CASRN: 15722-48-2
Drug Levels and Effects
Summary of Use during Lactation
Limited data indicate that olsalazine is poorly excreted into breastmilk. However, olsalazine is a mesalamine prodrug. Rather high levels of the mesalamine metabolite N-acetyl-5-ASA appear in breastmilk and its effects on breastfed infants are unknown. A few cases of diarrhea have been reported in infants exposed to mesalamine, although the rate is not high. Most experts consider mesalamine derivatives to be acceptable during breastfeeding.[1-6] If olsalazine is required by the mother, it is not a reason to discontinue breastfeeding, but carefully observe breastfed infants for diarrhea during maternal use of olsalazine.
Drug Levels
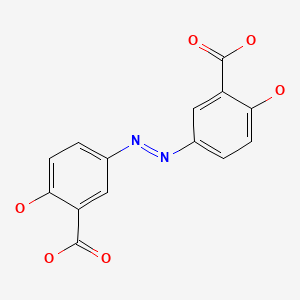
Olsalazine is a prodrug that liberates the active drug, mesalamine (5-aminosalicylic acid; 5-ASA), in the gastrointestinal tract. Mesalamine is metabolized to N-acetyl-5-ASA, which is inactive in treating inflammatory bowel disease, but its possible effects on the breastfed infant are unknown.
Maternal Levels. A lactating woman with a long history of Crohn's disease and 2 bowel resections at her terminal ileum was given a single oral dose of olsalazine 500 mg at 4 months postpartum. She was also taking prednisone 17.5 mg daily at the time of the study. Milk samples were taken periodically from 30 minutes to 48 hours after the dose. Olsalazine and its metabolites, olsalazine sulfate and mesalamine were undetectable (<170 mcg/L) in milk at all times during the study. The inactive mesalamine metabolite, N-acetyl-5-ASA, was detectable only at 10, 14 and 24 hours after the dose in concentrations of 170, 183 and 264 mcg/L, respectively.[7]
A woman taking olsalazine capsules 750 mg twice daily collected 5 breastmilk samples over one day at 2 to 4 weeks postpartum. Mesalamine was undetectable (<20 mcg/L) in milk. N-acetyl-5-ASA averaged 2.3 mg/L (range 1.2 to 3.8 mg/L) in the 5 samples. The highest milk level was in the 6 pm sample, but the times of the doses were not stated.[8]
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
One infant was breastfed during maternal therapy with olsalazine for Crohn's disease. After 2 and 3 weeks of therapy, no rash, wheezing, vomiting or diarrhea were noted in the infant.[7]
The active metabolite of olsalazine, mesalamine, was probably responsible for diarrhea in a 6-week-old whose diarrhea recurred 4 times after rechallenging the mother 4 times during breastfeeding.[9]
In a prospective telephone follow-up study, 8 nursing mothers reported taking mesalamine (dosage and route unspecified). One mother reported diarrhea in her infant. No other adverse reactions were reported in the infants by their mothers.[10]
A case-control study compared the infants of mothers taking mesalamine (n = 117; average dose, 2065 mg daily), olsalazine (n = 2) or sulfasalazine (n = 2) to infants of matched control mothers (n = 121) who were exposed to no treatment known to be harmful to a breastfed infant. Infants were exposed to mesalamine through milk for a mean of 5.3 months (range: 3 days-24 months). Infants were breastfed for an average of about 7.4 months and were followed up at an average age of about 22 months. No difference in the frequency or characteristics of maternally reported adverse events were found between exposed and control infants.[11,12]
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
Alternate Drugs to Consider
(Inflammatory Bowel Disease) Adalimumab, Azathioprine, Budesonide, Certolizumab Pegol, Infliximab, Mesalamine, Prednisone, Sulfasalazine
References
- 1.
- Nielsen OH, Maxwell C, Hendel J. IBD medications during pregnancy and lactation. Nat Rev Gastroenterol Hepatol 2014;11:116-27. [PubMed: 23897285]
- 2.
- Mahadevan U, Matro R. Care of the pregnant patient with inflammatory bowel disease. Obstet Gynecol 2015;126:401-12. [PubMed: 26241432]
- 3.
- Nguyen GC, Seow CH, Maxwell C, et al. The Toronto Consensus Statements for the Management of IBD in Pregnancy. Gastroenterology 2016;150:734-57.e1. [PubMed: 26688268]
- 4.
- van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis 2015;9:107-24. [PubMed: 25602023]
- 5.
- Torres J, Chaparro M, Julsgaard M, et al. European Crohn's and Colitis Guidelines on Sexuality, Fertility, Pregnancy, and Lactation. J Crohns Colitis 2023;17:1-27. [PubMed: 36005814]
- 6.
- Russell MD, Dey M, Flint J, et al. British Society for Rheumatology guideline on prescribing drugs in pregnancy and breastfeeding: Immunomodulatory anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford) 2023;62:e48-e88. [PMC free article: PMC10070073] [PubMed: 36318966]
- 7.
- Miller LG, Hopkinson JM, Motil KJ, et al. Disposition of olsalazine and metabolites in breast milk. J Clin Pharmacol 1993;33:703-6. [PubMed: 8408729]
- 8.
- Ambrosius Christensen L., Rasmussen SN, Hansen SH, et al. Salazosulfapyridine and metabolites in fetal and maternal body fluids with special reference to 5-aminosalicylic acid. Acta Obstet Gynecol Scand 1987;66:433-5. [PubMed: 2892343]
- 9.
- Nelis GF. Diarrhoea due to 5-aminosalicylic acid in breast milk. Lancet 1989;333:383. [PubMed: 2563532]
- 10.
- Ito S, Blajchman A, Stephenson M, et al. Prospective follow-up of adverse reactions in breast-fed infants exposed to maternal medication. Am J Obstet Gynecol 1993;168:1393-9. [PubMed: 8498418]
- 11.
- Moretti ME, Spiczynski Y, Hashemi G, et al. Prospective follow-up of infants exposed to 5-aminosalicylic acid containing drugs through maternal milk. J Clin Pharmacol 1998;38:867. doi:10.1177/009127009803800901 [CrossRef]
- 12.
- Moretti ME. Prospective follow-up of infants exposed to 5-aminosalicylic acid containing drugs through maternal milk. Theses Canada 1998. https:
//library-archives .canada.ca/eng/services /services-libraries /theses/Pages/item .aspx?idNumber=51446896
Substance Identification
Substance Name
Olsalazine
CAS Registry Number
15722-48-2
Drug Class
Breast Feeding
Lactation
Milk, Human
Gastrointestinal Agents
Anti-Inflammatory Agents, Non-Steroidal
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
Publication Details
Publication History
Last Revision: September 15, 2024.
Copyright
Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
Publisher
National Institute of Child Health and Human Development, Bethesda (MD)
NLM Citation
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-. Olsalazine. [Updated 2024 Sep 15].