NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
The identification of a patient at high risk of fracture should be followed by evaluation for factors contributing to low bone mass, skeletal fragility, falls, and fractures. Components of the evaluation include a bone density test, osteoporosis-directed medical history and physical exam, laboratory studies, and possibly skeletal imaging. A bone density test with dual-energy X-ray absorptiometry (DXA) helps with diagnostic classification, assessment of fracture risk, and provides a baseline for monitoring the skeletal effects of treatment. FRAX is a fracture risk algorithm that includes input of femoral neck bone mineral density measured by DXA. The DXA T-score, prior fracture history, and FRAX estimation of fracture risk are used with clinical practice guidelines to determine whether treatment is indicated. The medical history may reveal underlying causes of osteoporosis (e.g., nutritional deficiencies, gastric surgery, medications with adverse skeletal effects) and important risk factors for fracture (e.g., past history of fracture, family history of osteoporosis, or recent falls). Physical exam may show skeletal deformities due to unrecognized fractures (e.g., loss of height, kyphosis, or diminished rib-pelvis space), identify possible secondary causes of skeletal fragility (e.g., blue sclera with osteogenesis imperfecta, urticarial pigmentosa with systemic mastocytosis, dermatitis herpetiformis with celiac disease, or bone tenderness with osteomalacia), and help to recognize patients with poor balance and frailty that might lead to falls. Laboratory studies may show potentially reversible abnormalities (e.g., vitamin D deficiency, hypocalcemia, or impaired kidney function) that must be assessed and corrected, if possible, before starting pharmacological therapy. Disorders other than osteoporosis, requiring other types of treatment, may be found; for example, low serum alkaline phosphatase suggests hypophosphatasia, M-component may be due to myeloma, or hypocalciuria due to celiac disease. There are important safety considerations that can be derived from a pre-treatment assessment, as well. A patient with a blood clotting disorder should not be treated with raloxifene, a history of esophageal stricture is a contraindication for oral bisphosphonates, and previous skeletal radiation therapy precludes treatment with teriparatide or abaloparatide. Skeletal imaging may be helpful when a fracture, malignancy, or Paget’s disease of bone is suspected. Bone biopsy is rarely performed in clinical practice, but may be helpful in some situations, such as when it is necessary to determine the underlying bone disease in a patient with severe chronic kidney disease. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Osteoporosis is a common disease characterized by low bone strength that results in an increased risk of fracture (1). Fractures are associated with serious clinical consequences, including pain, disability, loss of independence, and death, as well as high healthcare costs. Early identification and intervention with patients at high risk for fracture is needed to reduce the burden of osteoporotic fractures (2). The management of a patient with a confirmed diagnosis of osteoporosis or low bone mass (osteopenia) includes assessment of fracture risk, evaluation for secondary causes of skeletal fragility, making decisions on initiation of treatment, and identification of all relevant clinical factors that may influence patient management. This is a review of the key components in the care of patients with osteoporosis prior to treatment.
DIAGNOSIS OF OSTEOPOROSIS
The World Health Organization (WHO) diagnostic classification (Table 1) (3) is made by bone mineral density (BMD) testing with dual-energy X-ray absorptiometry (DXA) using the T-score, calculated by subtracting the mean BMD (in g/cm2) of a young-adult reference population from the patient’s BMD and dividing by the standard deviation (SD) of the young-adult reference population. The International Society for Clinical Densitometry (ISCD) recommends that BMD be measured at the lumbar spine (ideally L1-L4), total hip, and femoral neck, with the 33% radius (1/3 radius) being measured when the lumbar spine and/or hip cannot be measured (e.g., obese patient who exceeds weight limit of table) or is invalid (e.g., patient with lumbar laminectomy or bilateral total hip replacements) (4). Osteoporosis cannot be diagnosed by BMD measurement at skeletal sites other than lumbar spine, total hip, femoral neck, and 33% radius or with technologies other than DXA, except for total hip and femoral neck T-scores calculated from 2D projections of quantitative computed tomography (QCT) data. The quality of DXA instrument maintenance, acquisition, analysis, interpretation, and reporting is important in obtaining valid results that can be used for making appropriate clinical decisions (4,5). In a patient with a fragility fracture, a clinical diagnosis of osteoporosis may be considered independently of BMD results, assuming that other causes of skeletal fragility (e.g., osteomalacia) are not responsible for the fracture. Establishing a diagnosis of osteoporosis is clinically useful because it facilitates communication among healthcare providers and patients concerning a disease with potentially serious consequences; in some countries, such as the United States (US), a diagnosis is necessary in order to select a numerical code for submission of insurance claims for reimbursement for medical services. The US National Bone Health Alliance (6) has recommended that osteoporosis be diagnosed in postmenopausal women and men over the age of 50 years in any of the following circumstances: T-score ≤ −2.5 at the lumbar spine or hip; low-trauma hip fracture; osteopenia by BMD with a low-trauma vertebral, proximal humerus, pelvis, or, in some cases, distal forearm fracture; and when FRAX shows fracture risk above the country-specific threshold for treatment (for the US, this is 10-year probability of major osteoporotic fracture ≥ 20% or 10-year probability of hip fracture ≥ 3%).
Table 1.
World Health Organization criteria for classification of patients with bone mineral density measured by dual-energy X-ray absorptiometry (3).
| Classification | T-score |
|---|---|
| Normal | -1.0 or greater |
| Low bone mass (osteopenia) | Between 1-.0 and -2.5 |
| Osteoporosis | -2.5 and below |
| Severe osteoporosis | -2.5 and below + fragility fracture |
The National Osteoporosis Foundation (NOF) indications for BMD testing in the US (7), which are similar to the ISCD Official Positions (4), are listed in Table 2. BMD testing should be done when it is likely to have an influence on patient management decisions. Other organizations and other countries with different economic resources and health care priorities have used a variety of methodologies to develop alternative recommendations (8-10).
Table 2.
National Osteoporosis Foundation recommends that bone mineral density testing be performed at DXA facilities using accepted quality assurance procedures for the following individuals (7).
| All women age 65 years and older and men age 70 years and older |
| Postmenopausal women and men above age 50-69 years, based on risk factor profile |
| Postmenopausal women and men over age 50 years who have had an adult-age fracture, to diagnose and determine the degree of osteoporosis |
FRACTURE RISK ASSESSMENT
There is a robust correlation between BMD and fracture risk, with approximately a 2-fold increase in fracture risk for every 1 standard deviation (SD) decrease in BMD (11). However, many or most patients with a hip fracture have a T-score better than -2.5 (12); although fracture risk is higher in patients with very low BMD, there are numerically many more patients with a T-score better than -2.5 than with a T-score of -2.5 or worse, therefore numerically more fractures in those with higher T-scores. The presence of clinical risk factors (CRFs) that are independent of BMD, particularly advancing age, prior fracture, and recency of fracture, can identify patients at high risk for fracture by providing information on fracture risk that is complementary to BMD. The NOF has provided an extensive list of CRFs (Table 3) for osteoporosis and fractures. Since most fractures occur as a result of a fall, it is helpful to recognize risk factors for falling (Table 4) so that appropriate interventions can be made, when possible, to reduce the chances of falling.
Table 3.
Conditions, diseases and medications that cause or contribute to osteoporosis and fractures (7).
| Lifestyle Factors | |||
| Low Calcium Intake | Vitamin D Insufficiency | Excess Vitamin A | High Caffeine Intake |
| High Salt Intake | Aluminum (in antacids) | Inadequate Physical Activity | Immobilization |
| Smoking | Falling | Thinness | Alcoholism |
| Genetic Factors | |||
| Cystic Fibrosis | Homocystinuria | Osteogenesis Imperfecta | Ehlers-Danlos Syndrome |
| Hypophosphatasia | Gaucher’s Disease | Idiopathic Hypercalciuria | Porphyria |
| Glycogen storage diseases | Marfan Syndrome | Riley-Day Syndrome | Hemochromatosis |
| Menkes Steely Hair Syndrome | Parental History of Hip Fracture | Androgen Insensitivity | Turner’s & Klinefelter’s Syndromes |
| Endocrine Disorders | |||
| Adrenal Insufficiency | Diabetes Mellitus | Hyperthyroidism | Cushing’s Syndrome |
| Hyperparathyroidism | Hypogonadal States | Panhypopituitarism | Athletic Amenorrhea |
| Anorexia Nervosa and Bulimia | Hyperprolactinoma | Premature Ovarian Failure | |
| Gastrointestinal disorders | |||
| Celiac Disease | Inflammatory Bowel Disease | Primary Biliary Cirrhosis | Gastric Bypass |
| Malabsorption | GI Surgery | Pancreatic Disease | |
| Hematologic Disorders | |||
| Hemophilia | Multiple Myeloma | Systemic Mastocytosis | Leukemia |
| Lymphoma | Sickle Cell Disease | Thalassemia | |
| Rheumatic and Autoimmune Diseases | |||
| Ankylosing Spondylitis | Lupus | Rheumatoid Arthritis | |
| Miscellaneous Conditions and Diseases | |||
| Chronic Obstructive Pulmonary Disease | Muscular Dystrophy | Amyloidosis | End Stage Renal Disease |
| Parenteral Nutrition | Chronic Metabolic Acidosis | Epilepsy | Post-Transplant Bone Disease |
| Congestive Heart Failure | Idiopathic Scoliosis | Prior Fracture as an Adult | Depression |
| Multiple Sclerosis | Sarcoidosis | HIV/AIDS | |
| Medications | |||
| Anticoagulants (heparin) | Cancer Chemotherapeutic Drugs | Gonadotropin Releasing Hormone Agonists | Anticonvulsants |
| Lithium | Aromatase Inhibitors | Depo-medroxyprogesterone | Barbiturates |
| Glucocorticoids (> 5mg of prednisone or equivalent for > 3 months) | Cyclosporine A | Tacrolimus | |
Table 4.
Risk factors for falls adapted from guidelines of the National Osteoporosis Foundation (7).
| Environmental Risk Factors |
| Lack of assistive devices in bathrooms, loose throw rugs, low level lighting, obstacles in the walking path, slippery outdoor conditions |
| Medical Risk Factors |
| Age, anxiety and agitation, arrhythmias, dehydration, depression, female gender, impaired transfer and mobility, malnutrition, orthostatic hypotension, poor vison and use of bifocals, previous fall, reduced mental acuity and diminished cognitive skills, urgent urinary incontinence, Vitamin D insufficiency (serum 25-OH-D < 30ng/ml (75nmol/l)), medications causing over-sedation (narcotic analgesics, anticonvulsants, psychotropics), diabetes |
| Neurological and Musculoskeletal Risk Factors |
| Kyphosis, poor balance, reduced proprioception, weak muscles |
| Other Risk Factors; Fear of falling |
| The presence of any of these risk factors should trigger consideration of further evaluation and treatment to reduce the risk of falls and fall-related injuries. |
VERTEBRAL FRACTURE ASSESSMENT (VFA)
VFA is a method for imaging the thoracic and lumbar spine by DXA for the purpose of detecting vertebral fracture deformities. Identification of a previously unrecognized vertebral fracture may alter diagnostic classification, change estimation of fracture risk, and influence treatment decisions (13). VFA compares favorably with standard radiographs of the spine, with good correlation for detecting moderate (grade 2) and severe (grade 3) vertebral fractures, a smaller dose of ionizing irradiation, greater patient convenience (i.e., it may be done at the same visit and with the same instrument as BMD testing by DXA), and lower cost. In a study of women age 65 and older, using the Genant semi-quantitative (SC) method of classifying vertebral deformities (14), the sensitivity of VFA for diagnosing moderate and severe vertebral fractures was 87-93%, with a specificity of 93-95% (15). Indications for vertebral imaging are listed in Table 5. Optimal use of DXA and VFA requires training and adherence to well established quality standards (4).
Table 5.
International Society for Clinical Densitometry (ISCD) indications for lateral spine imaging by standard radiography or vertebral fracture assessment (VFA).
| The ISCD Official Positions (4) state that vertebral imaging is indicated when the T-score is < -1.0 and one or more of the following is present: |
| Women > 70 years of age or men > 80 years of age |
| Historical height loss > 4cm (1.5 inches) |
| Self-reported but undocumented prior vertebral fracture |
| Glucocorticoid therapy equivalent to ≥ 5 mg of prednisone or equivalent per day for ≥ 3 months |
QUALITY OF DXA AND VFA
DXA and VFA should be performed by well-trained and experienced staff operating an instrument that has been maintained and calibrated according to the manufacturer’s standards. Precision assessment and least significant change (LSC) calculation by each DXA technologist are required in order to make quantitative comparisons of serial BMD measurements. The LSC is the smallest change in BMD that is statistically significant, usually with a 95% level of confidence. The use of the correct scan modes, proper patient positioning, consistent vertebral body labeling, and bone edge detection are among the essential elements for serial comparisons of BMD. VFA should be done by a technologist properly trained in acquisition techniques and interpreted by a clinician familiar with methods of diagnosing vertebral fractures using this technology. Bone densitometry facilities should be supervised by a clinician who knows current methods for BMD measurement and fully understands the standards for quality control, interpretation, and reporting of the findings. Poor quality studies may result in inappropriate clinical decisions, generate unnecessary healthcare expenses, and be harmful to patients (5). Assurances of high quality DXA can be attained through education, training, and certification of DXA technologists and interpreters by organizations such as the ISCD. DXA facilities should understand and adhere to ISCD Official Positions and DXA Best Practices; facility accreditation provides assurance of adherence to DXA quality standards (4,16,17).
TECHNOLOGIES FOR ASSESSMENT OF SKELETAL HEALTH
Dual-Energy X-Ray Absorptiometry (DXA)
Devices that measure or estimate BMD differ according to their clinical utility, cost, portability, and use of ionizing radiation (Table 6). DXA is the “gold standard” method for measuring bone density in clinical practice (18). There is a strong correlation between mechanical strength and BMD measured by DXA biomechanical studies (19). In observational studies of untreated patients, there is a robust relationship between fracture risk and BMD measured by DXA (11). The WHO diagnostic classification of osteoporosis is based primarily on reference data obtained by DXA (3), and femoral neck BMD provides input into the FRAX algorithm. Most randomized clinical trials showing reduction in fracture risk with pharmacological therapy have selected study participants according to BMD measured by DXA (20). There is a relationship between fracture risk reduction with drug therapy and increases in BMD measured by DXA (21). Accuracy and precision of DXA are excellent (22). Radiation exposure with DXA is very low (23). BMD of the 33% (one-third) radius, measured either by a dedicated peripheral DXA (pDXA) device or a central DXA instrument with appropriate software, may be used for diagnostic classification with the WHO criteria and to assess fracture risk, but is generally not clinically useful in monitoring the effects of treatment (23). DXA measures bone mineral content (BMC in grams [g]) and bone area (cm2), then calculates areal BMD in g/cm2 and derives parameters, such as the T-score and Z-score. DXA is used for diagnostic classification, assessment of fracture risk, and for monitoring changes in BMD over time.
Table 6.
Devices for measuring or estimating bone mineral density (BMD)
| DXA | pDXA | QUS | QCT | pQCT | |
|---|---|---|---|---|---|
| Diagnostic classification* | Yes | Limited** | No | Yes*** | No |
| Measurement | Areal BMD | Areal BMD | SOS, BUA | Volumetric BMD | Volumetric BMD |
| Prediction of fracture risk | Yes | Yes | Yes | Yes | Yes |
| Monitoring changes over time | Yes | No | No | Yes | No |
| Ionizing radiation | ++ | + | 0 | +++ | ++ |
| Cost | ++ | + | + | +++ | ++ |
Clinical applications of different technologies are listed with approximate comparison of associated radiation exposure and cost, with 0 = none, + = low, ++ moderate, +++ = highest.
DXA = dual-energy X-ray absorptiometry; pDXA = peripheral DXA; QUS = quantitative ultrasound; QCT = quantitative computed ultrasound; pQCT = peripheral QCT; SOS = speed of sound; BUA = broadband ultrasound attenuation; * World Health Organization classification; **pDXA of the distal one-third radius (33% radius) may be used with the WHO classification; *** Total hip and femoral neck T-scores calculated from 2D projections of QCT data may be used with the WHO classification
Quantitative Ultrasound (QUS)
QUS devices emit inaudible high frequency sound waves in the ultrasonic range, typically between 0.1 and 1.0 megahertz (MHz). The sound waves are produced and detected by means of high-efficiency piezoelectric transducers, which must have good acoustical contact with the skin over the bone being tested. Technical differences among QUS systems are great, with different instruments using variable frequencies, different transducer sizes, and sometimes measuring different regions of interest, even at the same skeletal site. The calcaneus is the skeletal site most often tested, although other bones, including the radius, tibia, and finger phalanges, can be used. Commercial QUS systems usually measure two parameters- the speed of sound (SOS) and broadband ultrasound attenuation (BUA). A proprietary value, such as the “quantitative ultrasound index” (QUI) with the Hologic Sahara or “stiffness index” with the GE Healthcare Achilles Express, may be calculated from a combination of these measurements. SOS varies according to the type of bone, with a typical range of 3000-3600 meters per second (m/sec) with cortical bone and 1650-2300 m/sec for trabecular bone (24). A higher bone density is associated with a higher SOS. BUA, reported as decibels per megahertz (dB/MHz), is a measurement of the loss of energy, or attenuation, of the sound wave as it passes through bone. As with SOS, a higher bone density is associated with a higher BUA. Values obtained from calculations using ultrasound parameters may be used to generate an estimated BMD and a T-score. The T-score derived from a QUS measurement is not the same as a T-score from a DXA. QUS cannot be used for diagnostic classification and is not clinically useful to monitor the effects of therapy (25).
Quantitative Computed Tomography (QCT) and Peripheral QCT (pQCT)
QCT and pQCT measure trabecular and cortical volumetric BMD at the axial skeleton and peripheral skeletal sites, respectively. QCT is a useful research tool to enhance understanding of the pathophysiology of osteoporosis and the mechanism of action of pharmacological agents used to treat osteoporosis. QCT predicts fracture risk, with the correlation varying according to skeletal site and bone compartment measured, type of fracture predicted, and population assessed (26). The ISCD Official Positions state that “spinal trabecular BMD as measured by QCT has at least the same ability to predict vertebral fractures as AP spinal BMD measured by central DXA in postmenopausal women with lack of sufficient evidence to support this position in men; pQCT of the forearm at the ultra-distal radius predicts hip, but not spine, fragility fractures in postmenopausal women with lack of sufficient evidence to support this position in men (26).” QCT is more expensive than DXA and QUS and uses higher levels of ionizing radiation than DXA. T-scores by QCT are typically lower than with DXA (27), thereby overestimating the prevalence of osteoporosis, with the exception of total hip and femoral neck T-scores calculated from 2D projections of QCT data, which are similar to DXA-derived T-scores at the same regions of interest and may be used for diagnosis of osteoporosis in accordance with the WHO criteria. T-scores and femoral neck BMD derived from 2D projections of QCT data may also be used as input for the FRAX algorithm to estimated 10-year fracture probabilities.
FRACTURE RISK ASSESSMENT TOOL (FRAX®)
The combination of BMD and clinical risk factors (CRFs) predicts fracture risk better than BMD or CRFs alone (28,29) (2). A fracture risk assessment tool (FRAX) combines CRFs and femoral neck BMD in a computer-based algorithm that estimates the 10-year probability of hip fracture and major osteoporotic fracture (i.e., clinical spine, hip, proximal humerus, and distal forearm fracture). FRAX can be accessed online at http://www.shef.ac.uk/FRAX (Figure 1), on most software versions of DXA systems, and on smartphones. FRAX is based on analysis of data from 12 large prospective observational studies in about 60,000 untreated men and women in different world regions, having over 250,000 person-years of observation and more than 5,000 reported fractures reported.
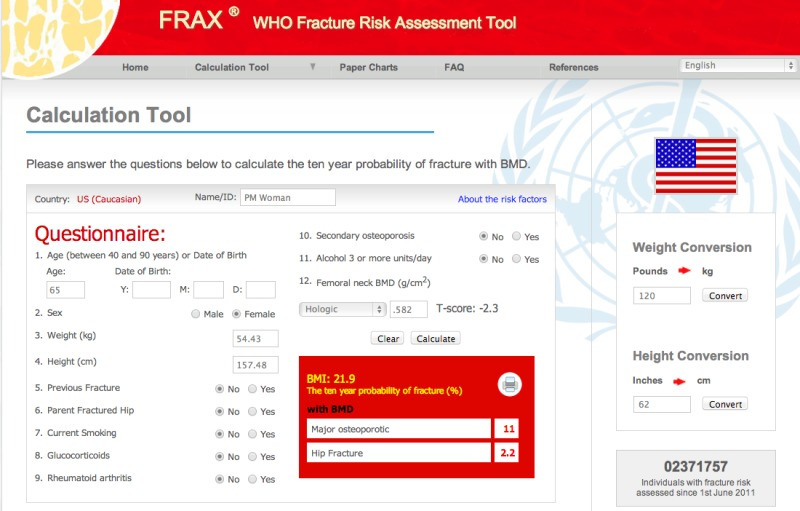
Figure 1.
FRAX online for US Caucasian patients. This example shows a 65-year-old woman who has no clinical risk factors for fracture and a femoral neck BMD of 0.582 g/cm2 with a Hologic instrument. The 10-year probability of major osteoporotic fracture is 11% and the 10-year probability of hip fracture is 2.2%. These levels do not meet the National Osteoporosis Foundation guidelines for initiation of pharmacological therapy in the US (7). Image reproduced with permission of the World Health Organization.
The input for FRAX is the patient’s age, sex, height, weight, a “yes” or “no” response indicating the presence or absence for each of 7 CRFs: 1. previous ‘spontaneous’ or fragility fracture as an adult; 2. parent with hip fracture; 3. current tobacco smoking; 4. ever use of chronic glucocorticoids at least 5 mg prednisolone for at least 3 months; 5. confirmed rheumatoid arthritis; 6. secondary osteoporosis, such as type 1 diabetes, osteogenesis imperfecta in adults, untreated longstanding hyperthyroidism and hypogonadism, or premature menopause (note: this is a “dummy” risk factor that has no effect on the fracture risk calculation unless no femoral neck BMD value is entered); 7. alcohol intake greater than 3 units per day, with a unit of alcohol defined as equivalent to a glass of beer, an ounce of spirits or a medium-sized glass of wine), and if available, femoral neck BMD and trabecular bone score (TBS). Since the introduction of FRAX, upgrades have been introduced to correct errors, enhance its usability, and incorporate new data that have become available.
Benefits of FRAX
The use of FRAX provides a quantitative estimation of fracture risk that is based on robust data in large populations of men and women with ethnic and geographic diversity. Expression of fracture risk as a probability provides greater clinical utility for than relative risk. When combined with cost-utility analysis, a fracture risk level at which it is cost-effective to treat may be derived. FRAX can be used to estimate fracture probability without femoral neck BMD, allowing it to be used when DXA in unavailable or inaccessible. FRAX is incorporated into many clinical practice guidelines.
Limitations of FRAX
To generate a valid FRAX output, the responses to CRF questions must be correct; for example, an incorrect entry of self-reported rheumatoid arthritis or use of glucocorticoids could skew the results toward overestimation of fracture risk. FRAX may underestimate or overestimate fracture risk due to dichotomized (yes or no) input for CRFs that in reality are associated with a range of risk that varies according to dose, duration of exposure, or severity; for example, fracture risk may be underestimated when a patient is on high-dose glucocorticoid therapy or has had multiple recent fragility fractures, even when a “yes” response is entered for these CRFs. FRAX is validated only in untreated patients and may overestimate fracture risk when the patient is being treated; the NOF/ISCD guidance on FRAX suggests that “untreated” may be interpreted as never treated or if previously treated, no bisphosphonate for the past 2 years (unless it is an oral agent taken for less than 2 months); and no estrogen, raloxifene, calcitonin, or denosumab for the past 1 year (7). In this context, calcium and vitamin D do not constitute treatment. FRAX in the US allows input for 4 ethnicities (Caucasian, Black, Hispanic, Asian); it is not clear how to use FRAX for patients of other ethnicities or a mix of these ethnicities. Answering “yes” for the category of secondary osteoporosis has no effect on the fracture risk calculation as long as a value for femoral neck BMD is entered. The range of error for a fracture probability generated by FRAX is unknown but may be substantial in some cases. Some important risk factors, such as falls and frailty, are not directly entered into FRAX, although they are indirectly included insofar as they are a component of aging. FRAX may underestimate fracture risk when the lumbar spine BMD is substantially lower than femoral neck BMD, as may occur in about 15% of patients (30).
Despite the numerous limitations of FRAX, it is a helpful clinical tool when used with a good understanding of factors that may result in underestimation or overestimation of fracture risk. FRAX may enhance discussion of risk with the patient and help to identify those who are at sufficiently high for fracture to benefit from therapy.
MEDICAL HISTORY
A thorough medical history may identify risk factors for osteoporosis and fractures, suggesting that a bone density test and/or further evaluation is indicated. The medical history may also reveal symptoms of potentially correctable causes of skeletal fragility (e.g., gluten intolerance with celiac disease) or co-morbidities that could influence treatment decisions (e.g., esophageal stricture suggests that oral bisphosphonates should not be given). A history of falls is a predictor of future falls, with that risk potentially modifiable though appropriate interventions. Finally, some symptoms may trigger further evaluation for the presence of fractures (e.g., historical height loss or development of kyphotic posture suggests the possibility of vertebral fractures that may warrant spine imaging). Table 7 provides examples of helpful information that might be obtained from a thoughtful interactive discussion with the patient.
Medical History for Patients with Osteoporosis
A thorough review of systems and history of relevant familial disorders, previous surgical procedures, medications, dietary supplements, food intolerances, and lifestyle provide helpful information in the management of patients with osteoporosis. Such historical information may play a role in determining who should have a bone density test, assessing fracture risk, providing input for FRAX®, evaluating for secondary causes of osteoporosis, selecting the most appropriate treatment to reduce fracture risk, and finding factors contributing to suboptimal response to therapy. Listed here are key components of the skeletal health history and examples of the potential impact on patient care.
Table 7.
Clinical Utility of the Medical History
| Clinical Utility | Medical History |
|---|---|
| Assist in determining who need a bone density test | See Table 3 |
| Assessing fracture risk | See Table 3 and 4 |
| Input for FRAX® | Age, sex, weight, height, previous fracture, parent with hip fracture, current tobacco smoking, ever use of glucocorticoids, rheumatoid arthritis, secondary osteoporosis, alcohol intake 3 or more units per day, and if available, femoral neck bone mineral density and trabecular bone score |
| Evaluating for secondary causes of osteoporosis | See Table 3 |
| Selecting most appropriate treatment | Identify co-morbidities of clinical significance. For example, high risk of breast cancer favors raloxifene use, while history of thrombophlebitis suggests that raloxifene should not be used; esophageal stricture is a contraindication for oral bisphosphonate use; a patient with a skeletal malignancy should not be treated with teriparatide. |
| Factors contributing to suboptimal response to therapy | Compliance and persistence to therapy; adequacy of calcium and vitamin D; comorbidities listed in Table3. |
PHYSICAL EXAM
Findings of importance on the physical exam of a patient with osteoporosis may be the sequelae of old fractures (e.g., kyphosis due to old vertebral fractures), a consequence of a recent fracture (e.g., localized vertebral spinous process tenderness with a new vertebral fracture), or abnormalities suggestive of a secondary cause of osteoporosis (e.g., thyromegaly with thyrotoxicosis). An accurate measurement of height with a wall-mounted stadiometer is a helpful office tool for evaluating patients at risk for fracture. A height loss of 1.5 inches (4.0 cm) or more compared to the historical maximum (31,32) or a loss of 0.75 inches (2.0 cm) or more compared to a previous measured height (33) suggests a high likelihood of vertebral fracture. Body weight measurement is part of the osteoporosis evaluation because low body weight (less than 127 lbs) (34), low BMI (20 kg/m2 or less) (35), and weight loss of 5% or more (36) are associated with increased risk of fracture. Localized tenderness of the spine, kyphosis, or diminished distance between the lower ribs and the pelvic brim may be the result of one or more vertebral fractures. Abnormalities of gait, posture, balance, muscle strength, or the presence of postural hypotension or impaired level of consciousness may be associated with increased risk of falling. Bone tenderness may be the caused by osteomalacia. Atrophic testicles suggest hypogonadism. Patients should be observed for stigmata of hyperthyroidism or Cushing’s syndrome. Blue sclera, hearing loss, and yellow-brown teeth are suggestive of osteogenesis imperfecta. Joint hypermobility and skin fragility could be due to Ehlers-Danlos syndrome. Urticaria pigmentosa may occur with systemic mastocytosis. Table 8 shows examples of abnormal physical exam findings with osteoporosis.
Table 8.
Focused Physical Examination in a Patient with Osteoporosis
| Component of physical exam | Example of finding of potential skeletal importance | Potential clinical implications for skeletal health |
|---|---|---|
| Vital signs | Low body weight or body mass index | Anorexia nervosa |
| Loss of height | Vertebral fracture | |
| Loss of weight | Malignancy, malabsorption | |
| Skin | Urticaria pigmentosa Dermatitis herpetiformis | Systemic mastocytosis Celiac disease |
| Striae, acne | Cushing’s syndrome, exogenous glucocorticoids | |
| Head | Cranial dysostosis | Hypophosphatasia |
| Eyes | Blue sclera | Osteogenesis imperfect |
| Ears | Hearing loss | Osteogenesis imperfecta, sclerosteosis |
| Nose | Anosmia | Kallmann syndrome |
| Throat | Poor dentition | Increased risk of osteonecrosis of the jaw |
| Neck | Thyromegaly | Thyrotoxicosis |
| Lungs | Decreased breath sounds | Chronic obstructive pulmonary disease |
| Heart | Aortic insufficiency | Marfan’s syndrome |
| Musculoskeletal | Kyphosis | Vertebral fractures |
| Spinous process tenderness | Acute vertebral fracture | |
| Decreased space between lower ribs and pelvis | Vertebral fractures | |
| Tender bones | Osteomalacia | |
| Inflammatory joint disease | Rheumatoid arthritis | |
| Hypermobility of joints | Ehlers-Danlos syndrome | |
| Muscle weakness | Vitamin D deficiency, osteomalacia | |
| Abdomen | Hepatomegaly | Chronic liver disease |
| Surgical scars | Bariatric surgery, gastrectomy | |
| Genitalia | Testicular atrophy | Hypogonadism |
| Neurological | Poor balance | High fall risk, vitamin D deficiency |
| Dementia | Poor adherence to therapy, high fall risk |
This table provides examples of findings on physical exam that may be helpful in the evaluation of skeletal health. It is not intended to show all findings of importance.
EVALUATION FOR SECONDARY CAUSES OF OSTEOPOROSIS
The possibility of previously unrecognized causes of skeletal fragility should be considered in every patient with osteoporosis (37). After an initial medical history is taken and physical exam is performed, appropriate laboratory testing and imaging may provide information that is critical for ongoing patient care. Osteoporosis is commonly divided into two categories according to etiology. “Primary osteoporosis” is due to time-appropriate postmenopausal estrogen deficiency (type I osteoporosis, preferentially involving trabecular bone loss) or to aging in men and women (type II osteoporosis, with a combination of trabecular and cortical bone loss). “Secondary osteoporosis” is osteoporosis caused by conditions, diseases, or medications other than estrogen deficiency or aging.
The reported prevalence of secondary osteoporosis varies depending on the study population, the extent of the medical evaluation, and definitions for laboratory abnormalities. It is likely that many or most patients with primary osteoporosis have clinically significant contributing factors that may influence patient management. In a study of North American women receiving osteoporosis therapy, it was found that 52% had vitamin D inadequacy, defined as serum 25-hydroxyvitamin D (25-OH-D) levels less than 30 ng/ml (38). In another study of patients referred to an osteoporosis clinic, over 60% were found to have elements of secondary osteoporosis when vitamin D deficiency was very conservatively defined as serum 25-OH-D level less than 12.5 ng/ml (39,40). In the same study, the number of patients with secondary osteoporosis was much higher when vitamin D inadequacy was more appropriately defined as serum 25-OH-D less than 33 ng/ml (41,42).
It has been proposed by some that a bone density that is less than expected compared to an age- and sex-matched population, as represented by a low Z-score (e.g., less than -2.0), suggests a high likelihood of secondary osteoporosis and should be one of the triggers for further investigation (43,44). While there may be some merit to this concept, there are few if any studies validating the use of a Z-score cutoff for this purpose. Since secondary osteoporosis is common, a more effective strategy is to screen all patients with osteoporosis for contributing factors (45). The results of a metabolic evaluation may identify previously unrecognized diseases and conditions that require treatment in addition to, or instead of, standard osteoporosis pharmacological therapy.
Depending on the patient population being studied, different causes of secondary osteoporosis may predominate. Calcium deficiency, vitamin D deficiency, and sedentary lifestyle are common contributing factors for all patients. In women referred to an osteoporosis clinic with previously recognized medications or diseases contributing to osteoporosis, the most common were history of glucocorticoid use (36%), premature ovarian failure (21%), history of unintentional weight loss (10%), history of alcoholism (10%), and history of liver disease (10%) (39). When patients without previously recognized contributing factors were evaluated at the same specialty clinic, most (55%) were found to have vitamin D deficiency or insufficiency (serum 25-OH-D less than 33 ng/ml) (42), while 10% had hypercalciuria, 8% had malabsorption, and 7% had primary or secondary hyperparathyroidism (39). In men, the most common secondary causes of osteoporosis are long-term glucocorticoid use, hypogonadism, and alcoholism (46,47). The increasing use of aromatase inhibitor therapy for breast cancer in women (48) and androgen deprivation therapy for prostate cancer in men (49) is now recognized as an important factor in the development of osteoporosis in these patients. Other common causes for low BMD and fractures include multiple myeloma (50), gastric bypass surgery (51) and gastric resection (52). Treatable but easily missed secondary causes of osteoporosis include asymptomatic primary hyperparathyroidism (53), subclinical hyperthyroidism (54), mild Cushing’s syndrome (55), and malabsorption due to unrecognized celiac disease (56). Table 9 lists some of the causes of low BMD by category.
Table 9.
Causes of Low Bone Mineral Density
| Inherited | Nutritional | Endocrine | Drugs | Other |
|---|---|---|---|---|
| Osteogenesis imperfecta | Malabsorption | Hypogonadism | Glucocorticoids | Multiple myeloma |
| Homocystinuria | Chronic liver disease | Hyperthyroidism | Anticonvulsants | Rheumatoid arthritis |
| Marfan’s syndrome | Alcoholism | Hyperparathyroidism | Long-term heparin | Systemic mastocytosis |
| Hypophosphatasia | Calcium deficient diet | Cushing’s syndrome | Excess thyroid | Immobilization |
| Vitamin D deficiency | Eating disorder | GnRH agonists | ||
| Aromatase inhibitors |
A variety of testing strategies have been proposed as screening for all patients with osteoporosis (37,39,42,45,57,58). A minimal cost-effective work-up for all patients consists of a complete blood count (CBC), serum calcium, phosphorus, creatinine with calculated or measured creatinine clearance, alkaline phosphatase, 24-hour urinary calcium, and serum 25-OH-D. Other laboratory tests may be indicated according to the patient’s clinical profile and the practice setting. A summary of useful common and uncommon laboratory studies with comments on their possible skeletal significance is provided below.
CLINICAL CASE
A 52-year-old postmenopausal woman with a history of irritable bowel syndrome (IBS) and a family history of osteoporosis (mother with hip fracture) is found to have osteoporosis on a DXA study. Evaluation for secondary causes of osteoporosis is unremarkable except for mild iron deficiency anemia (a long-standing problem, previously attributed to heavy menses) and a low 24-hour urinary calcium of 30 mg, with adequate calcium intake and normal renal function. Serum 25-OH-D is 29 ng/ml. Additional work-up shows a high titer of IgA endomysial antibodies consistent with celiac disease. This diagnosis is confirmed by a small bowel biopsy showing villous atrophy. She is started on a gluten-free diet, resulting in resolution of her “IBS” symptoms and correction of her anemia. One year later, with no pharmacological therapy for osteoporosis, there is a statistically significant BMD increase of 9% at the lumbar spine.
Celiac disease may result in osteoporosis due to calcium malabsorption, even in the absence of gastrointestinal symptoms. Treatment is strict lifelong adherence to a gluten-free diet, which may sometimes be followed by a substantial increase in BMD, as seen in this patient. A 24-hour urinary calcium is an inexpensive screening test for calcium malabsorption that should be considered a routine part of the initial evaluation of osteoporosis.
BASIC BLOOD TESTS
CBC- Anemia may be seen in patients with myeloma or malnutrition
Sedimentation rate- May be elevated with myeloma.
Calcium- Among the many causes of hypercalcemia are primary and secondary hyperparathyroidism, hyperthyroidism, renal failure, vitamin D intoxication, and Paget’s disease. Hypocalcemia may be seen with vitamin D deficiency and hyperphosphatemia.
Phosphorus- Hyperphosphatemia may occur with hypoparathyroidism, renal failure, and possibly with bisphosphonate therapy. Hypophosphatemia may be seen with primary or secondary hyperparathyroidism, vitamin D deficiency, tumor induced osteomalacia, and X-linked hypophosphatemia.
Alkaline phosphatase- High values can be seen with healing fractures, osteomalacia, and Paget’s disease, as well as occurring normally in growing children. Low values occur with hypophosphatasia, a rare genetic disorder that causes impaired mineralization of bone and dental tissue.
Vitamin D- The test that best reflects vitamin D stores is the serum 25-OH-D. While there is no consensus on the optimal range of serum 25-OH-D, a reasonable target for good skeletal health is approximately 30-50 ng/ml. This is likely to maximize intestinal absorption of calcium and minimize serum PTH levels. Interpretation of serum 25-OH-D levels is confounded by assay variability (59). Serum 1,25-(OH)2-D3 is usually not helpful in the evaluation of osteoporosis patients, unless there are concerns regarding renal conversion of 25-OH-D to 1,25-(OH)2-D3. Deficiency or insufficiency of vitamin D is very common and play a role in the pathogenesis of osteoporosis and osteomalacia.
Creatinine- Chronic kidney disease may cause an elevated creatinine level and renal osteodystrophy. Elderly patients with small muscle mass may have impaired renal function with a “normal” serum creatinine. An estimated glomerular filtration rate can be calculated using one of many formulae, such as that of Cockcroft and Gault (60) or modification of diet in renal disease study equation (61). Impaired renal function not only has adverse skeletal effects but also raises considerations regarding the type and dose of pharmacologic agents used.
TSH- Hyperthyroidism from any cause, including excess thyroid replacement, can usually be recognized by a low TSH. High bone turnover associated hyperthyroidism is associated with loss of bone mass.
Liver enzymes- Abnormalities may be caused by chronic liver disease, which is a risk factor for osteoporosis.
BASIC URINE TESTS
Urinalysis. Proteinuria may occur with multiple myeloma or chronic kidney disease. Abnormal cells may suggest kidney disease.
24-hour urine for calcium- A well-collected 24-hour urine for calcium is a helpful screening test for identifying patients with common disorders of calcium metabolism. The “normal” range of urinary calcium is not well established and varies according to many dietary factors and estrogen status in women (62,63). As a “rule of thumb,” urinary calcium may be considered elevated when it is greater than 250 mg per 24 hours in women; greater than 300 mg per 24 hours in men; or greater than 4 mg/kg body weight per 24 hours in either sex. It has been proposed that hypercalciuria can be easily classified as “renal” (renal calcium leak), “resorptive” (excess skeletal loss of calcium) or “absorptive” (increased intestinal absorption of calcium) (64). However, in clinical practice, these distinctions are not so easily established. Idiopathic hypercalciuria, perhaps the most common type of hypercalciuria (65), may be diagnosed if there are no underlying medical disorders (e.g., hyperparathyroidism, vitamin D toxicity, Paget’s disease of bone, multiple myeloma, sarcoidosis) and no obvious dietary excesses (e.g., calcium, sodium, protein, carbohydrates, alcohol) or deficiencies (e.g., phosphate, potassium) that are associated with hypercalciuria (63). In the absence of dietary calcium deficiency, vitamin D deficiency, malabsorption, liver disease, or chronic renal failure, low urinary calcium (less than 50 mg per 24 hours in women or men) is suggestive of calcium malabsorption and warrants further investigation. Celiac disease is a common (66) cause of asymptomatic malabsorption in osteoporosis that is treatable with a gluten-free diet (56).
ADDITIONAL STUDIES IN SELECTED PATIENTS
Celiac antibodies- Anti-endomysial antibody and tissue transglutaminase antibody are currently the serological markers of choice, with a higher sensitivity and specificity than anti-gliadin antibody and anti-reticulin antibody (67). If a serological marker is abnormal, or if there is a high clinical suspicion for celiac disease, the patient should be referred for endoscopy and small bowel biopsy.
Intact PTH- This may be elevated in patients with primary hyperparathyroidism, vitamin D deficiency, or renal failure.
Serum and urine protein electrophoresis- These are helpful tests to screen for possible multiple myeloma. If an M-component is identified, referral for bone marrow aspiration may be indicated.
Dexamethasone suppression test or 24-hour urinary free cortisol- This is helpful to evaluate patients with suspected Cushing’s syndrome.
Serum total or free testosterone level- May be helpful in the assessment of men with osteoporosis.
Serum homocysteine- Elevated circulating homocysteine levels are associated with increased risk of fracture (68,69). It is unknown whether reduction of homocysteine levels by increasing dietary intake of folic acid and vitamins B6 and B12 reduces the risk of fracture.
Serum tryptase and 24-hour urine for N-methylhistamine- Systemic mastocytosis is a rare cause of osteoporosis that can be diagnosed by a biopsy of typical skin lesions of urticaria pigmentosa, when present. Patients with systemic mastocytosis may sometimes present with osteoporosis and no other manifestations of the disease (70,71). When this disorder is suspected but skin lesions are not present, the finding of an elevated serum tryptase and/or urinary N-methyl histamine can be helpful, especially during or soon after a symptomatic episode of histamine release. However, normal values do not exclude the diagnosis. Bone marrow aspiration or biopsy, or non-decalcified double tetracycline labeled transiliac bone biopsy, may be necessary to confirm the diagnosis.
Serum bicarbonate- Renal tubular acidosis (RTA) has been associated with osteoporosis (72). With distal (type I) RTA, the serum bicarbonate is usually less than 15 mmol/l with a urine pH greater than 5.5.
BONE TURNOVER MARKERS
Bone turnover markers (BTMs) are noninvasive laboratory tests of serum and urine that are readily available in clinical practice. While BTMs cannot be used to diagnose osteoporosis or determine the cause to osteoporosis, they have been very helpful in the research to understand the pathophysiology of osteoporosis and other skeletal diseases and the mechanism of action of interventions used in the treatment of osteoporosis. In clinical practice, BTMs offer the potential of predicting fracture risk independently of BMD and may be useful in monitoring the metabolic effects of therapy (73). Drugs that are approved for the management of osteoporosis modulate bone remodeling in ways that are reflected by changes in BTMs. A decrease in BTMs with antiresorptive therapy is predictive of a subsequent increase in BMD (74) and reduction in fracture risk (75-77). The magnitude of BTM decrease with antiresorptive therapy is significantly associated with the level of fracture risk reduction, although the proportion of treatment effect due to the reduction in BTMs appears to vary according to the type of drug used (79). With teriparatide, a bone anabolic agent, an early increase in BTM levels is predictive of a subsequent increase in BMD (80).
Markers of bone resorption are mostly fragments of type I collagen, the main component of the organic bone matrix, that are released during osteoclastic bone resorption. These are measured in the serum or urine, with those available for clinical use including N-telopeptide of type I collagen (NTX), C-telopeptide of type I collagen (CTX), deoxypyridinoline (DPD), and pyridinoline (PYD). Bone formation markers are proteins secreted by osteoblasts or byproducts of type I collagen production by osteoblasts. They are measured in the serum and include bone specific alkaline phosphatase (BSAP), N-terminal propeptide of type I collagen (P1NP), and osteocalcin. CTX and P1NP have been proposed as the reference BTMs for clinical trials. (Samuel Vasikaran 1, Cyrus Cooper, Richard Eastell, Andrea Griesmacher, Howard A Morris, Tommaso Trenti, John A Kanis, International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis, Clin Chem Lab Med. 2011 Aug;49(8):1271-4)
Clinical use of BTMs requires knowledge of their limitations as well as benefits. BTMs are subject to pre-analytical (biological) and analytical variability. Uncontrollable sources of pre-analytical variability include age, sex, menopausal status, pregnancy, lactation, fractures, co-existing diseases (e.g., diabetes mellitus, impaired renal function, and liver disease), drugs (e.g., glucocorticoids, anticonvulsants, and gonadotropin hormone releasing agonists) and immobility (81). Controllable pre-analytical sources of variability include time of day (circadian variability), fasting status, and exercise (81). Analytical sources of variability include specimen processing (e.g., collection, handling, and storage) (82). Between-laboratory variability may be large (reported to be as much as a 7.3-fold difference), casting doubt on the validity of comparing specimens sent to different labs (83). Reference ranges for BTMs are not well established and may vary according to the population tested, the type of BTM, and the circumstances under which it is collected and processed.
In order to compare BTMs measurements longitudinally, it would be ideal to know the least significant change (LSC) and use this in a manner similar to what should be (but is probably not) common practice with DXA. However, the standards for calculating an LSC for a BTM are not as clear as with DXA, and the opportunity to do precision assessment for a BTM may not present itself. The NOF recommends calculating the LSC with a 95% level of confidence for each BTM used by multiplying the laboratory-provided precision error by 2.77 (84). The NOF also recommends that specimens be obtained in the early morning following an overnight fast to reduce biological variability, with serial measurements to be obtained at the same time of day and ideally during the same season of the year. The Belgian Bone Club suggests using an estimated LSC of assuming an LSC of about 30% for serum BTMs and about 50-60% for urine BTMs (73). While the LSC for BTMs is almost always greater than for DXA, the magnitude of likely change (85) is greater than DXA, with the ‘signal to noise ratio’ that may be as good or even better than DXA. One strategy for the use of BTMs to monitor patients on antiresorptive therapy is to use absolute values rather that percent changes, as follows: treatment effect can be considered optimal when serum CTX has decreased by 100 ng/L or is below 280 ng/L, or when P1NP has decreased by 10 mcg/L or is less than 35 mcg/L. (Andreas Fontalis, Richard Eastell, The challenge of long-term adherence: The role of bone turnover markers in monitoring bisphosphonate treatment of osteoporosis, Bone. 2020 Jul;136:115336).
Evidence-based guidelines for the clinical use of BTMs have been developed by organizations that include the NOF (7), Belgian Bone Club (73), and the Japan Osteoporosis Society (86). The NOF guidelines state that “suppression of biochemical markers of bone turnover after 3-6 months of specific antiresorptive osteoporosis therapies, and biochemical marker increases after 1-3 months of specific anabolic therapies, have been predictive of greater BMD responses and in some cases fracture risk reduction in large clinical trials. Biochemical marker changes in individuals must exceed the LSC in order to be clinically meaningful.” The Belgian Bone Club suggests that “early changes in BTM can be used to measure the clinical efficacy of an antiresorptive treatment and to reinforce patient compliance,” with goal of decreasing the BTM to the premenopausal range or at least achieving a decrease as great as the LSC. The Japanese guidelines indicate that “the argument for measuring bone turnover markers to evaluate the therapeutic effects of bone antiresorptive medications can be justified,” but go on to state that there is insufficient evidence for their use with medications having other mechanisms of action (86).
A significant change of a BTM level in the appropriate direction following therapy is evidence that the patient is taking the drug regularly, taking it correctly, and that it is being absorbed and having the expected effect in modulating bone remodeling. Failure to achieve such a change in the BTM level is cause for concern and suggests that evaluation and possibly a reconsideration of treatment should be considered (87). The use of BTMs allows assessment of drug effect sooner than with DXA, so that evaluation and corrective action, if needed, can be taken early in the course of therapy rather than later. Monitoring BTMs, especially in association with regular contact by a healthcare provider, may improve persistence with therapy (88). Despite the well-described limitations of BTMs (89), there is emerging support for their use in clinical practice, particularly in the assessment of response to therapy (90,91). Clinicians who are familiar with the benefits and limitations of BTMs may find them a helpful tool, in association with BMD testing, for managing patients with osteoporosis.
IMAGING STUDIES
Standard X-rays are used to diagnose fractures of all types and may sometimes suggest secondary causes of osteoporosis. Pseudofractures (Looser’s zones) are radiolucent lines running perpendicular to the bone cortex that may be seen in patients with osteomalacia. These probably represent stress fractures that have healed with poorly mineralized osteoid. Punctate radiolucencies may be seen in bone X-rays of patients with systemic mastocytosis. Primary hyperparathyroidism may cause bone cysts, subperiosteal bone resorption, brown tumors, and demineralization (‘salt and pepper’ pattern) of the skull. MRI, CT scanning, or nuclear imaging may be used to detect stress fractures not visible on X-ray. MRI of the spine is commonly used prior to vertebroplasty or kyphoplasty to determine the age of the fracture, the likelihood of the fracture being from causes other than osteoporosis, and whether there is retropulsion of bony fragments than could impair neurological function.
BONE BIOPSY
Non-decalcified double tetracycline labeled iliac crest bone biopsy is rarely used in clinical practice but may be helpful with difficult diagnostic problems. In the evaluation of renal osteodystrophy, a bone biopsy can distinguish between high turnover and low turnover bone disease, and possibly be an aid in the selection of therapy. With infiltrative disorders of bone, such as systemic mastocytosis, a bone biopsy or bone marrow aspiration may sometimes be the only way to make the diagnosis. In patients who are not responding to therapy as expected, or in patients with unusual presentations of osteoporosis, a bone biopsy may be indicated. Bone biopsies are required by the FDA for safety monitoring in clinical trials of osteoporosis drugs.
SUMMARY
Osteoporosis is a common skeletal disease with serious clinical consequences. Effective management of skeletal health includes appropriate selection of patients for bone density testing and assessment of risk factors for fracture. Prior to treatment, and when response to treatment is suboptimal, patients should be evaluated for secondary causes of osteoporosis. All reversible factors should be corrected and treatment should be individualized based on the clinical circumstances.
REFERENCES
- 1.
- Klibanski A, Adams-Campbell L, Bassford T, Blair SN, Boden SD, Dickersin K, Gifford DR, Glasse L, Goldring SR, Hruska K, Johnson SR, McCauley LK, Russell WE, Osteopor NCDP. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–795. [PubMed: 11176917]
- 2.
- US Department of Health and Human Services 2004 Bone Health and Osteoporosis: A Report of the Surgeon General. US Department of Health and Human Services, Office of the Surgeon General, Rockville, MD.
- 3.
- World Health Organisation 1994 Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO, Geneva, Switzerland.
- 4.
- Shuhart CR, Yeap SS, Anderson PA, Jankowski LG, Lewiecki EM, Morse LR, et al. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Periprosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J Clin Densitom. 2019;22(4):453–71. [PubMed: 31400968] [CrossRef]
- 5.
- Lewiecki EM, Binkley N, Petak SM. DXA Quality Matters. J Clin Densitom. 2006;9(4):388–392. [PubMed: 17097522]
- 6.
- Siris ES, Adler R, Bilezikian J, Bolognese M, Dawson-Hughes B, Favus MJ, Harris ST, Jan de Beur SM, Khosla S, Lane NE, Lindsay R, Nana AD, Orwoll ES, Saag K, Silverman S, Watts NB. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int. 2014;25(5):1439–43. [PMC free article: PMC3988515] [PubMed: 24577348]
- 7.
- Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–81. [PMC free article: PMC4176573] [PubMed: 25182228]
- 8.
- Qaseem A, Snow V, Shekelle P, Hopkins R Jr, Forciea MA, Owens DK. Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann.Intern.Med. 2008;148(9):680–684. [PubMed: 18458281]
- 9.
- Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A. Case finding for the management of osteoporosis with FRAX--assessment and intervention thresholds for the UK. Osteoporos.Int. 2008;19(10):1395–1408. [PubMed: 18751937]
- 10.
- Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster JY, Borgstrom F, Rizzoli R. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19(4):399–428. [PMC free article: PMC2613968] [PubMed: 18266020]
- 11.
- Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–1259. [PMC free article: PMC2351094] [PubMed: 8634613]
- 12.
- Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA, Hochberg MC, Vogt MT, Orwoll ES. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab. 2005;90(5):2787–2793. [PubMed: 15728213]
- 13.
- Lewiecki EM, Laster AJ. Clinical applications of vertebral fracture assessment by dual-energy X-ray absorptiometry. J Clin Endocrinol Metab. 2006;91(11):4215–4222. [PubMed: 16940447]
- 14.
- Genant HK, Wu CY, Van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–1148. [PubMed: 8237484]
- 15.
- Schousboe JT, DeBold CR. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int. 2006;17(2):281–289. [PubMed: 16172798]
- 16.
- Lewiecki EM, Binkley N, Morgan SL, Shuhart CR, Camargos BM, Carey JJ, et al. Best Practices for Dual-Energy X-ray Absorptiometry Measurement and Reporting: International Society for Clinical Densitometry Guidance. J Clin Densitom. 2016;19(2):127–40. [PubMed: 27020004]
- 17.
- International Society for Clinical Densitometry, Facility Accreditation. Available at www
.iscd.org/accreditation. - 18.
- Lewiecki EM. Update on bone density testing. Curr.Osteoporos Rep. 2005;3(4):136–142. [PubMed: 16303113]
- 19.
- Lotz JC, Cheal EJ, Hayes WC. Fracture prediction for the proximal femur using finite element models: Part I--Linear analysis. J Biomechan. Eng. 1991;113:353–360. [PubMed: 1762430]
- 20.
- Cranney A, Tugwell P, Wells G, Guyatt G. Systematic reviews of randomized trials in osteoporosis: Introduction and methodology. Endocrine Reviews. 2002;23(4):497–507. [PubMed: 12202464]
- 21.
- Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. Journal of Clinical Endocrinology and Metabolism. 2000;85(1):231–236. [PubMed: 10634392]
- 22.
- Mazess R, Chesnut CH III, McClung M, Genant H. Enhanced precision with dual-energy X-ray absorptiometry. Calcif.Tissue Int. 1992;51(1):14–17. [PubMed: 1393769]
- 23.
- Njeh CF, Fuerst T, Hans D, Blake GM, Genant HK. Radiation exposure in bone mineral density assessment. Applied Radiation & Isotopes. 1999;50(1):215–236. [PubMed: 10028639]
- 24.
- Bonnick SL 2004 Bone densitometry in clinical practice- application and interpretation, 2nd ed ed. Humana Press, Totowa, N.J.
- 25.
- Lewiecki EM, Richmond B, Miller PD. Uses and misuses of quantitative ultrasonography in managing osteoporosis. Cleve.Clin J Med. 2006;73(8):742–752. [PubMed: 16913198]
- 26.
- Engelke K, Adams JE, Armbrecht G, Augat P, Bogado CE, Bouxsein ML, Felsenberg D, Ito M, Prevrhal S, Hans DB, Lewiecki EM. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J.Clin.Densitom. 2008;11(1):123–162. [PubMed: 18442757]
- 27.
- Faulkner KG, von Stetten E, Miller P. Discordance in patient classification using T-scores. J.Clin.Densitom. 1999;2:343–350. [PubMed: 10548828]
- 28.
- Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S, Eisman JA, Fujiwara S, Gluer C, Goltzman D, Hans D, Krieg MA, La CA, McCloskey E, Mellstrom D, Melton LJ, III, Pols H, Reeve J, Sanders K, Schott AM, Silman A, Torgerson D, van ST, Watts NB, Yoshimura N 2007 The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos.Int. 18(8):1033-1046.
- 29.
- Kanis JA, on behalf of the World Health Organization Scientific Group 2007 Assessment of osteoporosis at the primary health-care level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK: Printed by the University of Sheffield.
- 30.
- Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA. Spine-hip discordance and fracture risk assessment: a physician-friendly FRAX enhancement. Osteoporos Int. 2011;22:839–847. [PubMed: 20959961]
- 31.
- Vogt TM, Ross PD, Palermo L, Musliner T, Genant HK, Black D, Thompson DE. Vertebral fracture prevalence among women screened for the Fracture Intervention Trial and a simple clinical tool to screen for undiagnosed vertebral fractures. Fracture Intervention Trial Research Group. Mayo Clin Proc. 2000;75(9):888–896. [PubMed: 10994823]
- 32.
- Gunnes M, Lehmann EH, Mellstrom D, Johnell O. The relationship between anthropometric measurements and fractures in women. Bone. 1996;19(4):407–413. [PubMed: 8894148]
- 33.
- Siminoski K, Jiang G, Adachi JD, Hanley DA, Cline G, Ioannidis G, Hodsman A, Josse RG, Kendler D, Olszynski WP, Ste Marie LG, Eastell R. Accuracy of height loss during prospective monitoring for detection of incident vertebral fractures. Osteoporos Int. 2005;16(4):403–410. [PubMed: 15309381]
- 34.
- Margolis KL, Ensrud KE, Schreiner PJ, Tabor HK. Body size and risk for clinical fractures in older women. Study of Osteoporotic Fractures Research Group. Ann.Intern Med. 2000;133(2):123–127. [PubMed: 10896638]
- 35.
- de Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ III, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos.Int. 2005;16(11):1330–1338. [PubMed: 15928804]
- 36.
- Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR, Gr SOFR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. Journal of the American Geriatrics Society. 2003;51(12):1740–1747. [PubMed: 14687352]
- 37.
- Lewiecki EM 2013 Evaluation of the patient at risk for osteoporosis. In: Marcus R, Feldman D, Dempster DW, Luckey M, Cauley JA (eds.) Osteoporosis, Fourth ed., vol. 2. Elsevier, Waltham, Massachusetts, USA, pp 1481-1504.
- 38.
- Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, De Papp AE. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90(6):3215–3224. [PubMed: 15797954]
- 39.
- Tannenbaum C, Clark J, Schwartzman K, Wallenstein S, Lapinski R, Meier D, Luckey M. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab. 2003;87:4431–4437. [PubMed: 12364413]
- 40.
- Wagman RB, Marcus R. Beyond bone mineral density-navigating the laboratory assessment of patients with osteoporosis. J Clin Endocrinol Metab. 2002;87(10):4429–4430. [PubMed: 12364412]
- 41.
- Barzel US. Recommended testing in patients with low bone density. J Clin Endocrinol Metab. 2003;88(3):1404–1405. [PubMed: 12629143]
- 42.
- 42 Luckey MM, Tannenbaum C 2003 Authors' response: Recommended testing in patients with low bone density. J Clin Endocrinol Metab 88(3):1405.
- 43.
- Harper KD, Weber TJ. Secondary osteoporosis - Diagnostic considerations. Endocrinology and Metabolism Clinics of North America. 1998;27(2):325–348. [PubMed: 9669141]
- 44.
- Eastell R. Treatment of postmenopausal osteoporosis. N.Engl.J.Med. 1998;338(11):736–746. [PubMed: 9494151]
- 45.
- Favus MJ. Postmenopausal osteoporosis and the detection of so-called secondary causes of low bone density. J Clin Endocrinol Metab. 2005;90(6):3800–3801. [PubMed: 15917489]
- 46.
- Looker AC, Orwoll ES, Johnston CCJ, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J.Bone Miner.Res. 1997;12:1761–1768. [PubMed: 9383679]
- 47.
- Orwoll ES, Klein RF. Osteoporosis in men. Endocr.Rev. 1995;16:87–116. [PubMed: 7758434]
- 48.
- Lester J, Coleman R. Bone loss and the aromatase inhibitors. Br.J Cancer. 2005;93 Suppl 1:S16–S22. [PMC free article: PMC2361688] [PubMed: 16100521]
- 49.
- Daniell HW. Osteoporosis due to androgen deprivation therapy in men with prostate cancer. Urology. 2001;58(2) Suppl 1:101–107. [PubMed: 11502461]
- 50.
- Melton LJ III, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV. Fracture risk with multiple myeloma: a population-based study. J Bone Miner Res. 2005;20(3):487–493. [PubMed: 15746994]
- 51.
- De Prisco C, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci. 2005;329(2):57–61. [PubMed: 15711420]
- 52.
- Adachi Y, Shiota E, Matsumata T, Iso Y, Yoh R, Kitano S. Osteoporosis after gastrectomy: bone mineral density of lumbar spine assessed by dual-energy X-ray absorptiometry. Calcif.Tissue Int. 2000;66(2):119–122. [PubMed: 10652959]
- 53.
- Cormier C, Souberbielle JC, Kahan A. Primary hyperparathyroidism and osteoporosis in 2004. Joint Bone Spine. 2004;71(3):183–189. [PubMed: 15182788]
- 54.
- Földes J, Tarján G, Szathmari M, Varga F, Krasznai I, Horvath C. Bone mineral density in patients with endogenous subclinical hyperthyroidism: Is this thyroid status a risk factor for osteoporosis. Clin.Endocrinol.(Oxf.). 1993;39:521–527. [PubMed: 8252739]
- 55.
- Mancini T, Doga M, Mazziotti G, Giustina A. Cushing's Syndrome and Bone. Pituitary. 2004;7:249–252. [PubMed: 16010458]
- 56.
- Bianchi ML, Bardella MT. Bone and celiac disease. Calcified Tissue International. 2002;71(6):465–471. [PubMed: 12232681]
- 57.
- Freitag A, Barzel US. Differential diagnosis of osteoporosis. Gerontology. 2002;48(2):98–102. [PubMed: 11867932]
- 58.
- Orlic ZC, Raisz LG. Causes of secondary osteoporosis. J Clin Densitom. 1999;2(1):79–92. [PubMed: 23547317]
- 59.
- Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, Deluca HF, Drezner MK. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89(7):3152–3157. [PubMed: 15240586]
- 60.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. [PubMed: 1244564]
- 61.
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van LF. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann.Intern.Med. 2006;145(4):247–254. [PubMed: 16908915]
- 62.
- Heaney RP, Recker RR, Ryan RA. Urinary calcium in perimenopausal women: normative values. Osteoporos Int. 1999;9(1):13–18. [PubMed: 10367024]
- 63.
- Audran M, Legrand E. Hypercalciuria. Joint Bone Spine. 2000;67(6):509–515. [PubMed: 11195313]
- 64.
- Pak CY, Kaplan R, Bone H, Townsend J, Waters O. A simple test for the diagnosis of absorptive, resorptive and renal hypercalciurias. N.Engl.J Med. 1975;292(10):497–500. [PubMed: 163960]
- 65.
- Pacifici R. Idiopathic hypercalciuria and osteoporosis--distinct clinical manifestations of increased cytokine-induced bone resorption? J Clin Endocrinol Metab. 1997;82(1):29–31. [PubMed: 8989227]
- 66.
- Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman SS, Murray JA, Horvath K. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch.Intern.Med. 2003;163(3):286–292. [PubMed: 12578508]
- 67.
- Alaedini A, Green PH. Narrative review: celiac disease: understanding a complex autoimmune disorder. Ann.Intern Med. 2005;142(4):289–298. [PubMed: 15710962]
- 68.
- McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, Hannan MT, Cupples LA, Kiel DP. Homocysteine as a predictive factor for hip fracture in older persons. N.Engl.J Med. 2004;350(20):2042–2049. [PubMed: 15141042]
- 69.
- van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, van der KM, de JR, Lindemans J, de Groot LC, Hofman A, Witteman JC, van Leeuwen JP, Breteler MM, Lips P, Pols HA, Uitterlinden AG 2004 Homocysteine levels and the risk of osteoporotic fracture. N.Engl.J Med 350(20):2033-2041.
- 70.
- Chines A, Pacifici R, Avioli LV, Teitelbaum SL, Korenblat PE. Systemic mastocytosis presenting as osteoporosis: a clinical and histomorphometric study. J Clin Endocrinol Metab. 1991;72(1):140–144. [PubMed: 1986013]
- 71.
- Lidor C, Frisch B, Gazit D, Gepstein R, Hallel T, Mekori YA. Osteoporosis as the sole presentation of bone marrow mastocytosis. J Bone Miner Res. 1990;5(8):871–876. [PubMed: 2239371]
- 72.
- Weger M, Deutschmann H, Weger W, Kotanko P, Skrabal F. Incomplete renal tubular acidosis in 'primary' osteoporosis. Osteoporos Int. 1999;10(4):325–329. [PubMed: 10692983]
- 73.
- Bergmann P, Body JJ, Boonen S, Boutsen Y, Devogelaer JP, Goemaere S, Kaufman JM, Reginster JY, Gangji V. Evidence-based guidelines for the use of biochemical markers of bone turnover in the selection and monitoring of bisphosphonate treatment in osteoporosis: a consensus document of the Belgian Bone Club. Int J Clin Pract. 2009;63(1):19–26. [PMC free article: PMC2705815] [PubMed: 19125989]
- 74.
- Greenspan SL, Parker RA, Ferguson L, Rosen HN, Maitland-Ramsey L, Karpf DB. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res. 1998;13:1431–1438. [PubMed: 9738515]
- 75.
- Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051–1056. [PubMed: 12817758]
- 76.
- Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, Thompson DE, Ewing SK, Delmas PD. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004;19(8):1250–1258. [PubMed: 15231011]
- 77.
- Sarkar S, Reginster JY, Crans GG, Diez-Perez A, Pinette KV, Delmas PD. Relationship between changes in biochemical markers of bone turnover and BMD to predict vertebral fracture risk. J Bone Miner Res. 2004;19(3):394–401. [PubMed: 15040827]
- 78.
- Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol.Metab. 2002;87(4):1586–1592. [PubMed: 11932287]
- 79.
- Bouxsein ML, Delmas PD. Considerations for development of surrogate endpoints for antifracture efficacy of new treatments in osteoporosis: a perspective. J Bone Miner Res. 2008;23(8):1155–1167. [PMC free article: PMC2680170] [PubMed: 18318643]
- 80.
- Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, Wagman RB. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005;20(6):962–970. [PubMed: 15883636]
- 81.
- Hannon R, Eastell R. Preanalytical variability of biochemical markers of bone turnover. Osteoporos Int. 2000;11 Suppl 6:S30–S44. [PubMed: 11193238]
- 82.
- Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem.Rev. 2005;26(4):97–122. [PMC free article: PMC1320175] [PubMed: 16648882]
- 83.
- Seibel MJ, Lang M, Geilenkeuser WJ. Interlaboratory variation of biochemical markers of bone turnover. Clin Chem. 2001;47(8):1443–1450. [PubMed: 11468235]
- 84.
- National Osteoporosis Foundation 2013, Version 3, Released February 25, 2014 Clinician's Guide to Prevention and Treatment of Osteoporosis, .
- 85.
- Srivastava AK, Vliet EL, Lewiecki EM, Maricic M, Abdelmalek A, Gluck O, Baylink DJ. Clinical use of serum and urine bone markers in the management of osteoporosis. Curr.Med Res Opin. 2005;21(7):1015–1026. [PubMed: 16004668]
- 86.
- Nishizawa Y, Nakamura T, Ohta H, Kushida K, Gorai I, Shiraki M, Fukunaga M, Hosoi T, Miki T, Chaki O, Ichimura S, Nakatsuka K, Miura M. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004). J Bone Miner Metab. 2005;23(2):97–104. [PubMed: 15750686]
- 87.
- Lewiecki EM, Watts NB. Assessing response to osteoporosis therapy. Osteoporos Int. 2008;19(10):1363–1368. [PubMed: 18546030]
- 88.
- Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(3):1117–1123. [PubMed: 15001596]
- 89.
- Meier C, Seibel MJ, Kraenzlin ME. Use of bone turnover markers in the real world: are we there yet? J Bone Miner Res. 2009;24(3):386–388. [PubMed: 19138133]
- 90.
- Lewiecki EM, Baim S, Bilezikian JP, Eastell R, LeBoff MS, Miller PD. 2008 Santa Fe Bone Symposium: update on osteoporosis. J Clin Densitom. 2009;12(2):135–157. [PubMed: 19426925]
- 91.
- Singer FR, Eyre DR. Using biochemical markers of bone turnover in clinical practice. Cleve.Clin J Med. 2008;75(10):739–750. [PubMed: 18939390]
- ABSTRACT
- INTRODUCTION
- DIAGNOSIS OF OSTEOPOROSIS
- FRACTURE RISK ASSESSMENT
- VERTEBRAL FRACTURE ASSESSMENT (VFA)
- QUALITY OF DXA AND VFA
- TECHNOLOGIES FOR ASSESSMENT OF SKELETAL HEALTH
- MEDICAL HISTORY
- PHYSICAL EXAM
- EVALUATION FOR SECONDARY CAUSES OF OSTEOPOROSIS
- CLINICAL CASE
- BASIC BLOOD TESTS
- BASIC URINE TESTS
- ADDITIONAL STUDIES IN SELECTED PATIENTS
- BONE TURNOVER MARKERS
- IMAGING STUDIES
- BONE BIOPSY
- SUMMARY
- REFERENCES
- Utilization of DXA Bone Mineral Densitometry in Ontario: An Evidence-Based Analysis.[Ont Health Technol Assess Ser....]Utilization of DXA Bone Mineral Densitometry in Ontario: An Evidence-Based Analysis.Medical Advisory Secretariat. Ont Health Technol Assess Ser. 2006; 6(20):1-180. Epub 2006 Nov 1.
- Summary of AHRQ's comparative effectiveness review of treatment to prevent fractures in men and women with low bone density or osteoporosis: update of the 2007 report.[J Manag Care Pharm. 2012]Summary of AHRQ's comparative effectiveness review of treatment to prevent fractures in men and women with low bone density or osteoporosis: update of the 2007 report.Levis S, Theodore G. J Manag Care Pharm. 2012 May; 18(4 Suppl B):S1-15; discussion S13.
- Review [Diagnosis and treatment of osteoporosis in patients with chronic kidney disease : Joint guidelines of the Austrian Society for Bone and Mineral Research (ÖGKM), the Austrian Society of Physical and Rehabilitation Medicine (ÖGPMR) and the Austrian Society of Nephrology (ÖGN)].[Wien Med Wochenschr. 2023]Review [Diagnosis and treatment of osteoporosis in patients with chronic kidney disease : Joint guidelines of the Austrian Society for Bone and Mineral Research (ÖGKM), the Austrian Society of Physical and Rehabilitation Medicine (ÖGPMR) and the Austrian Society of Nephrology (ÖGN)].Cejka D, Wakolbinger-Habel R, Zitt E, Fahrleitner-Pammer A, Amrein K, Dimai HP, Muschitz C. Wien Med Wochenschr. 2023 Oct; 173(13-14):299-318. Epub 2022 Dec 21.
- Preoperative bone health assessment and optimization in spine surgery.[Neurosurg Focus. 2020]Preoperative bone health assessment and optimization in spine surgery.Anderson PA, Kadri A, Hare KJ, Binkley N. Neurosurg Focus. 2020 Aug; 49(2):E2.
- Review Bone health in childhood and adolescence: an overview on dual-energy X-ray absorptiometry scanning, fracture surveillance and bisphosphonate therapy for low-middle-income countries.[Front Endocrinol (Lausanne). 2...]Review Bone health in childhood and adolescence: an overview on dual-energy X-ray absorptiometry scanning, fracture surveillance and bisphosphonate therapy for low-middle-income countries.Madhuchani D, Seneviratne SN, Ward LM. Front Endocrinol (Lausanne). 2023; 14:1082413. Epub 2023 Apr 17.
- Osteoporosis: Clinical Evaluation - EndotextOsteoporosis: Clinical Evaluation - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
