMC6997251] [PubMed: 31802143]
- 24.
- Nouwen A, Adriaanse MC, van Dam K, Iversen MM, Viechtbauer W, Peyrot M, Caramlau I, Kokoszka A, Kanc K, de Groot M, Nefs G, Pouwer F. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet Med 2019; 36:1562-1572 [PubMed: 31215077]
- 25.
- Omer Gilon M, Balmakov Y, Gelman S, Twig G. Adolescent Immigration and Type-2 Diabetes. Current diabetes reports 2021; 21:60 [PubMed: 34902101]
- 26.
- Hamiel U, Pinhas-Hamiel O, Vivante A, Bendor C, Bardugo A, Afek A, Beer Z, Derazne E, Tzur D, Behar D, Itzhak A, Skorecki K, Tirosh A, Grossman E, Twig G. Impact of Immigration on Body Mass Index and Blood Pressure Among Adolescent Males and Females: A Nationwide Study. Hypertension 2019; 74:1316-1323 [PubMed: 31630574]
- 27.
- Khanolkar AR, Amin R, Taylor-Robinson D, Viner RM, Warner J, Stephenson T. Inequalities in glycemic control in childhood onset type 2 diabetes in England and Wales-A national population-based longitudinal study. Pediatr Diabetes 2019; 20:821-831 [PubMed: 31329349]
- 28.
- Magge SN, Wolf RM, Pyle L, Brown EA, Benavides VC, Bianco ME, Chao LC, Cymbaluk A, Balikcioglu PG, Halpin K, Hsia DS, Huerta-Saenz L, Kim JJ, Kumar S, Levitt Katz LE, Marks BE, Neyman A, O'Sullivan KL, Pillai SS, Shah AS, Shoemaker AH, Siddiqui JAW, Srinivasan S, Thomas IH, Tryggestad JB, Yousif MF, Kelsey MM. The Coronavirus Disease 2019 Pandemic is Associated with a Substantial Rise in Frequency and Severity of Presentation of Youth-Onset Type 2 Diabetes. J Pediatr 2022; 251:51-59.e52 [PMC free article: PMC9383958] [PubMed: 35985535]
- 29.
- DeLacey S, Arzu J, Levin L, Ranganna A, Swamy A, Bianco ME. Impact of SARS-CoV2 on youth onset type 2 diabetes new diagnoses and severity. J Diabetes 2022; 14:532-540 [PMC free article: PMC9426273] [PubMed: 36040204]
- 30.
- Denzer C, Rosenbauer J, Klose D, Körner A, Reinehr T, Baechle C, Schröder C, Wiegand S, Holl RW, Prinz N. Is COVID-19 to Blame? Trends of Incidence and Sex Ratio in Youth-Onset Type 2 Diabetes in Germany. Diabetes Care 2023; 46:1379-1387 [PubMed: 37140887]
- 31.
- Chang TH, Chen YC, Chen WY, Chen CY, Hsu WY, Chou Y, Chang YH. Weight Gain Associated with COVID-19 Lockdown in Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients 2021; 13 [PMC free article: PMC8540321] [PubMed: 34684669]
- 32.
- Srinivasan S, Chen L, Todd J, Divers J, Gidding S, Chernausek S, Gubitosi-Klug RA, Kelsey MM, Shah R, Black MH, Wagenknecht LE, Manning A, Flannick J, Imperatore G, Mercader JM, Dabelea D, Florez JC. The First Genome-Wide Association Study for Type 2 Diabetes in Youth: The Progress in Diabetes Genetics in Youth (ProDiGY) Consortium. Diabetes 2021; 70:996-1005 [PMC free article: PMC7980197] [PubMed: 33479058]
- 33.
- Hao S, Cossen K, Westbrook AL, Umpierrez GE, Vellanki P. Diabetic Ketoacidosis and Long-term Insulin Requirements in Youths with Newly Diagnosed Type 2 Diabetes During the SARS-CoV-2 Pandemic. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2023; 29:754-761 [PMC free article: PMC10910395] [PubMed: 37451650]
- 34.
- Klingensmith GJ, Connor CG, Ruedy KJ, Beck RW, Kollman C, Haro H, Wood JR, Lee JM, Willi SM, Cengiz E, Tamborlane WV. Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr Diabetes 2016; 17:266-273 [PubMed: 25951940]
- 35.
- Klingensmith GJ, Lanzinger S, Tamborlane WV, Hofer SE, Cheng P, de Beaufort C, Gal RL, Reinehr T, Kollman C, Holl RW. Adolescent type 2 diabetes: Comparing the Pediatric Diabetes Consortium and Germany/Austria/Luxemburg Pediatric Diabetes Prospective registries. Pediatr Diabetes 2018; 19:1156-1163 [PubMed: 29923263]
- 36.
- Otaigbe BE, Imafidon EE. Type 2 diabetes mellitus in a Nigerian child: a case report. African health sciences 2011; 11:454-456 [PMC free article: PMC3260995] [PubMed: 22275939]
- 37.
- Kevat D, Wilson D, Sinha A. A 5-year-old girl with type 2 diabetes. Lancet 2014; 383:1268 [PubMed: 24703566]
- 38.
- Greenup E, Sunil B, Barr MM, Ashraf AP. Glycaemic control and outcomes in children with type 2 diabetes diagnosed at or before 10 years of age. Endocrinology, diabetes & metabolism 2021; 4:e00192 [PMC free article: PMC8029508] [PubMed: 33855201]
- 39.
- Curran JA, Haynes A, Davis EA. Clinical characteristics of Western Australian children diagnosed with type 2 diabetes before 10 years of age. The Medical journal of Australia 2020; 212:95-95.e91 [PubMed: 31808549]
- 40.
- Astudillo M, Tosur M, Castillo B, Rafaey A, Siller AF, Nieto J, Sisley S, McKay S, Nella AA, Balasubramanyam A, Bacha F, Redondo MJ. Type 2 diabetes in prepubertal children. Pediatr Diabetes 2021; 22:946-950 [PubMed: 34363430]
- 41.
- Hutchins J, Barajas RA, Hale D, Escaname E, Lynch J. Type 2 diabetes in a 5-year-old and single center experience of type 2 diabetes in youth under 10. Pediatr Diabetes 2017; 18:674-677 [PubMed: 27807935]
- 42.
- Amutha A, Unnikrishnan R, Anjana RM, Mohan V. Prepubertal Childhood Onset Type 2 Diabetes Mellitus: Four Case Reports. The Journal of the Association of Physicians of India 2017; 65:43-46 [PubMed: 28457031]
- 43.
- Al Amiri E, Abdullatif M, Abdulle A, Al Bitar N, Afandi EZ, Parish M, Darwiche G. The prevalence, risk factors, and screening measure for prediabetes and diabetes among Emirati overweight/obese children and adolescents. BMC Public Health 2015; 15:1298-1298 [PMC free article: PMC4690431] [PubMed: 26704130]
- 44.
- Alsaffar Y, Hussain AM, Selman NAJAAVdFyT. Prevalence of type 2 diabetes in pediatrics and adolescents newly diagnosed with diabetes in Babylon Governorate, Iraq. 2020; 39
- 45.
- Moussa MA, Alsaeid M, Abdella N, Refai TM, Al-Sheikh N, Gomez JE. Prevalence of type 2 diabetes mellitus among Kuwaiti children and adolescents. Medical principles and practice : international journal of the Kuwait University, Health Science Centre 2008; 17:270-275 [PubMed: 18523392]
- 46.
- Elkum N, Al-Arouj M, Sharifi M, Shaltout A, Bennakhi A. Prevalence of childhood obesity in the state of Kuwait. Pediatr Obes 2016; 11:e30-e34 [PubMed: 26663908]
- 47.
- AlBlooshi A, Shaban S, AlTunaiji M, Fares N, AlShehhi L, AlShehhi H, AlMazrouei A, Souid AK. Increasing obesity rates in school children in United Arab Emirates. Obesity science & practice 2016; 2:196-202 [PMC free article: PMC5074293] [PubMed: 27818779]
- 48.
- Pearson ER. Treating type 2 diabetes in youth: a depressing picture. The journal of the Royal College of Physicians of Edinburgh 2012; 42:228 [PubMed: 22953318]
- 49.
- Kriska A, El Ghormli L, Copeland KC, Higgins J, Ievers-Landis CE, Levitt Katz LE, Trief PM, Wauters AD, Yasuda PM, Delahanty LM. Impact of lifestyle behavior change on glycemic control in youth with type 2 diabetes. Pediatr Diabetes 2018; 19:36-44 [PMC free article: PMC5628101] [PubMed: 28378429]
- 50.
- Alfaraidi H, Samaan MC. Metformin therapy in pediatric type 2 diabetes mellitus and its comorbidities: A review. Frontiers in endocrinology 2022; 13:1072879 [PMC free article: PMC9939509] [PubMed: 36814831]
- 51.
- Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care 2002; 25:89-94 [PubMed: 11772907]
- 52.
- Gottschalk M, Danne T, Vlajnic A, Cara JF. Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomized, single-blind comparative study. Diabetes Care 2007; 30:790-794 [PubMed: 17392540]
- 53.
- Laffel L, Chang N, Grey M, Hale D, Higgins L, Hirst K, Izquierdo R, Larkin M, Macha C, Pham T, Wauters A, Weinstock RS. Metformin monotherapy in youth with recent onset type 2 diabetes: experience from the prerandomization run-in phase of the TODAY study. Pediatr Diabetes 2012; 13:369-375 [PMC free article: PMC3371323] [PubMed: 22369102]
- 54.
- Kelsey MM, Geffner ME, Guandalini C, Pyle L, Tamborlane WV, Zeitler PS, White NH. Presentation and effectiveness of early treatment of type 2 diabetes in youth: lessons from the TODAY study. Pediatr Diabetes 2016; 17:212-221 [PMC free article: PMC4539288] [PubMed: 25690268]
- 55.
- Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse Effects of GLP-1 Receptor Agonists. The review of diabetic studies : RDS 2014; 11:202-230 [PMC free article: PMC5397288] [PubMed: 26177483]
- 56.
- Tamborlane WV, Barrientos-Pérez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, Jalaludin MY, Kovarenko M, Libman I, Lynch JL, Rao P, Shehadeh N, Turan S, Weghuber D, Barrett T. Liraglutide in Children and Adolescents with Type 2 Diabetes. N Engl J Med 2019; 381:637-646 [PubMed: 31034184]
- 57.
- Tamborlane WV, Bishai R, Geller D, Shehadeh N, Al-Abdulrazzaq D, Vazquez EM, Karoly E, Troja T, Doehring O, Carter D, Monyak J, Sjostrom CD. Once-Weekly Exenatide in Youth With Type 2 Diabetes. Diabetes Care 2022; 45:1833-1840 [PMC free article: PMC9346995] [PubMed: 35679098]
- 58.
- Arslanian SA, Hannon T, Zeitler P, Chao LC, Boucher-Berry C, Barrientos-Perez M, Bismuth E, Dib S, Cho JI, Cox D, Investigators A-P. Once-Weekly Dulaglutide for the Treatment of Youths with Type 2 Diabetes. N Engl J Med 2022; 387:433-443 [PubMed: 35658022]
- 59.
- Padda IS, Mahtani AU, Parmar M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Arun Mahtani declares no relevant financial relationships with ineligible companies. Disclosure: Mayur Parmar declares no relevant financial relationships with ineligible companies.: StatPearls Publishing, Copyright © 2023, StatPearls Publishing LLC.; 2023.
- 60.
- Laffel LM, Danne T, Klingensmith GJ, Tamborlane WV, Willi S, Zeitler P, Neubacher D, Marquard J. Efficacy and safety of the SGLT2 inhibitor empagliflozin versus placebo and the DPP-4 inhibitor linagliptin versus placebo in young people with type 2 diabetes (DINAMO): a multicentre, randomised, double-blind, parallel group, phase 3 trial. The lancet Diabetes & endocrinology 2023; 11:169-181 [PMC free article: PMC10851109] [PubMed: 36738751]
- 61.
- Tamborlane WV, Laffel LM, Shehadeh N, Isganaitis E, Van Name M, Ratnayake J, Karlsson C, Norjavaara E. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. The lancet Diabetes & endocrinology 2022; 10:341-350 [PMC free article: PMC10851108] [PubMed: 35378069]
- 62.
- Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocrine reviews 2014; 35:992-1019 [PMC free article: PMC7108477] [PubMed: 25216328]
- 63.
- Shankar RR, Zeitler P, Deeb A, Jalaludin MY, Garcia R, Newfield RS, Samoilova Y, Rosario CA, Shehadeh N, Saha CK, Zhang Y, Zilli M, Scherer LW, Lam RLH, Golm GT, Engel SS, Kaufman KD. A randomized clinical trial of the efficacy and safety of sitagliptin as initial oral therapy in youth with type 2 diabetes. Pediatr Diabetes 2022; 23:173-182 [PubMed: 34779087]
- 64.
- Bensignor MO, Kelly AS, Arslanian S. Anti-obesity pharmacotherapy for treatment of pediatric type 2 diabetes: Review of the literature and lessons learned from adults. Frontiers in endocrinology 2022; 13:1043650 [PMC free article: PMC9647073] [PubMed: 36387846]
- 65.
- Stretton B, Kovoor J, Bacchi S, Chang S, Ngoi B, Murray T, Bristow TC, Heng J, Gupta A, Ovenden C, Maddern G, Thompson CH, Heilbronn L, Boyd M, Rayner C, Talley NJ, Horowtiz M. Weight loss with subcutaneous semaglutide versus other glucagon-like peptide 1 receptor agonists in type 2 diabetes: a systematic review. Internal medicine journal 2023; 53:1311-1320 [PubMed: 37189293]
- 66.
- Weghuber D, Barrett T, Barrientos-Pérez M, Gies I, Hesse D, Jeppesen OK, Kelly AS, Mastrandrea LD, Sørrig R, Arslanian S. Once-Weekly Semaglutide in Adolescents with Obesity. N Engl J Med 2022; 387:2245-2257 [PMC free article: PMC9997064] [PubMed: 36322838]
- 67.
- Zeitler P, El Ghormli L, Arslanian S, Caprio S, Isganaitis E, Kelsey MK, Weinstock RS, White NH, Drews K. Deterioration of Glycemic Control in Youth-Onset Type 2 Diabetes: What Are the Early and Late Predictors? J Clin Endocrinol Metab 2022; 107:e3384-e3394 [PMC free article: PMC9653021] [PubMed: 35486388]
- 68.
- ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA, on behalf of the American Diabetes A. 14. Children and Adolescents: Standards of Care in Diabetes-2023. Diabetes Care 2023; 46:S230-S253 [PMC free article: PMC9810473] [PubMed: 36507640]
- 69.
- Asiri A, Alzahrani F, Alghamdi H, Alamri Z. Effect of Laparoscopic Sleeve Gastrectomy on HbA1C Level in Children with Type 2 Diabetes Mellitus. Medicina (Kaunas, Lithuania) 2022; 58 [PMC free article: PMC9318732] [PubMed: 35888681]
- 70.
- Inge TH, Laffel LM, Jenkins TM, Marcus MD, Leibel NI, Brandt ML, Haymond M, Urbina EM, Dolan LM, Zeitler PS. Comparison of Surgical and Medical Therapy for Type 2 Diabetes in Severely Obese Adolescents. JAMA pediatrics 2018; 172:452-460 [PMC free article: PMC5875354] [PubMed: 29532078]
- 71.
- Zhu X, Zhou G, Gu X, Jiang X, Huang H, You S, Zhang G. Comparison of bariatric surgery and medical therapy for obese adolescents with type 2 diabetes. Asian journal of surgery 2022; [PubMed: 36369137]
- 72.
- Bjornstad P, Hughan K, Kelsey MM, Shah AS, Lynch J, Nehus E, Mitsnefes M, Jenkins T, Xu P, Xie C, Inge T, Nadeau K. Effect of Surgical Versus Medical Therapy on Diabetic Kidney Disease Over 5 Years in Severely Obese Adolescents With Type 2 Diabetes. Diabetes Care 2020; 43:187-195 [PMC free article: PMC6925577] [PubMed: 31685489]
- 73.
- Bjornstad P, Nehus E, van Raalte D. Bariatric surgery and kidney disease outcomes in severely obese youth. Seminars in pediatric surgery 2020; 29:150883 [PMC free article: PMC7125208] [PubMed: 32238288]
- 74.
- Ryder JR, Xu P, Nadeau KJ, Kelsey MM, Xie C, Jenkins T, Inge TH, Bjornstad P. Effect of surgical versus medical therapy on estimated cardiovascular event risk among adolescents with type 2 diabetes and severe obesity. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2021; 17:23-33 [PMC free article: PMC8007282] [PubMed: 33071178]
- 75.
- Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, Coskun T, Haupt A, Milicevic Z, Hartman ML. Triple-Hormone-Receptor Agonist Retatrutide for Obesity - A Phase 2 Trial. N Engl J Med 2023; 389:514-526 [PubMed: 37366315]
- 76.
- Medicine TSGJNEJo. Long-term complications in youth-onset type 2 diabetes. 2021; 385:416-426 [PMC free article: PMC8697255] [PubMed: 34320286]
- 77.
- Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 2013; 36:1735-1741 [PMC free article: PMC3661847] [PubMed: 23704672]
- 78.
- Bjornstad P, Drews KL, Caprio S, Gubitosi-Klug R, Nathan DM, Tesfaldet B, Tryggestad J, White NH, Zeitler P. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med 2021; 385:416-426 [PMC free article: PMC8697255] [PubMed: 34320286]
- 79.
- Cioana M, Deng J, Hou M, Nadarajah A, Qiu Y, Chen SSJ, Rivas A, Banfield L, Chanchlani R, Dart A, Wicklow B, Alfaraidi H, Alotaibi A, Thabane L, Samaan MC. Prevalence of Hypertension and Albuminuria in Pediatric Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Netw Open 2021; 4:e216069 [PMC free article: PMC8087958] [PubMed: 33929524]
- 80.
- Chu PY, Campbell MJ, Miller SG, Hill KD. Anti-hypertensive drugs in children and adolescents. World journal of cardiology 2014; 6:234-244 [PMC free article: PMC4062129] [PubMed: 24944754]
- 81.
- Siddiqi N, Shatat IF. Antihypertensive agents: a long way to safe drug prescribing in children. Pediatric nephrology (Berlin, Germany) 2020; 35:2049-2065 [PMC free article: PMC7515858] [PubMed: 31676933]
- 82.
- Dabelea D, Sauder KA, Jensen ET, Mottl AK, Huang A, Pihoker C, Hamman RF, Lawrence J, Dolan LM, Agostino R, Jr., Wagenknecht L, Mayer-Davis EJ, Marcovina SM. Twenty years of pediatric diabetes surveillance: what do we know and why it matters. Ann N Y Acad Sci 2021; [PMC free article: PMC8282684] [PubMed: 33543783]
- 83.
- Amatruda M, Gembillo G, Giuffrida AE, Santoro D, Conti G. The Aggressive Diabetic Kidney Disease in Youth-Onset Type 2 Diabetes: Pathogenetic Mechanisms and Potential Therapies. Medicina (Kaunas, Lithuania) 2021; 57 [PMC free article: PMC8467670] [PubMed: 34577791]
- 84.
- Naaman SC, Bakris GL. Diabetic Nephropathy: Update on Pillars of Therapy Slowing Progression. Diabetes Care 2023; 46:1574-1586 [PMC free article: PMC10547606] [PubMed: 37625003]
- 85.
- Blazek O, Bakris GL. Slowing the Progression of Diabetic Kidney Disease. Cells 2023; 12 [PMC free article: PMC10417620] [PubMed: 37566054]
- 86.
- Jackson S, Creo A, Kumar S. Are Clinicians Aggressive Enough in Treating Diabetes-Related Hyperlipidemia in Youth? Current atherosclerosis reports 2022; 24:471-481 [PubMed: 35404039]
- 87.
- Cioana M, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, Rivas A, Banfield L, Alfaraidi H, Alotaibi A, Thabane L, Samaan MC. Prevalence of Polycystic Ovary Syndrome in Patients With Pediatric Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Network Open 2022; 5:e2147454-e2147454 [PMC free article: PMC8848210] [PubMed: 35166782]
- 88.
- Pregnancy Outcomes in Young Women With Youth-Onset Type 2 Diabetes Followed in the TODAY Study. Diabetes Care 2021; 45:1038-1045 [PMC free article: PMC9174960] [PubMed: 34880068]
- 89.
- Shah RD, Chernausek SD, El Ghormli L, Geffner ME, Keady J, Kelsey MM, Farrell R, Tesfaldet B, Tryggestad JB, Van Name M, Isganaitis E. Maternal Diabetes in Youth-Onset Type 2 Diabetes Is Associated With Progressive Dysglycemia and Risk of Complications. J Clin Endocrinol Metab 2023; 108:1120-1131 [PMC free article: PMC10306086] [PubMed: 36446741]
- 90.
- Merino PM, Codner E. Contraception for Adolescents and Young Women with Type 2 Diabetes-Specific Considerations. Current diabetes reports 2022; 22:77-84 [PubMed: 35150410]
- 91.
- Cioana M, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, Rivas A, Toor PP, Banfield L, Thabane L, Chaudhary V, Samaan MC. Global Prevalence of Diabetic Retinopathy in Pediatric Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Netw Open 2023; 6:e231887 [PMC free article: PMC10024209] [PubMed: 36930156]
- 92.
- Mititelu M, Uschner D, Doherty L, Bjornstad P, Domalpally A, Drews KL, Gubitosi-Klug R, Levitsky LL, Pak JW, White NH, Blodi BA. Retinal Thickness and Morphology Changes on OCT in Youth with Type 2 Diabetes: Findings from the TODAY Study. Ophthalmol Sci 2022; 2:100191 [PMC free article: PMC9754955] [PubMed: 36531589]
- 93.
- Wright AD, Dodson PM. Medical management of diabetic retinopathy: fenofibrate and ACCORD Eye studies. Eye (London, England) 2011; 25:843-849 [PMC free article: PMC3178166] [PubMed: 21436845]
- 94.
- Dabelea D, Stafford JM, Mayer-Davis EJ, D'Agostino R, Jr., Dolan L, Imperatore G, Linder B, Lawrence JM, Marcovina SM, Mottl AK, Black MH, Pop-Busui R, Saydah S, Hamman RF, Pihoker C. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. Jama 2017; 317:825-835 [PMC free article: PMC5483855] [PubMed: 28245334]
- 95.
- Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier Onset of Complications in Youth With Type 2 Diabetes. Diabetes Care 2014; 37:436-443 [PubMed: 24130346]
- 96.
- Amutha A, Datta M, Unnikrishnan R, Anjana RM, Mohan V. Clinical profile and complications of childhood- and adolescent-onset type 2 diabetes seen at a diabetes center in south India. Diabetes Technol Ther 2012; 14:497-504 [PubMed: 22551567]
- 97.
- Jaiswal M, Lauer A, Martin CL, Bell RA, Divers J, Dabelea D, Pettitt DJ, Saydah S, Pihoker C, Standiford DA, Rodriguez BL, Pop-Busui R, Feldman EL. Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for Diabetes in Youth follow-up cohort: a pilot study. Diabetes Care 2013; 36:3903-3908 [PMC free article: PMC3836139] [PubMed: 24144652]
- 98.
- Jaiswal M, Divers J, Dabelea D, Isom S, Bell RA, Martin CL, Pettitt DJ, Saydah S, Pihoker C, Standiford DA, Dolan LM, Marcovina S, Linder B, Liese AD, Pop-Busui R, Feldman EL. Prevalence of and Risk Factors for Diabetic Peripheral Neuropathy in Youth With Type 1 and Type 2 Diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care 2017; 40:1226-1232 [PMC free article: PMC5566278] [PubMed: 28674076]
- 99.
- Akinci G, Savelieff MG, Gallagher G, Callaghan BC, Feldman EL. Diabetic neuropathy in children and youth: New and emerging risk factors. Pediatr Diabetes 2021; 22:132-147 [PubMed: 33205601]
- 100.
- Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 2003; 26:1553-1579 [PubMed: 12716821]
- 101.
- Duque A, Mediano MFF, De Lorenzo A, Rodrigues LF, Jr. Cardiovascular autonomic neuropathy in diabetes: Pathophysiology, clinical assessment and implications. World J Diabetes 2021; 12:855-867 [PMC free article: PMC8192252] [PubMed: 34168733]
- 102.
- Varley BJ, Gow ML, Cho YH, Benitez-Aguirre P, Cusumano J, Pryke A, Chan A, Velayutham V, Donaghue KC, Craig ME. Higher frequency of cardiovascular autonomic neuropathy in youth with type 2 compared to type 1 diabetes: Role of cardiometabolic risk factors. Pediatr Diabetes 2022; 23:1073-1079 [PMC free article: PMC9805172] [PubMed: 35856852]
- 103.
- Jaiswal M, Divers J, Urbina EM, Dabelea D, Bell RA, Pettitt DJ, Imperatore G, Pihoker C, Dolan LM, Liese AD, Marcovina S, Linder B, Feldman EL, Pop-Busui R. Cardiovascular autonomic neuropathy in adolescents and young adults with type 1 and type 2 diabetes: The SEARCH for Diabetes in Youth Cohort Study. Pediatr Diabetes 2018; 19:680-689 [PMC free article: PMC5938122] [PubMed: 29292558]
- 104.
- Wadwa RP, Urbina EM, Anderson AM, Hamman RF, Dolan LM, Rodriguez BL, Daniels SR, Dabelea D, Group ftSS. Measures of Arterial Stiffness in Youth With Type 1 and Type 2 Diabetes: The SEARCH for Diabetes in Youth study. Diabetes Care 2010; 33:881-886 [PMC free article: PMC2845046] [PubMed: 20067960]
- 105.
- Shah AS, Gidding SS, El Ghormli L, Tryggestad JB, Nadeau KJ, Bacha F, Levitt Katz LE, Willi SM, Lima J, Urbina EM. Relationship between Arterial Stiffness and Subsequent Cardiac Structure and Function in Young Adults with Youth-Onset Type 2 Diabetes: Results from the TODAY Study. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography 2022; 35:620-628.e624 [PMC free article: PMC9177714] [PubMed: 35149207]
- 106.
- Urbina EM, Isom S, Dabelea D, D'Agostino R, Daniels SR, Dolan LM, Imperatore G, Lustigova E, Marcovina S, Mottl A, Pihoker C, Shah AS. Association of Elevated Arterial Stiffness With Cardiac Target Organ Damage and Cardiac Autonomic Neuropathy in Young Adults With Diabetes: The SEARCH for Diabetes in Youth Study. Diabetes Care 2023; 46:786-793 [PMC free article: PMC10090911] [PubMed: 36730642]
- 107.
- Urbina EM, Kimball TR, McCoy CE, Khoury PR, Daniels SR, Dolan LM. Youth with obesity and obesity-related type 2 diabetes mellitus demonstrate abnormalities in carotid structure and function. Circulation 2009; 119:2913-2919 [PMC free article: PMC2741387] [PubMed: 19470890]
- 108.
- Shah AS, Urbina EM. Vascular and Endothelial Function in Youth with Type 2 Diabetes Mellitus. Current diabetes reports 2017; 17:36-36 [PMC free article: PMC5800521] [PubMed: 28432570]
- 109.
- Lee HS, Yoon JS, Park KJ, Lim JS, Hwang JS. The Relationship Between Bone Mineral Density and Type 2 Diabetes in Obese Children and Adolescents at the Time of Initial Diagnosis. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 2019; 51:42-46 [PubMed: 30340234]
- 110.
- Kindler JM, Kelly A, Khoury PR, Levitt Katz LE, Urbina EM, Zemel BS. Bone Mass and Density in Youth With Type 2 Diabetes, Obesity, and Healthy Weight. Diabetes Care 2020; 43:2544-2552 [PMC free article: PMC7510020] [PubMed: 32778556]
- 111.
- Monaghan M, Mara CA, Kichler JC, Westen SC, Rawlinson A, Jacobsen LM, Adams RN, Stone JY, Hood KK, Mulvaney SA. Multisite Examination of Depression Screening Scores and Correlates Among Adolescents and Young Adults With Type 2 Diabetes. Can J Diabetes 2021; 45:411-416 [PMC free article: PMC8238813] [PubMed: 33722492]
- 112.
- McVoy M, Hardin H, Fulchiero E, Caforio K, Briggs F, Neudecker M, Sajatovic M. Mental health comorbidity and youth onset type 2 diabetes: A systematic review of the literature. International journal of psychiatry in medicine 2023; 58:37-55 [PubMed: 35026126]
- 113.
- Hoffman RP, Damilano CP, Hong KMC, Glick BA, Kamboj MK. Glycemic control, depression, diabetes distress among adolescents with type 2 diabetes: effects of sex, race, insurance, and obesity. Acta Diabetol 2022; 59:1083-1089 [PubMed: 35648254]
- 114.
- Gulley LD, Shomaker LB. Depression in Youth-Onset Type 2 Diabetes. Current diabetes reports 2020; 20:51 [PubMed: 32857299]
- 115.
- Wilfley D, Berkowitz R, Goebel-Fabbri A, Hirst K, Ievers-Landis C, Lipman TH, Marcus M, Ng D, Pham T, Saletsky R, Schanuel J, Van Buren D. Binge eating, mood, and quality of life in youth with type 2 diabetes: baseline data from the today study. Diabetes Care 2011; 34:858-860 [PMC free article: PMC3064041] [PubMed: 21357794]
- 116.
- Nip ASY, Reboussin BA, Dabelea D, Bellatorre A, Mayer-Davis EJ, Kahkoska AR, Lawrence JM, Peterson CM, Dolan L, Pihoker C. Disordered Eating Behaviors in Youth and Young Adults With Type 1 or Type 2 Diabetes Receiving Insulin Therapy: The SEARCH for Diabetes in Youth Study. Diabetes Care 2019; 42:859-866 [PMC free article: PMC6489106] [PubMed: 30862656]
- 117.
- Reynolds K, Saydah SH, Isom S, Divers J, Lawrence JM, Dabelea D, Mayer-Davis EJ, Imperatore G, Bell RA, Hamman RF. Mortality in youth-onset type 1 and type 2 diabetes: The SEARCH for Diabetes in Youth study. Journal of diabetes and its complications 2018; 32:545-549 [PMC free article: PMC6089078] [PubMed: 29685480]
- 118.
- Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. Estimated morbidity and mortality in adolescents and young adults diagnosed with Type 2 diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association 2012; 29:453-463 [PubMed: 22150528]
- Review Cardiovascular Risk Reduction in Youth with Diabetes- Opportunities and Challenges.[Endotext. 2000]Review Cardiovascular Risk Reduction in Youth with Diabetes- Opportunities and Challenges.Shah AS, Wilson DP. Endotext. 2000
- Review Treatment of Diabetes Mellitus in Children and Adolescents.[Endotext. 2000]Review Treatment of Diabetes Mellitus in Children and Adolescents.Yau M, Sperling MA. Endotext. 2000
- Review Etiology and Pathogenesis of Diabetes Mellitus in Children and Adolescents.[Endotext. 2000]Review Etiology and Pathogenesis of Diabetes Mellitus in Children and Adolescents.Yau M, Maclaren NK, Sperling MA. Endotext. 2000
- Review Diabetes in the Elderly.[Endotext. 2000]Review Diabetes in the Elderly.Milanesi A, Weinreb JE. Endotext. 2000
- Review Genetic Disorders Causing Hypertriglyceridemia in Children and Adolescents.[Endotext. 2000]Review Genetic Disorders Causing Hypertriglyceridemia in Children and Adolescents.Shah AS, Wilson DP. Endotext. 2000
- Type 2 Diabetes in Children and Adolescents- A Focus on Diagnosis and Treatment ...Type 2 Diabetes in Children and Adolescents- A Focus on Diagnosis and Treatment - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...