NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010-.
A scaffold hopping approach afforded ML218 (CID 45115620) a selective T-Type Ca2+ (Cav3.1, Cav3.2, Cav3.3) inhibitor (Cav3.2, IC50 = 150 nM in Ca2+ flux; Cav3.2 IC50 = 310 nM and Cav3.3 IC50 = 270 nM in patch clamp electrophysiology) suitable for in vitro and in vivo studies. ML218 possess excellent dystrophia myotonica protein kinase (DMPK) properties, acceptable in vivo rat PK, excellent brain levels and was efficacious upon oral dosing in a preclinical Parkinsonian model. Thus, ML218 is a powerful new probe to study T-Type Ca2+ function in vitro and in vivo.
Assigned Assay Grant #: NS050771-01
Screening Center Name & PI: Vanderbilt Screening Center for GPCRs, Ion Channels and Transporters (MLSCN), David Weaver; Follow-up by Johns Hopkins Ion Channel Center, Min Li
Chemistry Center Name & PI: Vanderbilt Specialized Chemistry Center, Craig Lindsley
Assay Submitter & Institution: Xinmin (Simon) Xie, SRI International
PubChem Summary Bioassay Identifier (AID): 463807
Resulting Publications
- 1.
- Xiang Z, Thompson AD, Brogan JT, Schulte ML, Melancon BJ, Mi D, Lewis LM, Zou B, Yang L, Morrison R, Santomango T, Byers F, Brewer K, Aldrich JS, Yu H, Dawson ES, Li M, McManus O, Jones CK, Daniels JS, Hopkins CR, Xie XS, Conn PJ, Weaver CD, Lindsley CW. The Discovery and Characterization of ML218: A Novel, Centrally Active T-Type Calcium Channel Inhibitor with Robust Effects in STN Neurons and in a Rodent Model of Parkinson's Disease. ACS chemical neuroscience. 2011 Dec 21;2(12):730–42. [PubMed: 22368764]
Probe Structure & Characteristics
![3,5-dichloro-N-(((1R,5S,6S)-3-(3,3-dimethylbutyl)-3-azabicylco[3.1.0]hexan-6-yl)methyl)benzamide, ML218.](/books/NBK143195/bin/ml218fu1.jpg)
3,5-dichloro-N-(((1R,5S,6S)-3-(3,3-dimethylbutyl)-3-azabicylco[3.1.0]hexan-6-yl)methyl)benzamide, ML218
| CID/ML# | Target Name | EC50 (nM) [SID, AID] | Anti-target Name(s) | IC50/EC50 (μM) [SID, AID] | Fold Selective | Secondary Assay(s) Name: IC50/EC50 (nM) [SID, AID] |
|---|---|---|---|---|---|---|
| CID 45115620/ML218 | Cav3.2 (Ca2+flux) | 150, [SID 92310249, AID 449739, AID 489005, AID 493021, AID 493022, AID 493023, AID 493041, AID 504619] | L/N-Type Ca2+ hERG Na+/K+ current in rat DRG | >10, [SID 92310249, AID 504584] >10 [SID 92310249, AID 504584] >10 [SID 92310249, AID 504579] | >60 >60 >60 | Cav3.2_IWS: 310 [SID 92310249, AID 504425] Cav3.3_IWS: 270 [SID 92310249, AID 504425] |
1. Recommendations for Scientific Use of the Probe
This probe (ML218, CID 45115620) can be used to investigate the role of selective T-Type Ca2+ (Cav3.1, Cav3.2, Cav3.3) inhibition in vitro and in vivo. There are many known T-Type Ca2+ inhibitors, but the potent and selective ones are patented. This was known prior to running the HTS screen, and NIH desired we develop a T-Type Ca2+ inhibitor free of IP issues so that the biomedical community could possess a potent and selective probe without concern over use restrictions. ML218 is a potent inhibitor (Cav3.2, IC50 = 150 nM in Ca2+ flux; Cav3.2 IC50 = 310 nM and Cav3.3 IC50 = 270 nM in patch clamp electrophysiology) and selective against the other members of the calcium family of ion channels (L- and N-type calcium channels) as well as multiple sodium and potassium channels. Lastly, ML218 was tested at Ricerca’s (formerly MDS Pharma’s) Lead Profiling Screen (binding assay panel of 68 GPCRs, ion channels and transporters screened at 10 μM) and was found to significantly bind to only 2 of the 68 targets; importantly, ML218 was found to be selective against hERG and many other ion channels. ML218 possess excellent DMPK properties, acceptable in vivo rat PK, excellent brain levels and was efficacious in a haloperidol-induced catalepsy assay, a preclinical Parkinsonian model. Thus, ML218 is a powerful new probe to study T-Type Ca2+ function in vitro and in vivo.
2. Materials and Methods
- HEK-293 cell line expressing T-type (CaV3.2) channels
- Native HEK-293 cell line
- Fluo-4 AM dye (Invitrogen, Cat #F14202)
- KK-3-118 (positive control T-type inhibitor)
- Cell plates: BD Biocoat, Poly-D-Lysine coated, black/clear bottom (cat # 356936) 384 well assay plates
- Compound daughter plates: 384-well Greiner round bottom, clear, custom barcoded (cat# 781281)
- BioTek ELx405 plate washer
- Thermo Fisher Combi
- Labcyte Echo555
- Hammamatsu FDSS 6000 kinetic imaging plate reader’
- HEK293 cell lines stably expressing Cav3.2 and Cav3.3 channels
- Parental HEK cell line
- Population Patch Clamp patch plates from Molecular Devices
2.1. Assays
T-type calcium channel Cell-based FDSS Ca2+ flux Assay
T Type Primary (AID 449739)
- Cell culture: Cells are routinely cultured in D-MEM/F12 (Gibco 11330-032) supplemented with 10% heat-inactivated FBS (Sigma F2442), 0.5% Sodium Pyruvate (Gibco 11360), and 1mg/ml G418 (Cellgro 30-234-CR)
- Cell plating: Add 20 μl/well of 1000 cells/μl suspended in D-MEM/F12 (Gibco 11330-032) supplemented with 10% heat-inactivated FBS (Sigma F2442) and 0.5% Sodium Pyruvate (Gibco 11360).
- Incubate overnight at 37 °C and 5% CO2.
- Remove medium with ELx405 and replace with 20 μl/well of Assay Buffer (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 0.5 mM CaCl2, 10 mM glucose, 10 mM HEPES pH 7.3).
- Add 20 μl/well Dye Loading Solution (4.6 μM Fluo-4 AM with 0.02% w/v Pluronic F-127 in Assay Buffer) with Combi
- Incubate 60 minutes at room temperature (RT)
- Prepare 2X compound plates by transferring 80 nL/well from stock plates containing compounds at 10 mM in DMSO to compound daughter plates and add 40μl/well Assay Buffer using Combi.
- Negative (vehicle) control and positive control wells are 0.2% DMSO in Assay Buffer and EC80 of KK-3-118 in Assay Buffer, respectively in every other well of columns 1–2, 23–24.
- Replace Dye Loading Solution with 20 μL/well of Assay Buffer using ELx405.
- Load cell plates to Hamamatsu FDSS 6000 kinetic imaging plate reader.
- Measure fluorescence for 10 seconds at 1Hz to establish baseline.
- Add 20 μl/well test compounds from daughter plates and continue to collect data for 50 seconds at 1Hz.
- Remove plates from FDSS and incubate for 20 min at RT
- Reload plates to FDSS and resume collecting data at 1Hz
- After 10 seconds add 10 μl/well 5 × Stimulus Buffer (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 50 mM CaCl2, 5 mM glucose, 10 mM HEPES pH 7.3) and continue collecting data for 2 min. Calculate ratio readout as F/F0 to normalize the data and reduce the waveform data to reflect the maximum normalized fluorescence/well. Calculate the average and standard deviation for negative and positive controls in each plate, as well as Z′.
- Calculate Z and B scores for test compounds using ratios calculated in Step 15.
- Outcome assignment: If the Z or B score of the test compound was more than 3 standard deviations below the average of the test population the compounds were denoted as “Active”. If compounds were less than 3 standard deviations from the average of the test population they were labeled as “Inactive”
- Score assignment: “Active” compounds were assigned a score of “100”. “Inactive” assigned a score of “0”.
Cav3.2 and Cav3.3 calcium channel IonWorks Electrophysiology Assays
Cav3 channel activity was examined in electrophysiological assays using the population patch clamp mode on the Ionworks Quattro (MDC, Sunnyvale, CA), an automated patch clamp instrument. The HEK293 cells stably expressing Cav3.2 and Cav3.3 channels were freshly dislodged from flasks and dispensed into a 384-well population patch clamp (PPC) plate, separately. The cell plating density was 7,000 cells/well suspended in the extracellular solution, composed of (in mM): 137 NaCl, 4 KCl, 1 MgCl2, 1.8 CaCl2, 10 HEPES, and 10 glucose, pH 7.4 adjusted with NaOH.
After dispensing, seal resistance of cells was measured for each well and cells were perforated by incubation with 50μg/mL amphotericin B (Sigma, St. Louis, MO) on the intracellular side, which was dissolved in the internal solution composed of (in mM): 40 KCl, 100 K-Gluconate, 1 MgCl2, 5 HEPES, 2 mM CaCl2 pH 7.2 adjusted with KOH. The channel activity was then measured with the recording protocol as described below. Leak currents were linearly subtracted by extrapolating the current elicited by a 100-ms step to −110 mV from a holding potential of −100 mV. During the voltage pulse protocol, cells were held at −100 mV, followed by a depolarization step to −30mV for 1 s, and then back to −90mV. The currents were measured at the peak of the inward currents before and after the application of compounds for 3 minutes. Only cells with a current amplitude greater than 200 pA at −30 mV and a seal resistance >30 MΩ were included in the data analysis.
Compound effects were assessed by the percentage changes in the inward currents, which were calculated by dividing the difference of current amplitude between pre- and post-compound recordings by the respective pre-compound currents in the same well. No corrections for liquid junction potentials were applied. The current signal was sampled at 0.625 kHz.
DMPK Methods
In vitro: The metabolism of ML218 was investigated in rat hepatic microsomes (BD Biosciences, Billerica, MA) using substrate depletion methodology (% test article remaining). A potassium phosphate-buffered reaction mixture (0.1 M, pH 7.4) of test article (1 μM) and microsomes (0.5 mg/mL) was pre-incubated (5 min) at 37°C prior to the addition of NADPH (1 μM). The incubations, performed in 96-well plates, were continued at 37 °C under ambient oxygenation and aliquots (80 μL) were removed at selected time intervals (0, 3, 7, 15, 25 and 45 min). Protein was precipitated by the addition of chilled acetonitrile (160 μL), containing glyburide as an internal standard (50 ng/mL), and centrifuged at 3000 rpm (4°C) for 10 minutes. Resulting supernatants were transferred to new 96-well plates in preparation for LC/MS/MS analysis. The in vitro half-life (t1/2, min, Eq. 1), intrinsic clearance (CLint, mL/min/kg, Eq. 2) and subsequent predicted hepatic clearance (CLhep, mL/min/kg, Eq. 3) was determined employing the following equations:
In vivo: Male Sprague-Dawley rats (n=2) weighing around 300g were purchased from Harlon laboratories (Indianapolis, IN) and implanted with catheters in the carotid artery and jugular vein. The cannulated animals were acclimated to their surroundings for approximately one week before dosing and provided food and water ad libitum. ML218 was administered intravenously (IV) to rats via the jugular vein catheter in 20% DMSO/80% saline at a dose of 1 mg/kg and a dose volume of 1 mL/kg. Blood collections via the carotid artery were performed at pre-dose, at 2, 7, 15, and 30 minutes, and at 1, 2, 4, 7 and 24 hrs post dose. Samples were collected into chilled, EDTA-fortified tubes, centrifuged for 10 minutes at 3000 rpm (4 °C), and resulting plasma aliquoted into 96-well plates for LC/MS/MS analysis. All pharmacokinetic analysis was performed employing noncompartmental analysis. For oral exposure studies, measuring both systemic plasma and CNS tissue exposure, ML218 was administered (oral gavage) to fasted rats (n=2) as suspensions in 10% tween 80/0.5% methylcellulose at a dose of 10 mg/kg and in a dosing volume of 10 mL/kg; blood and whole brain samples were collected at 1.5h post dose. Blood was collected into chilled, EDTA-fortified tubes, centrifuged for 10 minutes at 3000 rpm (4 °C) and stored at −80 °C until LC/MS/MS analysis. The brain samples were rinsed in PBS, snap frozen and stored at −80 °C. Prior to LC/MS/MS analysis, brain samples were thawed to room temperature and subjected to mechanical homogenation employing a Mini-Beadbeater™ and 1.0 mm Zirconia/Silica Beads (BioSpec Products). All animal studies were approved by the Vanderbilt University Medical Center Institutional Animal Care and Use Committee. The animal care and use program is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Plasma Protein Binding
Protein binding of ML218 was determined in rat plasma via equilibrium dialysis employing Single-Use RED Plates with inserts (ThermoFisher Scientific, Rochester, NY). Briefly, plasma (220 μL) was added to the 96 well plate containing test article (5 μL) and mixed thoroughly. Subsequently, 200 μL of the plasma-test article mixture was transferred to the cis chamber (red) of the RED plate, with an accompanying 350 μL of phosphate buffer (25 mM, pH 7.4) in the trans chamber. The RED plate was sealed and incubated 4 h at 37 °C with shaking. At completion, 50 μL aliquots from each chamber were diluted 1:1 (50 μL) with either plasma (cis) or buffer (trans) and transferred to a new 96 well plate, at which time ice-cold acetonitrile (2 volumes) was added to extract the matrices. The plate was centrifuged (3000 rpm, 10 min) and supernatants transferred to a new 96 well plate. The sealed plate was stored at −20°C until LC/MS/MS analysis.
Liquid Chromatography/Mass Spectrometry Analysis
In vivo experiments: ML218 was analyzed via electrospray ionization (ESI) on an AB Sciex API-4000 (Foster City, CA) triple-quadrupole instrument that was coupled with Shimadzu LC-10AD pumps (Columbia, MD) and a Leap Technologies CTC PAL auto-sampler (Carrboro, NC). Analytes were separated by gradient elution using a Fortis C18 2.1 × 50 mm, 3.5 μm column (Fortis Technologies Ltd, Cheshire, UK) thermostated at 40°C. HPLC mobile phase A was 0.1% NH4OH (pH unadjusted), mobile phase B was acetonitrile. The gradient started at 30% B after a 0.2 min hold and was linearly increased to 90% B over 0.8 min; held at 90% B for 0.5 min and returned to 30% B in 0.1 min followed by a re-equilibration (0.9 min). The total run time was 2.5 min and the HPLC flow rate was 0.5 mL/min. The source temperature was set at 500°C and mass spectral analyses were performed using multiple reaction monitoring (MRM) utilizing a Turbo-Ionspray® source in positive ionization mode (5.0 kV spray voltage). All data were analyzed using AB Sciex Analyst 1.4.2 software.
In vitro experiments. ML218 was analyzed similarly to that described above (In vivo) with the following exceptions: LC/MS/MS analysis was performed employing a TSQ QuantumULTRA that was coupled to a ThermoSurveyor LC system (Thermoelectron Corp., San Jose, CA) and a Leap Technologies CTC PAL auto-sampler (Carrboro, NC). Chromatographic separation of analytes was achieved with an Acquity BEH C18 2.1 × 50 mm, 1.7 μm column (Waters, Taunton, MA).
Haloperidol-Induced Catalepsy Methods
Animals. Male Sprague-Dawley rats weighing between 275 and 299 grams (Harlan Laboratories, Inc Indianapolis, IN) were used for the behavioral studies and were housed under a 12-h light/dark cycle (lights on at 6 AM, lights off at 6 PM) with free access to food and water. The experimental protocols, which were performed during the light cycle, were approved by the Institutional Animal Care and Use Committee of Vanderbilt University and conformed to the guidelines established by the National Research Council Guide for the Care and Use of Laboratory Animals. Rats were administered haloperidol (0.75mg/kg, i.p., dissolved in 8.5% lactic acid) 60 minutes prior to vehicle (10% Tween 80 in 0.5% methylcellulose) or ML218 (0.1–30mg/kg). After an additional 30 minute pretreatment interval, all rats were assessed in the catalepsy model. Catalepsy was measured by placing the forepaws of each rat gently onto a horizontal placed 6cm from the testing surface with the body positioned at an angle of ~45° to the testing surface. The latency in seconds required for the rat to remove one or both forepaws from the bar was measured with a testing cutoff of 30 seconds.
2.2. Probe Chemical Characterization

Probe compound ML218 (CID: 45115620, SID: 92310249) was prepared according to the above scheme and provided the following characterization data: 1H NMR (400 MHz, CDCl3) δ (ppm): 7.78 (d, J = 1.82 Hz, 2H); 7.57 (br, 1H); 7.42 (ap. t, J = 1.76 Hz, 1H); 3.64 (d, J = 10.56 Hz, 2H); 3.32 (t, J = 5.98 Hz, 2H); 2.95–2.83 (m,4H); 1.95 (m, 1H); 1.74 (s, 2H); 1.62 (m, 2H); 0.88 (s, 9H). 13C NMR (100 MHz, CD3OD) δ (ppm): 165.41, 137.46, 135.50, 131.46, 126.36, 55.69, 53.11, 41.94, 39.42, 30.07, 29.44, 21.71; HRMS calc’d for C19H26Cl2N2O, 369.1500 [M+H]; found 369.1501.
Solubility. Solubility (kinetic solubility) in PBS was determined to be 542 μM.
GSH Conjugates. No glutathione conjugates detected.
Stability. Stability was determined for ML218 at 23 °C in PBS (no antioxidants or other protectorants and DMSO concentration below 0.1%) and is shown in Table 1. We were surprised by the lack of stability in phosphate buffer, and Merck’s 724 behaved similarly. Due to the high logP (clogP 5.2, experimentally derived logP 4.9), we proposed that over time, the thermodynamic nature of this assay, the compound precipitates out of solution. In the solubility assay in pH 7.4 phosphate buffer, ML218 shows high solubility, but this is kinetic versus thermodynamic conditions. Upon closer inspection, it does appear that ML218, as well as 7,24 do precipitate out of solution over the time course in the 48 hour PBS assay. Moreover, the ML218 is stable in rat plasma and shows good stability in vivo. Therefore, the physical properties of ML218 appear to be the culprit for the apparent lack of stability in the 48 hour PBS assay, which is actually a lack of solubility and not instability. In vivo, ML218 is stable.
Table 1
Stability in Phosphate Buffer.
Table 2Calculated Property Comparison with MDDR Compounds
| Property | ML218 | MDDR Phase I | MDDR Launched |
|---|---|---|---|
| MW | 369.33 | 438.98 | 415.20 |
| cLogP | 5.28 | 3.21 | 2.21 |
| TPSA | 32.34 | 97.06 | 91.78 |
| Hdon | 1.00 | 2.12 | 2.13 |
| Hacc | 2.00 | 7.06 | 6.47 |
| LogS | −5.43 | −4.96 | −3.73 |
| NrotB | 6.00 | 7.08 | 5.71 |
Compounds added to the SMR collection (MLS#s): 003431854 (ML 218, CID 45115620, 20 mg), 003431850 (CID 44251479, 5.3 mg), 003431851 (CID 44251485, 5.0 mg), 003431852 (CID 45115609, 9.4 mg), 003431849 (CID 46943243, 5.5 mg), 003431853 (CID 46943258, 8.4 mg).
2.3. Probe Preparation
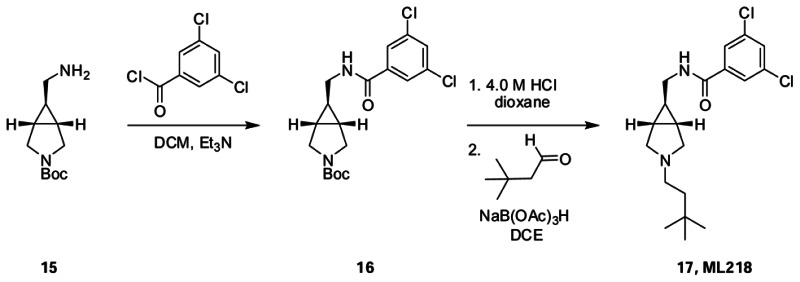
3,5-dichloro-N-((3-(3,3-dimethylbutyl)-3-azabicyclo[3.1.0]hexan-6-yl)methyl)benzamide (ML218)). tert-butyl 6-((3,5-dichlorobenzamido)methyl)-3 azabicyclo[3.1.0]hexane-3-carboxylate (16). To a solution of exo-3-Boc-6-aminomethyl-3-azabicyclo[3.1.0]hexane (1.0 eq.) and triethylamine (1.0 eq.) in CH2Cl2 at 0 °C was added a solution of 3,5-dichlorobenzoyl chloride (1.0 eq.) in CH2Cl2. The reaction stirred at 0 °C for approximately 4 hrs. The mixture was then concentrated in vacuo and purified by reverse phase chromatography (MeCN/H2O/0.1% TFA) to afford the product as a white solid (93% yield). 1H NMR (400 MHz, CD3OD) δ (ppm): 7.83 (d, J = 1.92 Hz, 2H); 7.66 (t, J = 1.88 Hz, 1H); 3.57 (m, 2H); 3.35 (m, 4H); 1.60 (m, 2H); 1.46 (s,9H); 0.90 (m, 1H). 13C NMR (125 MHz, CD3OD) δ (ppm): 167.96, 157.61, 139.75, 137.33, 133.02, 127.99, 81.86, 43.65, 29.56, 24.46, 24.02, 23.23. To a vial containing tert-butyl 6-((3,5-dichlorobenzamido)methyl)-3-azabicyclo[3.1.0]hexane-3-carboxylate 16, was added 4.0 M HCl in dioxane. This mixture was allowed to stir at room temp for 2 hrs. The reaction was then concentrated in vacuo and purified by reverse phase chromatography (MeCN/H2O/0.1% TFA) to afford the product as a white solid (95% yield). 1H NMR (400 MHz, CD3OD) δ (ppm): 7.82 (d, J = 1.92 Hz, 2H); 7.68 (m, 1H); 3.45 (s, 4H); 3.34 (m, 2H); 1.90 (m, 2H); 1.21 (m,1H). 13C NMR (100 MHz, CD3OD) δ (ppm): 168.04, 163.95, 139.57, 137.40, 133.17, 127.97, 43.02, 23.35, 22.48. 3,5-dichloro-N-((3-(3,3-dimethylbutyl)-3-azabicyclo[3.1.0]hexan-6-yl)methyl)benzamide (ML218): To a solution of 3,3-dimethylbutyraldehyde (1.0 eq.) in CH2Cl2 was added a solution of N-(3-azabicyclo[3.1.0]hexan-6-ylmethyl)-3,5-dichlorobenzamide (1.0 eq.) in CH2Cl2 followed by addition of polymer-supported sodium triacetoxyborohydride (1.2 eq.). The reaction was left to stir overnight at room temperature after which the mixture was filtered through a pad of Celite eluting with CH2Cl2 and concentrated in vacuo resulting in a crude oil which was then purified by reverse phase chromatography (MeCN/H2O/0.1% TFA) to afford the product as an off-white solid (88% yield). 1H NMR (400 MHz, CDCl3) δ (ppm): 7.78 (d, J = 1.82 Hz, 2H); 7.57 (br, 1H); 7.42 (ap. t, J = 1.76 Hz, 1H); 3.64 (d, J = 10.56 Hz, 2H); 3.32 (t, J = 5.98 Hz, 2H); 2.95–2.83 (m,4H); 1.95 (m, 1H); 1.74 (s, 2H); 1.62 (m, 2H); 0.88 (s, 9H). 13C NMR (100 MHz, CD3OD) δ (ppm): 165.41, 137.46, 135.50, 131.46, 126.36, 55.69, 53.11, 41.94, 39.42, 30.07, 29.44, 21.71; HRMS calc’d for C19H26Cl2N2O, 369.1500 [M+H]; found 369.1501.
3. Results
The Vanderbilt Screening/Chemistry Center completed the primary screen using fluorescence-based assay provided by the PI Dr. Xie. AfaSci Lab and JHU have been undertaking secondary screening using electrophysiologic techniques. The primary screen using the Cav3.2 channel expressed in HEK293 cells (provided by the project Consultant Dr. Perez-Reyes, University of Virginia) was performed in a 384-well format using an FDSS 6000 kinetic imaging plate reader (Hamamatsu). The 2008 collection of MLSCN library containing 110,720 compounds was screened at 10μM in a single measurement. Data quality was controlled by the known T channel inhibitor 7 as the positive control and the 0.1% DMSO vehicle as the negative control. The assay quality control used a screening window coefficient “Z-factor”. The Z′ values were between 0.6–0.8, indicating excellent assay quality. Primary data analysis was performed by comparing the activity of any test compound with all the other test compounds on the same plate, taking the ratio max in the kinetic time window of 12–40 sec with 0–1 sec as the baseline. Primary hits were defined as any outliers that were different from all the others based on a z-score threshold of 3. That is, after hits were picked using z-score, those z-scores were calculated again on the remaining compounds. Total 4,246 initial hits were identified from these 2 iterations.
3.1. Dose Response Curves for Probe
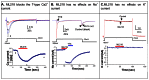
ML218 inhibition of Cav3 channels was evaluated in electrophysiological and fluorescent assays (Figure 1). Currents through Cav3 channels were recorded using the IonWorks Quattro (patch EP). ML218 is a potent and well-behaved inhibitor of Cav3 channels.
3.2. Cellular Activity
All assays (fluorescence and EP) for Cav3 and other ion channels described in this report are cell-based assays. The activity of ML218 in these assays indicates that the probe compound exhibits adequate cell permeability to support further studies with native cells or tissues. No acute toxicity was observed in the cell based assays. ML218 was also evaluated in our tier 1 DMPK profiling assays as well as in in vivo PK and brain level studies in rats. No acute toxicity was observed in any cell based assay or in vivo. No toxicity was observed in a haloperiodol-induced catalepsy model up to 30 mg/kg p.o.
3.3. Profiling Assays
Beyond the basic CRCs in Figure 1, we assessed ML218 activity on the native T-type currents for selectivity over other ion channels in rat DRG neurons (small diameter 10–20μm) in which the Cav3.2 channel is prominently expressed. Using pharmacological tools to isolate the current of interest, ML218 at 1μM produced approximately 70% inhibition of the T-type Ca2+ current without affecting the tetrodotoxin (TTX)-sensitive voltage-gated Na+ current and K+ currents (mainly delay rectifier, Figure 2). Although the potency of ML218 in blocking the native T-type current is less potent compared to its inhibition on the recombinant Cav3.2 channel expressed in HEK cells, it shows great selectivity over other major membrane ion channels in native neurons. The effects of ML218 on high-voltage-activated Ca2+ channels (e.g., L- and N- type) in the DRG neurons will be investigated in an extended probe characterization project.
ML218 was evaluated in the parental HEK fluorescent assay which contains native Ca2+ channels, presumably both L- and N-Type Ca2+ channels. ML218 had no affect on the parental line and was therefore thought to be selective versus L- and N-calcium channels (later confirmed at Ricerca panel, where ML218 showed no significant activity in radioligand binding at L- and N-type Ca2+ channels, 49%@10 μM and 17%@10 μM, respectively). As mentioned above, the effects of ML218 on high-voltage-activated Ca2+ channels (e.g., L- and N- type) in the DRG neurons via EP will be investigated in a later extended probe characterization effort. To more fully characterize this novel Cav3 inhibitor probe molecule, ML218 was tested on Ricerca’s (formerly MDS Pharma’s) Lead Profiling Screen (binding assay panel of 68 GPCRs, ion channels and transporters screened at 10 μM), and was found to only significantly bind two targets (sodium channel site 2 and sigma 1) of the 68 assays conducted (no inhibition of radio ligand binding > 50% at 10 μM). Interestingly, the Merck T-type inhibitors also showed similar activity in binding assays at sodium channel site 2 and sigma 1, but neither Merck T-type inhibitors were active in functional sodium channel EP assays. In radioligand binding assays at Ricerca,43 ML218 had no significant inhibition of L- and N-type calcium channels (17–49% inhibition @10 μM, respectively), KATP potassium channel (4% inhibition @10 μM) or hERG (48% inhibition @10 μM). Thus, from the Ricerca selectivity profile and the selectivity versus closely related ion channels in which ML218 affords greater than 60-fold selectivity for block of related ion channels and other distantly related potassium channels.

DMPK Profiling. ML218 was evaluated in our tier 1 in vitro DMPK panel. In plasma protein binding studies (equilibrium dialysis), ML218 possessed good free fraction in both rat (fu = 9.1%) and human (fu = 3.3%). The CYP profile across four major CYP450s was also good: 3A4 (IC50 >30 μM), 2C9 (IC50 >30 μM), 1A2 (IC50 = 10.8 μM), 2D6 (IC50 = 1.7 μM). Intrinsic clearance experiments in liver microsomes indicated that ML218 was highly cleared in rat (rat CLINT = 115 ml/min/kg), but low to moderate clearance in human (human CLINT = 12.7 ml/min/kg). In vivo rat PK mirrored the in vitro work, with a CL of 56 ml/min/kg; however, the t1/2 of ML218 was 6.8 hours with a mean residence time of ~7 hours. In a 10 mg/kg po plasma/brain study at a 1 hour time point, the plasma concentration of ML218 was 145 ng/mL and the brain concentration of ML218 was 1018 ng/mL. Importantly, ML218 was found to be highly brain penetrant with a BrainAUC/PlasmaAUC of ~7.4, making ML218 an exciting tool compound for CNS studies. The profile of ML218 is virtually identical to Merck’s, which showed poor rat PK, but excellent dog, rhesus and predicted human PK. Thus, ML218 (CID 45115620) is highly selective and can be used to dissect the role of Ca T-type inhibition in vitro and potentially in vivo.
In Vivo Efficacy in a Preclinical Model of Parkinson’s disease. Parkinson’s disease (PD) is therapeutic indication for T-Type Ca2+ channel inhibitors, and Merck’s displayed robust efficacy in a preclinical cataleptic PD model. As we have this same model, haloperidol-induced catalepsy, running for our other PD programs, we evaluated Merck’s and ML218 in this model, wherein reversal of the cataleptic state is consistent with an anti-Parkinsonian phenotype.24,42 Here, we dosed i.p. in dose response and observed robust efficacy comparable to an A2A standard.24,42 Excitingly, ML218 showed similar efficacy upon oral dosing, with a 30 mg/kg dose (1.8 μM plasma (160 nM free) with 17.7 μM brain) essentially equivalent to a 56.6 mg/kg dose of the A2A standard (Figure 3). Thus, ML218 is comparable to Merck’s (both in vitro and in vivo), and a valuable probe to study T-Type Ca2+ channel inhibition in vivo without IP restrictions.
4. Discussion
4.1. Comparison to Existing Art and How the New Probe is an Improvement
A number of structural classes of compounds have been described in the scientific and patent literature that act as Ca2+ T-Type channel inhibitors (vide supra). At least 20 structural classes of compounds that inhibit Ca2+ T-Type channel have been described in the scientific and/or patent literature. The known compounds either lack the required selectivity for the Cav3 channels, or are covered by patents.11–26,40 This was known prior to running the HTS screen, and NIH desired that we develop a Ca2+ T-Type inhibitor free of IP issues so that the biomedical community could possess a potent and selective probe without concern over use restrictions. ML218 is a potent inhibitor (Cav3.2, IC50 = 150 nM in Tl+ flux; Cav3.2 IC50 = 310 nM and Cav3.3 IC50 = 270 nM in patch clamp electrophysiology) and selective against the other members of the calcium family of ion channels (L- and N-type calcium channels) as well as multiple sodium and potassium channels. Lastly, ML218 was tested at Ricerca’s (formerly MDS Pharma’s) Lead Profiling Screen (binding assay panel of 68 GPCRs, ion channels and transporters screened at 10 μM) and was found to significantly bind to only 2 of the 68 targets; importantly, ML218 was found to be selective against hERG and many other ion channels. ML218 possess excellent DMPK properties, acceptable in vivo rat PK, excellent brain levels and was efficacious in a haloperidol-induced catalepsy assay, a preclinical Parkinsonian model. Thus, ML218 is a powerful new probe to study Ca2+ T-Type function in vitro and in vivo.
5. References
- 1.
- 2.
- Barrow JC, Duffy JL. Ann Reports Med Chem. 2010;35:3–18.
- 3.
- Mohan CG, Gandhi T. Mini-Reviews in Med Chem. 2008;8:1285–1290. [PubMed: 18855741]
- 4.
- Tringham E, Snutch TP. Voltage-gated N-type and T-type calcium channels and Excitability disorders. Structure, Function and Modulation of Neuronal Voltage-Gated Ion Channels. 2009:35–66.
- 5.
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Neuron. 2000;25:533–535. [PubMed: 10774722]
- 6.
- Catterall WA. Annu Rev Cell Bio. 2000;16:521. [PubMed: 11031246]
- 7.
- Ono K, Iijima T. J Mol Cell Cardiology. 2010;48:65–70. [PubMed: 19729018]
- 8.
- Cribbs LL, et al. FEBS Lett. 2000;466(1):54–88. [PubMed: 10648811]
- 9.
- Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E. Circ Res. 1998;83:103–109. [PubMed: 9670923]
- 10.
- Choi SN, Kim J, Lee J, Lee S, Kim D, Park J, Chen CC, Campbell KP, Shin HS. Genes, Brain and Behavior. 2007;6:425–431. [PubMed: 16939637]
- 11.
- Anderson MP, Mochizuki T, Xie J, Fischler W, Manger JP, Talley EM, Scammell TE, Tonegawa S. Proc Natl Acad Sci U S A. 2005;102:1743–1748. [PMC free article: PMC547889] [PubMed: 15677322]
- 12.
- Avdonin PV, Buhler FR, Tkachuk VA. Membr Cell Biol. 2000;13:645–55. [PubMed: 10987388]
- 13.
- Baxter DF, Kirk M, Garcia AF, Raimondi A, Holmqvist MH, Flint KK, Bojanic D, Distefano PS, Curtis R, Xie Y. J Biomol Screen. 2002;7:79–85. [PubMed: 11897058]
- 14.
- Becker AJ, Pitsch J, Sochivko D, Opitz T, Staniek M, Chen CC, Campbell KP, Schoch S, Yaari Y, Beck H. J Neurosci. 2008;28:13341–13353. [PMC free article: PMC6671595] [PubMed: 19052226]
- 15.
- Bourinet E, Alloui A, Monteil A, Barrere C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J. Embo J. 2005;24:315–324. [PMC free article: PMC545807] [PubMed: 15616581]
- 16.
- Jagodic MM, Pathirathna S, Joksovic PM, Lee W, Nelson MT, Naik AK, Su P, Jevtovic-Todorovic V, Todorovic SM. J Neurophysiol. 2008;99:3151–3156. [PMC free article: PMC2667888] [PubMed: 18417624]
- 17.
- Jevtovic-Todorovic V, Todorovic SM. Cell Calcium. 2006;40:197–203. [PubMed: 16777222]
- 18.
- Khosravani H, Altier C, Simms B, Hamming KS, Snutch TP, Mezeyova J, McRory JE, Zamponi GW. J Biol Chem. 2004;279:9681–9684. [PubMed: 14729682]
- 19.
- Kim D, Park D, Choi S, Lee S, Sun M, Kim C, Shin HS. Science. 2003;302:117–119. [PubMed: 14526084]
- 20.
- Lee J, Shin HS. CNS Neurol Disord Drug Targets. 2007;6:63–69. [PubMed: 17305554]
- 21.
- Messinger RB, Naik AK, Jagodic MM, Nelson MT, Lee WY, Choe WJ, Orestes P, Latham JR, Todorovic SM, Jevtovic-Todorovic V. Pain. 2009;145:184–195. [PMC free article: PMC2735619] [PubMed: 19577366]
- 22.
- Giordanetto F, Knerr L, Walberg A. Exp Opin Ther Patents. 2011;21:85–101. [PubMed: 21087200]
- 23.
- Shipe WD, Barrow JC, Yang Z-Q, Lindsley CW, Yang FV, Schlegel KS, Shu Y, Rittle KE, Zrada MM, Bock MG, Hartman GD, Tang C, Ballard JE, Kuo Y, Adarayan ED, Prueksaritanont Y, Uebele VN, Nuss CE, Connolly TM, Doran SM, Fox SV, Kraus RL, Marino MJ, Graufelds VK, Vargas HM, Bunting PB, Hasbun-Manning M, Evans RM, Koblan KS, Renger JJ. J Med Chem. 2008;51:3692–3695. [PubMed: 18540666]
- 24.
- Yang Z-Q, Barrow JC, Shipe WD, Schlegel KS, Shu Y, Yang FV, Lindsley CW, Rittle KE, Bock MG, Hartman GD, Uebele VN, Nuss CE, Fox SV, Kraus RL, Doran SM, Connolly TM, Tang C, Ballard JE, Kuo Y, Adarayan ED, Prueksaritanont Y, Zrada MM, Marino MJ, DiLella AG, Reynolds IJ, Vargas HM, Bunting PB, Woltman MM, Koblan KS, Renger JJ. J Med Chem. 2008;51:6471–6477. [PubMed: 18817368]
- 25.
- Barrow JC, Lindsley CW, Shipe WD, Yang Z, Wisnoski DD. WO 0216841. 4-Fluoro-Piperdine T-Type Calcium Channel Antagonists. 2010
- 26.
- Barrow JC, Lindsley CW, Shipe WD, Yang Z. WO 0222387. 3-Fluoro-Piperdine T-Type Calcium Channel Antagonists. 2010
- 27.
- Leuranguer V, Mangoni ME, Nargeot RS. J. CardioVasc Pharmacol. 2001;37:649–661. [PubMed: 11392461]
- 28.
- Mehrke G, Zong XG, Flockerzi V, Hofmann F. J Pharmacol Exp Ther. 1994;271:1483–1488. [PubMed: 7996461]
- 29.
- Bezprozvanny I, Tsien RW. Mol Pharmacol. 1995;48:540–549. [PubMed: 7565636]
- 30.
- Masuda Y, Tanaka S. Cardiovasc Drug Res. 1994;12:123–129.
- 31.
- Richelson E, Souder T. Life Sci. 2000;68:29–34. [PubMed: 11132243]
- 32.
- Renger TS, Yang ZQ, Schlegel KS, Shu Y, Mattern C, et al. Bioorg Med Chem Lett. 2011;21:1692–1696. [PubMed: 21316226]
- 33.
- Smith EM, Sorota S, Kim HM, McKittrick BA, et al. Bioorg Med Chem Lett. 2010;20:4602–4606. [PubMed: 20580233]
- 34.
- Choi YH, Baek DJ, Lee JK, Pae AN, Cho YS, Min SJ. Bioorg Med Chem Lett. 2011;21:215–219. [PubMed: 21126876]
- 35.
- Hangeland JJ, Cheney DL, Friends TJ, Swartz S, et al. Bioorg Med Chem Lett. 2008;18:474–478. [PubMed: 18160281]
- 36.
- Gu SJ, Lee JK, Pae AN, et al. Bioorg Med Chem Lett. 2010;20:2705–2708. [PubMed: 20382529]
- 37.
- Fritch PC, Krajewski J. Bioorg Med Chem Lett. 2010;20:6375–6378. [PubMed: 20934333]
- 38.
- Lee JE, Koh HY, Seo SH, et al. Bioorg Med Chem Lett. 2010;20:4219–4222. [PubMed: 20621730]
- 39.
- Schlegel KS, Yang ZQ, Reger TS, et al. Bioorg Med Chem Lett. 2010;20:5147–5152. [PubMed: 20673719]
- 40.
- www
.uspto.com, search string T-Type calcium inhibitors - 41.
- Jain AM. J Computer-Aided Drug Design. 2000;14:199–213. [PubMed: 10721506]
- 42.
- Niswender CM, Myers-Johnson KA, Weaver CD, Jones CK, Luo Q, Rodriguez AL, Marlo JE, de Paulis T, Thompson A, Days E, Nalywajko NT, Austin C, Williams M, Ayala JE, Williams R, Lindsley CW, Conn PJ. Mol Pharm. 2008;74:1345–1358. [PMC free article: PMC2574552] [PubMed: 18664603]
- 43.
- For details on the Lead Profiing Screen see: www
.ricerca.com
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- The Discovery and Characterization of ML218: A Novel, Centrally Active T-Type Calcium Channel Inhibitor with Robust Effects in STN Neurons and in a Rodent Model of Parkinson's Disease.[ACS Chem Neurosci. 2011]The Discovery and Characterization of ML218: A Novel, Centrally Active T-Type Calcium Channel Inhibitor with Robust Effects in STN Neurons and in a Rodent Model of Parkinson's Disease.Xiang Z, Thompson AD, Brogan JT, Schulte ML, Melancon BJ, Mi D, Lewis LM, Zou B, Yang L, Morrison R, et al. ACS Chem Neurosci. 2011 Dec 21; 2(12):730-742.
- Review Development of a Selective, Allosteric PLD1/2 Inhibitor in a Novel Scaffold.[Probe Reports from the NIH Mol...]Review Development of a Selective, Allosteric PLD1/2 Inhibitor in a Novel Scaffold.Scott SA, O’Reilly MC, Daniels JS, Morrison R, Ptak R, Dawson ES, Tower N, Engers JL, Engers DW, Oguin T, et al. Probe Reports from the NIH Molecular Libraries Program. 2010
- Review Development of a Selective, Allosteric PLD2 Inhibitor.[Probe Reports from the NIH Mol...]Review Development of a Selective, Allosteric PLD2 Inhibitor.Scott SA, O’Reilly MC, Daniels JS, Morrison R, Ptak R, Dawson ES, Tower N, Engers JL, Engers DW, Oguin T, et al. Probe Reports from the NIH Molecular Libraries Program. 2010
- Review A Next generation PLD2 inhibitor with improved physiochemical properties and DMPK profile for translational in vivo.[Probe Reports from the NIH Mol...]Review A Next generation PLD2 inhibitor with improved physiochemical properties and DMPK profile for translational in vivo.O'Reilly MC, Scott SA, Daniels JS, Morrison R, Engers JL, Oguin T, Thomas P, Brown HA, Lindsley CW. Probe Reports from the NIH Molecular Libraries Program. 2010
- Protein kinase A regulation of T-type Ca2+ channels in rat cerebral arterial smooth muscle.[J Cell Sci. 2013]Protein kinase A regulation of T-type Ca2+ channels in rat cerebral arterial smooth muscle.Harraz OF, Welsh DG. J Cell Sci. 2013 Jul 1; 126(Pt 13):2944-54. Epub 2013 Apr 23.
- Scaffold Hopping Affords a Highly Selective in vitro and in vivo T-Type Calcium ...Scaffold Hopping Affords a Highly Selective in vitro and in vivo T-Type Calcium Inhibitor Probe Free From IP Issues - Probe Reports from the NIH Molecular Libraries Program
Your browsing activity is empty.
Activity recording is turned off.
See more...

 .
.