NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Management of abnormal muscle tone in adults aged 19 and over with cerebral palsy
Review question
A3 Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB)) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
Introduction
Dystonia is a pattern of sustained disturbed muscle contraction causing abnormal posture and frequent involuntary movements in some adults with cerebral palsy. There can be environmental, physical or psychological factors that aggravate dystonia and once they have been removed there are enteral and intramuscular pharmacological agents that can be used to manage dystonia. Neurosurgical procedures, such as intrathecal baclofen therapy, and in severe intractable cases Deep Brain Stimulation (DBS) are currently available options. Both procedures require anaesthetic, and have surgical, recovery and long-term risks. This review question examines the effectiveness of these interventions, including patient experience and quality of life and in the case of DBS the potential complications of brain surgery as well as on-going maintenance costs.
PICO table
Please see Table 1 for a summary of the Population, Intervention, Comparison and Outcome (PICO) characteristics of this review.
Table 1
Summary of the protocol (PICO table).
For full details see the review protocol in appendix A
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines 2014: the manual. Methods specific to this review question are described in the review protocol in appendix A and for a full description of the methods see supplementary document C.
Declaration of interests were recorded according to NICE’s 2014 conflicts of interest policy from May 2016 until April 2018. From April 2018 onwards they were recorded according to NICE’s 2018 conflicts of interest policy. Those interests declared until April 2018 were reclassified according to NICE’s 2018 conflicts of interest policy (see Interests Register).
Clinical evidence
Included studies
Five studies (number of participants, N=51) were included in the review: 1 randomised trial (Pozin 2014) and 4 before-and-after observational studies (Koy 2014, Marks 2011, Romito 2015, and Vidailhet 2009).
The clinical studies included in this evidence review are summarised in Table 2.and evidence from these is summarised in the clinical evidence profiles (Table 3 and Table 4).
Pozin 2014 compared levodopa with placebo. The remaining studies (Koy 2014, Marks 2011, Romito 2015, and Vidailhet 2009) compared pre and post-operative outcomes in people receiving bilateral pallidal deep brain stimulation.
See also the literature search strategy in appendix B, study selection flow chart in appendix C, forest plots in appendix E and study evidence tables in appendix D.
Excluded studies
Studies excluded from this systematic review, with reasons for their exclusion, are provided in appendix K.
Summary of clinical studies included in the evidence review are provided in Table 2.
Table 2
Summary of included studies.
See appendix D for the full evidence tables.
Quality assessment of clinical outcomes included in the evidence review
The clinical evidence profiles for this review question are presented in Table 3 and Table 4.
Table 3
Summary clinical evidence profile: Comparison 1: levodopa versus placebo.
Table 4
Summary clinical evidence profile: Comparison 2: bilateral pallidal deep brain stimulation (DBS) – pre versus post-operative.
See appendix F for the full GRADE tables.
Economic evidence
Included studies
A systematic review of the economic literature was conducted but no studies were identified which were applicable to this review question.
Excluded studies
No studies were identified which were applicable to this review question.
Summary of studies included in the economic evidence review
No economic evaluations were included in this review.
Economic model
See appendix J for the full report of the economic model.
A decision analytical model in the form of a state transition model was developed to estimate the cost effectiveness of deep brain stimulation (DBS) compared to usual care of trihexyphenidyl 5mg daily in adults aged over 19 with cerebral palsy and dystonia. The main outcome of the economic model was incremental cost per QALY. Costing was undertaken using a NHS and Personal Social Services (PSS) perspective. The model had a lifetime time horizon. Costs and QALYs were discounted at 3.5% per annum.
During the procedure for DBS patients may experience a seizure, infection, intracranial haemorrhage (ICH), or die. Patients who experience an infection could either remain on DBS, or abandon DBS and receive “usual care”. Patients who experience a seizure or minor ICH remain on DBS treatment. Following a successful procedure for DBS, patients remain on DBS and receive a routine implanted pulse generator (IPG) replacement every 5 years. Each year patients on DBS are at risk of a hardware failure which will incur additional surgery to correct. Patients in usual care receive pharmacological treatment in the base case. It was assumed patients in usual care are not at risk of any adverse events.
The structure of the model is illustrated in Figure 1.

Figure 1
Model structure.
Evidence identified during the accompanying clinical evidence review had small numbers and were not representative of adverse events seen in practice. Alternative papers that analysed DBS were sought to inform the probability of complications in the model. Two studies were identified Boviatsis 2010 and Voges 2006 reviewed the complications of DBS experienced by their departments; from 2003 to 2010 in 106 patients and from 1996 to 2003 in 262 patients, respectively. Both also compared their own results to others reported in the literature. The model assumed an annual probability of hardware failure of 4%. The probability of adverse events are listed in Table 5.
Table 5
Probability of perioperative DBS-related complications.
Health related quality of life data was taken from 2 before and after type studies (Vidailhet 2009 and Romito 2015) that reported the results for each of the 8 domains of the SF-36, pre- and post- DBS treatment. Given heterogeneity in the results of the two studies they were used separately to inform the economic model. The SF-36 was mapped on to the EQ-5D, NICE’s preferred measure of quality of life, for use in the economic model (discussed in detail in appendix J.) Given that no comparative data was identified, it was assumed the utility pre-DBS is equivalent to the utility associated with “usual care”. It was also assumed that the utility post-DBS holds when patients remain on DBS care. A disutility was applied for patients undergoing surgery for DBS. A disutility was also applied for all adverse events in the model (Table 6). In the absence of evidence to the contrary overall survival was assumed identical between the two interventions considered.
Table 6
Disutility from DBS-related complications.
All DBS related resource use and unit costs were taken from Yianni 2005 and inflated to current costs. This was study of quality of life and costs in 26 patients with dystonia (not exclusively cerebral palsy) from 1 UK centre. The committee considered that costs reported in this paper maybe an underestimate of the true costs as they do not reflect the latest innovations in DBS. These costs were therefore explored extensively during sensitivity analysis.
Table 7
Cost of DBS reproduced from Yianni 2005.
The annual costs of usual care were £77.82 and an annual follow up appointment in neurology at £161 taken from the NHS Electric Drug Tariff and NHS Reference Costs respectively.
A series of sensitivity analyses were undertaken in order to test how sensitive the results were to uncertainty in individual parameters. Probabilistic sensitivity analysis (PSA) was conducted in the model to take account of the simultaneous effect of uncertainty relating to model parameter values.
Results of the economic model
Base case results
When Romito 2015 was used to inform the model, DBS was more costly and more effective than usual care, with an ICER of £20,169 per QALY (Table 8). DBS was also more costly than usual care when Vidailhet 2009 was used, but less effective than Romito 2015. As a result, the ICER was higher at £77,181 per QALY.
Table 8
Base case results (deterministic).

Figure 2
Cost-effectiveness plane (base case).
Sensitivity analysis results
The total QALYs increased for DBS when utility decrements were removed and when the risk of complications were removed. This reduced the ICER for Vidailhet 2009 and Romito 2015, but the ICER for Vidailhet 2009 remained above NICE’s upper threshold of £30,000 per QALY.
Reducing the time horizon reduced the number of QALYs that could be accrued and amplified the cost of the DBS procedure. This analysis increased the ICER above NICE’s upper threshold in both studies.
When usual care consisted of botulinum toxin (a more costly treatment than trihexyphenidyl) the incremental cost reduced. This reduced the ICER for Vidailhet 2009 and Romito 2015, but the ICER for Vidailhet 2009 remained above NICE’s upper threshold of £30,000 per QALY.
The results of each analysis are provided in Table 9 for Romito 2015 and Table 10 for Vidailhet 2009.
Table 9
Results of sensitivity analysis (Romito 2015).
Table 10
Results of sensitivity analysis (Vidailhet 2009).
Using Romito 2015, the worst case scenario, raising the cost of the total procedure by 50%, increased the ICER to £23,918. In the best case scenario, lowering the total cost of the procedure by 50% reduced the ICER to £16,420. The most influential parameters were related to the replacement of the IPG. When the cost to replace the IPG was varied by 50% the ICER ranged from £16,342 to £23,750. When the frequency of replacements was changed from every 5 years to every 2 or 8 years, the ICER ranged from £16,761 to £33,351. (Figure 3, Figure 4) For Vidailhet 2009 all ICERs remained above a cost-effectiveness threshold of £30,000 per QALY when parameters were varied (Figure 5, Figure 6). Similarly to Romito 2015, the most influential parameters included the total cost of the procedure (namely stimulation equipment) and IPG replacements.

Figure 3
Tornado diagram of the costs associated with the procedure and monitoring (Romito 2015).
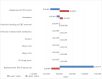
Figure 4
Tornado diagram of the costs to treat the complications of DBS (Romito 2015)).

Figure 5
Tornado diagram of the costs associated with the procedure and monitoring (Viadilhet 2009).

Figure 6
Tornado diagram of the costs to treat the complications of DBS (Vidailhet 2009).
Probabilistic results
For Romito 2015, all simulations found DBS to be more effective and more expensive than usual care with a mean probabilistic ICER of £20,077. Furthermore, 739 of 1,000 simulations had ICER’s below £20,000 and 927 below £30,000. This is illustrated in Figure 7Figure 3 where simulations cross the £20,000 threshold in the north-east quadrant. The cost effectiveness acceptability curve (CEAC) also illustrated that DBS would be considered as the most optimal treatment for any threshold over £17,000 per QALY (Figure 8). In Figure 7Figure 3 the simulations do not fall below an incremental cost of £60,000 the cost to provide DBS.

Figure 7
PSA simulations (Romito 2015).

Figure 8
CEAC (Romito 2015).
When Vidailhet 2009 was used to inform the model, the mean ICER was £72,323 with almost all simulations (996 of 1,000) in the north-east quadrant above NICE’s threshold (Figure 9). The CEAC also illustrated that usual care would be considered as the most optimal treatment for thresholds up to £65,000 per QALY (Figure 8).

Figure 9
PSA simulations (Vidailhet 2009).
Conclusions
DBS is more effective but also more costly than usual care when either study is used to inform the model. When the ICER is considered, DBS could be considered cost effective according to Romito 2015, who produce an ICER just above NICE’s advisory threshold of £20,000 per QALY in the base case with many iterations of the PSA being below it. The opposite was the case when Vidailhet 2009 was used to inform the model with both the base case and nearly all iterations of the PSA, DBS was above NICE’s conventionally held threshold of £20,000 per QALY and therefore not considered cost effective.
Given the large uncertainty inherent in the clinical evidence it is difficult to make strong conclusions. Greater certainty around cost effectiveness would be obtained through further research. Given the evidence around cost effectiveness DBS should only be considered for use when all other medical and surgical interventions have been considered and exhausted – in line with the current NHS commissioning policy on DBS for dystonia.
Resource impact
In the absence of economic evidence for all the interventions considered in the review question unit costs were presented to the committee to aid in their consideration of resource impact and cost effectiveness.
Pharmacological treatments
According to NHS Reference Costs 2015/16 the first attendance for a pre-assessment in neurology would cost £217 (currency code WF01B, service code 400, non-admitted face-to-face attendance, first, neurology), but the committee advised that pharmacological treatments for dystonia could be initiated by a specialist clinic neurologist, rehabilitation medicine consultant, specialist nurse or specialist prescribing physiotherapist.
Drug acquisition costs for all pharmacological interventions for which evidence was searched, were taken from the NHS Electronic Drug Tariff May 2017 and dosages from the BNF August 2017. (Not presented) Dosages were verified with the committee to ensure they were appropriate for this patient group.
Often, oral treatments for dystonia do not incur administration costs as they are administered at home, without health care professional assistance. However, if families or carers administer oral treatments via PEG, they will require additional training and equipment. Oral treatments may be monitored by the patient’s GP and community team at routine visits, but advice from a rehabilitation medicine or neurologist on increasing or decreasing medication would be sought if they were not directly responsible for monitoring the treatment. Furthermore, for levodopa, an additional review with the patient’s GP or neurologist after the initial 3 months of treatment would be incurred to assess efficacy.
Botulinum toxin involves a day–case admission performed by a neurologist, rehabilitation medicine doctor, or a specially trained physiotherapist or nurse in a specialist clinic. Adults with cerebral palsy are unlikely to be sedated, but ultrasound or electromyography (EMG) may be used for guidance.
The appointment for the injection of botulinum has a NHS reference cost assigned – Torsion dystonia and other involuntary movements drugs band 1 (code XD09Z). This reference cost (£324) will include all costs related to the procedure, the day case admission, drug costs and staff costs.
Following the injections, patients would be monitored every 3 to 4 months by the specialist clinic at a cost of £161 (NHS Reference Costs 2015/16, currency code WF01A, service code 400, non-admitted face-to-face attendance follow-up, neurology) to assess their response and need for repeat injections.
Dynamic orthotics
Healthcare Improvement Scotland identified no published cost-effectiveness evidence on dynamic lycra splinting. For completeness they provided the cost of body suits currently available from personal communications. Those costs are presented alongside costs converted to 2015/16 using the hospital and community health services pay and prices index uplift (Curtis 2015) in Table 11.
Table 11
Cost of dynamic orthotic equipment.
Dynamic orthotic equipment would be offered after an assessment with an orthotist (NHS Reference Costs 2015/16 WF01B 658, £77) following a referral from an occupational therapist or physiotherapist. Orthotic equipment should be reviewed annually by an occupational therapist (regarding upper limb and hand orthotics), physiotherapist (regarding body suits or legs and feet orthotics) or orthotist. If there is a ‘change’ or ‘problem’ 2 or 3 of those healthcare professional may complete a joint review (Table 12).
Table 12
Follow-up costs, orthotics.
The committee advised that orthotic equipment would typically last between 6 to 24 months before it needs to be replaced, but reiterated that the lifespan would depend on how much it is used and during which activities.
Intrathecal baclofen (ITB)
Sampson 2002 published a study on ITB in which detailed cost estimates were derived from 3 centres in the UK where the procedure was being performed. The costs included in the study were obtained in 1999 and have been converted to 2015/16 costs using the hospital and community health services pay and prices index uplift (Curtis 2015) in Table 13.
Table 13
Cost of intrathecal baclofen reproduced from Sampson 2002.
The East Midlands Specialised Commissioning Group also produced detailed paediatric and adult costs for ITB treatment in 2009. They assumed the admission for the test dose usually takes 2 days whilst the admission for the implant usually takes an additional 5 days. The test dose, implant and refills were worked out using the contract code AB05Z (for intermediate pain procedures), at 2009/2010 prices. Those prices are presented alongside 2015/16 costs in Table 14.
Table 14
Cost of ITB treatment based on East Midlands commissioning policy 2009.
The total costs over 5 years are similar in the Sampson 2002 study and in the East Midlands Commissioning Policy; however, it is likely that the costs from the latter source are more accurate as costs were based on an HRG code, reflecting more recent UK practice.
Evidence statements
Comparison 1. Levodopa versus placebo
Critical outcomes
Health related quality of life
- No evidence was found for this outcome.
Dystonia
- No evidence was found for this outcome.
Patient or carer reported satisfaction
- No evidence was found for this outcome.
Important outcomes
Motor function using functional measures
- Low quality evidence from 1 randomised trial including 9 people with cerebral palsy and dystonia suggested no clinically important effect of levodopa as compared to placebo on motor function assessed using the QUEST score.
Goal attainment scores
- No evidence was found for this outcome.
Adverse events
- Very low quality evidence from 1 randomised trial including 9 people with cerebral palsy and dystonia identified no adverse effects associated with levodopa.
Pain
- No evidence was found for this outcome.
Comparison 2. bilateral pallidal deep brain stimulation (DBS) – pre versus post-operative
Critical outcomes
Health related quality of life
- Very low quality evidence from 2 before and after studies of DBS in 28 people with cerebral palsy and dystonia indicated a clinically important improvement in some of the subscales of the SF-36 health related quality of life measure following DBS.
Dystonia
- Very low quality evidence from 4 before and after studies of bilateral pallidal deep brain stimulation (DBS) in 42 people with cerebral palsy and dystonia indicated a clinically important reduction in dystonia following DBS.
Patient or carer reported satisfaction
- No evidence was found for this outcome.
Important outcomes
Motor function using functional measures
- No evidence was found for this outcome.
Goal attainment scores
- No evidence was found for this outcome.
Adverse events
- Very low quality evidence about adverse events following DBS came from 2 before and after studies of DBS in 28 people with cerebral palsy and dystonia. Adverse events included: hypophonia, dysarthria, localised pain, paraesthesia, anxiety, requirement to adjust the stimulator due to ineffectiveness and stimulator failure following exposure to magnetic field.
Pain
- Very low quality evidence from 1 before and after study of DBS in 13 people with cerebral palsy and dystonia indicated no clinically important reduction in pain following DBS.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
The critical outcomes for consideration in dystonia were health related quality of life and patient satisfaction. These were prioritised due to the disruptive effect of uncontrolled muscle spasms on daily life. Motor function, reduction of pain, goal attainment and treatment related adverse events were important outcomes. Health related quality of life was reported in studies that assessed the effectiveness of deep brain stimulation. However, in the trial on levodopa only change in motor function and adverse events were reported. No evidence was found for other potential antidystonic pharmacological treatments such as trihexyphenidyl, botulinum toxin injections, gabapentin/pregabalin, tetrabenazine, intrathecal baclofen; or orthotic use to improve physical function for dystonia in adults with cerebral palsy (such as Lycra garments).
The quality of the evidence
Evidence for outcomes comparing treatments was very low to low quality according to GRADE and was only available for levodopa compared to placebo and for pre-postoperative comparison of deep brain stimulation.
The evidence had several limitations. The trial on levodopa included people with dystonia related to cerebral palsy who were quadriplegic with GMFCS ranging from III to V. This means that they were severely impaired and is the committee therefore noted that the results of this trial could not be generalised to all people with cerebral palsy who have dystonia.
Study design was also a factor that lowered the committee’s confidence in the evidence. The evidence to assess the effectiveness of deep brain stimulation came from before and after observational studies. It was often not clearly described what kinds of treatments people have had prior to having deep brain stimulation and it is also not clear whether the benefits or risks would have been the same or different to any other type of intervention since there was no comparison group.
Benefits and harms
Based on their experience the committee discussed that the relationship between spasticity and dystonia is not always clear to healthcare professionals and that better knowledge of this would lead to more effective shared decision. To highlight the complexity of conditions of abnormal muscle tone they therefore decided to describe that adults with cerebral palsy can have both spasticity as well as dystonia and that symptom severity may vary.
The committee, based on their experience and expertise, agreed that there are a number of factors that can contribute to, or exacerbate, both spasticity and dystonia. They highlighted those factors that are most commonly associated with spasticity or dystonia and that are not always recognised as such. Identifying and addressing these improves the effectiveness of any multidisciplinary spasticity treatment strategy by focusing the management plan (for example if dystonia is exacerbated by pressure sores or constipation then a treatment plan should address these factors first).
Based on their experience and expertise the committee considered that treatment of both spasticity and dystonia can reduce pain and improve sleep, has an impact on motor function and can improve quality of life. The difference between spasticity, voluntary resistance and contractures requires careful assessment and it may not be possible to tell them apart in one assessment, or until treatment is initiated where movement is severely restricted. The committee discussed that spasticity as well as dystonia can have a positive impact on motor function. Some people with cerebral palsy make functional use of their increased muscle tone from spasticity and dystonia, for example to help them walk. For these people reduction in spasticity or dystonia could have a negative impact on certain motor functions, for example loss of their ability to transfer independently. However, severe spasticity can also have a negative impact on motor function as increased muscle tone can limit function.
The committee agreed that the risks and benefits should be discussed with each person before treatment and specific treatment goals are agreed. In relation to potentially positive or negative effects of increased tone, the committee highlighted that goals need to be clearly set out and that this should also feature in multidisciplinary team discussions to assess potential changes in function. This would also lead to better shared decision making and would inform the assessment of whether or not treatments are effective.
Apart from limited evidence related to levondopa and bilateral pallidal deep brain stimulation there was no evidence identified for other medicines or neurosurgical procedures. The committee noted that that it is a specialist clinical area. Based on their experience they acknowledged that there are enteral drug treatments available (such as trihexyphenidyl and gabapentin) that might be beneficial for some people. To balance the benefits and harms of these, and other more invasive options they agreed that treatments should only be considered by a specialist service and would depend on the person’s symptoms and treatment goals. Therefore, the committee agreed that adults with cerebral palsy should be referred for specialist management if they have problematic dystonia.
There was some evidence that levodopa was not effective in adults with cerebral palsy and dystonia and severe impairment. Due to the lack of evidence for effectiveness, the potential for side effects the committee agreed that levodopa should not be prescribed routinely for dystonia in cerebral palsy. However, they decided that a trial of levodopa can be useful to identify the rare, but treatable, condition of dopa-responsive dystonia.
Based on their expertise and experience the committee noted that stopping antidystonic drugs too quickly could lead to severe symptoms (for example anxiety and panic attacks) particularly if it has been taken for a few weeks. Therefore they agreed that the dose of the medication should be gradually reduced before stopping it to minimise risk.
The committee made a recommendation, based on experience and knowledge, for the use of botulinum toxin type A as a treatment for focal dystonia, particularly when it is causing pain and is affecting their care or function. They recommended such treatment should be supervised by a tone or spasticity management service because expert assessment and a wider management programme (that may include physiotherapy and splinting) is also needed to get optimise the benefit of the treatment.
The committee noted, based on their experience and expertise that focal interventions in some individuals with dystonia and cerebral palsy may alter the balance of motor function, adversely affecting the outcomes. The committee acknowledged this and recommended it should be taken into account during consideration of botulinum toxin type A therapy.
No evidence was identified for the use of continuous pump administered intrathecal baclofen. Given the risks associated with this surgical procedure the committee decided that this should only be considered when all other options have been exhausted. They agreed that the specific benefits and harms of this procedure should be discussed and as well as the test dose and how to assess the response with the adults with cerebral palsy (and their family or carer, if appropriate) and therefore cross referred to the relevant recommendations in the section on neurosurgical treatments to reduce spasticity (A2.2 to A2.5).
Although there was limited evidence for deep brain stimulation, it did suggest some improvement in dystonia after treatment. However, some serious complications were noted, including problems with speech, pain, numbness and anxiety, as well as problems with the equipment. The committee therefore agreed that this should only be considered after referral to a specialist centre with experience in providing this procedure. The committee acknowledged that there are not many of these centres who provide this, but agreed that there would only be a small proportion of adults with cerebral palsy who may benefit from this.
Cost effectiveness and resource use
The committee noted that no relevant published economic evaluations had been identified for this topic.
Dystonia is aggravated by factors such as pain and anxiety which if not identified and managed appropriately, can negatively impact on the patients’ health-related quality of life. Therefore, knowing what factors can aggravate dystonia may lead to increased vigilance and thus more timely management with potential cost savings. Estimating the costs to manage those factors would go beyond the scope of the guideline although they were likely to offset the cost of the recommendations.
The committee discussed the evidence that levodopa provided no additional benefit compared to placebo and agreed that relatively cheap treatments should not be recommended if they are ineffective or have the potential to incur adverse effects. For this reason, the committee made a recommendation to not routinely prescribe levodopa to stop potentially cost-ineffective practices. However, other pharmacological treatments such as gabapentin and trihexyphendyl are currently available in practice and could be considered before more costly and more invasive options. Given that no evidence was identified on those alternatives, the committee made a recommendation to refer adults with problematic dystonia to a specialist movement disorder or spasticity service to consider treatment in line with their experience and expertise. The committee noted that a recommendation to refer adults to a specialist tone management team would not increase current resource use as it would be beyond the remit of GPs to initiate treatments for dystonia in primary care. The committee also noted it would be cost-ineffective to refer people with asymptomatic or tolerable dystonia as treatment would not provide any additional benefits to justify the cost, burden, or potential adverse effects of treatment.
The committee noted that no one should remain on cheap, ineffective treatments as the burden of treatment and long-term cost, including the cost to manage their adverse events could be substantial. However, treatments for dystonia should be discontinued gradually, to minimise withdrawal symptoms such as anxiety and distress, as those symptoms would offset the cost of immediate discontinuation.
Some centres would consider splinting (including dynamic Lycra) following an assessment with occupational therapy, before more invasive treatments such as botulinum toxin or intrathecal baclofen are considered. However, the committee acknowledged the high cost to provide orthotic equipment and agreed there was no clinical evidence it provided a cost effective use of resources to implement its wider use. For this reason, the committee made a research recommendation to assess the clinical and cost effectiveness of splinting options.
The committee stated botulinum toxin was frequently used in current practice to manage focal dystonia when it is causing discomfort or affecting care or function and cannot be managed effectively using cheaper, less invasive treatments. The committee agreed it was important to state those criteria in their recommendation to prevent practises which were unlikely to be cost effective. However, in the absence of comparative high quality evidence, the committee did not make a strong recommendation.
The committee agreed intrathecal baclofen therapy was an expensive but successful option provided by specialists to manage dystonia when other options have been exhausted. The committee also added that additional research on its effectiveness in a population of adults with cerebral palsy would not change current practice unless it was shown to be harmful as the benefits have been shown to outweigh the costs in other populations. In the absence of published evidence on intrathecal baclofen therapy in adults with cerebral palsy, the committee made a recommendation based on their clinical experience and expertise which they considered to reinforce best practice.
Deep brain stimulation (DBS) is a relatively new and expensive treatment used to manage dystonia in England and Wales and is commissioned in the NHS for patients with generalised dystonia, status dystonicus, laryngeal dystonia and cervical dystonia if the criteria set out in the commissioning policy are met. However, the cost effectiveness of DBS had not been assessed in adults with cerebral palsy when that policy was produced.
Two studies included in the clinical evidence review provided SF-36 data, before and after deep brain stimulation treatment which enabled a cost utility analysis to be developed. The Committee stated that it was crucial the complications of deep brain stimulation were taken into consideration when making their recommendations, as they may outweigh the benefits deep brain stimulation can provide. As a result, the economic modelling was used by the Committee as one of many ways to assess those trade-offs.
The results of the model were sensitive to the study used to inform the improvement in utility as one study included participants with a much lower utility pre-DBS who saw a much greater improvement in their utility post-DBS. The committee agreed that DBS would only be considered when all pharmacological treatments had failed and for this reason placed more weight on the study who included participants with a lower utility value pre-DBS. The committee however noted that the patient group in Vidailhet 2009, for which DBS was not cost effective, was of people with dystonia which had become unresponsive to pharmacological treatment. Whilst this more closely reflected the patient group in the recommendation, Romito 2015 (individuals with persistent dystonia) was more recent better reflecting current technology and was a larger study with longer follow-up. The larger increases in utility values from Romito 2015 also better reflected what the committee experienced in clinical practise.
Uncertain parameters were varied in deterministic one-way sensitivity analyses to assess the robustness of the results. Those parameters included the cost of the procedure, inclusion and consequences of complications and frequency of battery replacements. The results of those analyses provided ICERs below or within NICE’s advisory threshold for cost-effectiveness when parameters set out in Romito 2015 were used to inform the model, providing evidence that DBS could be a cost-effective option. Those results were also reiterated in probabilistic analysis with 739 of 1,000 simulations below an ICER of £20,000.
Based on the economic evidence and their clinical expertise, the committee agreed that deep brain stimulation should be recommended in line with the current NHS commissioning policy as a cost effective option. As a result, the committee made a recommendation to consider a referral to a specialised tone management service with experience in providing deep brain stimulation for adults with intractable dystonia that is severe and painful.
Other factors the committee took into account
The committee also took into account the recommendations made in the NICE interventional procedure guideline Deep brain stimulation for tremor and dystonia (excluding Parkinson’s disease) IPG188 (2006). It is recommended in IPG188 that deep brain stimulation can be used provided that the normal arrangements are in place for consent, audit and clinical governance and only in the context of a multidisciplinary team specialising in the long-term care of patients with movement disorders. The committee therefore believed that the recommendation that they made aligns with IPG188 and made a cross-reference to it.
References
Koy 2014
Koy, A., Pauls, K. A., Flossdorf, P. Young adults with dyskinetic cerebral palsy improve subjectively on pallidal stimulation, but not in formal dystonia, gait, speech and swallowing testing, European Neurology, 72, 340–8, 2014 [PubMed: 25322688]Marks 2011
Marks, W. A., Honeycutt, J., Acosta, F. Dystonia due to cerebral palsy responds to deep brain stimulation of the globus pallidus internus, Movement Disorders, 26, 1748–1751, 2011 [PubMed: 21491490]Pozin 2014
Pozin, I., Bdolah-Abram, T., Ben-Pazi, H., Levodopa does not improve function in individuals with dystonic cerebral palsy, Journal of Child Neurology, 29, 534–7, 2014 [PubMed: 23349519]Romito 2015
Romito, L. M., Zorzi, G., Marras, C. E. Pallidal stimulation for acquired dystonia due to cerebral palsy: beyond 5 years, European Journal of Neurology, 22, 426–e32, 2015 [PubMed: 25382808]Vidailhet 2009
Vidailhet, M., Yelnik, J., Lagrange, C. Bilateral pallidal deep brain stimulation for the treatment of patients with dystonia-choreoathetosis cerebral palsy: a prospective pilot study, The Lancet Neurology, 8, 709–717, 2009 [PubMed: 19576854]
Appendices
Appendix A. Review protocols
Review protocol for review question A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
Table 15. Review protocol for interventions for dystonia (PDF, 343K)
Appendix B. Literature search strategies
Literature search strategies for review question A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
This appendix is a combined search strategy and will be the same for all the evidence reviews for the A review questions as listed below:
- A1: Which pharmacological treatments for spasticity (for example, enteral baclofen, tizanidine, diazepam, cannabinoids, and botulinum toxin injections) are most effective for improving motor function, participation and quality of life in adults with cerebral palsy?
- A2: Are neurosurgical procedures (intrathecal baclofen pump and selective dorsal rhizotomy) effective in adults aged 19 and over with cerebral palsy to reduce spasticity and or dystonia?
- A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB)) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
Database: Medline & Embase (Multifile)
Database(s): Embase 1974 to 2018 March 22, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to Present
Database: Cochrane Library
Database: Web of Science
Appendix C. Clinical evidence study selection
Clinical evidence study selection strategies for review question A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
Figure 10. Flow diagram of clinical article selection for interventions for dystonia review
Appendix D. Clinical evidence tables
Clinical evidence tables for review question A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
Table 19. Studies included in the evidence review for interventions for dystonia (PDF, 295K)
Appendix E. Forest plots
Forest plots for review question A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
Comparison 1. Levodopa versus placebo
Comparison 2. Bilateral pallidal deep brain stimulation: post versus pre-operative
Figure 14. Adverse events during 1 to 4 years of bilateral pallidal deep brain stimulation
Figure 15. Pain after one year of bilateral pallidal deep brain stimulation compared to baseline
Appendix F. GRADE tables
GRADE tables for review question A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
Table 20. Clinical evidence profile: levodopa versus placebo (PDF, 327K)
Table 21. Clinical evidence profile: bilateral pallidal deep brain stimulation versus pre-operative (PDF, 468K)
Appendix G. Economic evidence study selection
Economic evidence study selection for review question A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
No economic evidence was identified for this review
Appendix H. Economic evidence tables
Economic evidence tables for review question A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
No economic evidence was identified for this review.
Appendix I. Health economic evidence profiles
Health economic evidence profiles for review question A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
No economic evidence was identified for this review.
Appendix J. Health economic analysis
Health economic analysis for review question A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
Model structure
A decision analytic model was developed in Microsoft Excel® (2013) from the perspective of the UK NHS and using 2015/16 costs. The model takes the form of a state transition model. The first cycle lasts 2 weeks to reflect the duration of the procedure and complications associated with the procedure, whilst the second cycle lasts 1 month to reflect the risk of postoperative events. Subsequent cycles are 12 months long.
The model takes a lifetime horizon since cerebral palsy is a chronic conditions associated with on-going medical management, rather than a cure. DBS is a permanent procedure, hence it is important to capture those benefits that may persist for the remainder of the individual’s life. Adults with dystonia enter the model aged 19 as the committee considered patients to be eligible for DBS from this age.
Cost-effectiveness results should reflect the present value of the stream of costs and benefits accruing over the time horizon of the analysis. NICE considers that it is usually appropriate to discount costs and health effects at the same annual rate of 3.5%, based on the recommendations of the UK Treasury for the discounting of costs (NICE 2017 Methods Manual). Consequently the model has adopted a discount rate of 3.5% for both costs and benefits (QALYs, quality adjusted life years), but this input can be varied by the user in the model.
During the first cycle (the procedure) patients may experience a seizure, infection, intracranial haemorrhage (ICH), or die. Patients who experience an infection could either remain on DBS, or abandon DBS and receive “usual care”. Patients who experience a seizure remain on DBS treatment based on the assumption that seizures stabilise following immediate treatment. Patients who experience a symptomatic ICH with recovery or asymptomatic ICH (referred to as “minor ICH” in this document) continue with DBS, or transition to “usual care”. Patients who experience a symptomatic ICH with deficit (referred to as “major ICH” in this document) abandon DBS and receive long-term ICH care. Following a successful procedure for DBS, patients remain on DBS and receive a routine implanted pulse generator (IPG) replacement every 5 years (frequency varied in sensitivity analysis). Each year patients on DBS are at risk of a hardware failure which will incur additional surgery to correct. Patients in usual care receive pharmacological treatment in the base case, but alternative treatments are explored in sensitivity analysis.
It is important to note that the severity of an ICH will depend on symptoms and the subsequent effect on function and quality of life. Whereas, the volume of blood, location of the blood, and timing of the bleed intraoperatively would determine if the DBS hardware is abandoned or not. However, it was not possible to capture all of these eventualities in the model, as evidence was not available to inform those possibilities. As a result, committee opinion was used alongside the best available evidence to justify the assumptions this model has made with regards to this complication and others.
Patients in usual care are at a low risk of many minor adverse events such as drowsiness, confusion, urinary problems and at a lower risk of serious adverse events such as allergic reactions, seizures and arrhythmias. The committee also added that adults with cerebral palsy who receive other (non-dystonia related) treatments would also be at risk of those adverse events. Given the small number of people that would enter those health states, the total treatment cost and QALY loss attached to them would be negligible. Moreover, the clinical evidence review identified no studies that reported the adverse effects of pharmacological treatment for dystonia in adults with cerebral palsy. As a result, it was assumed patients in usual care are not at risk of any adverse events as the added complexity to the model would have a negligible impact on the results. For completeness, implications of omitting the adverse effects of usual care are discussed.
The structure of the model is illustrated in Figure 16 and described in more detail in Table 22.
Clinical effectiveness
Probability of DBS-related complications
DBS-related complications were included in the model as they can have important cost and QALY implications. The trials included in the clinical evidence review were small and unrepresentative of the adverse effects seen in practice, so alternative papers that analysed DBS were sought to inform the probability of complications in the model.
Boviatsis 2010 and Voges 2006 reviewed the complications of DBS experienced by their departments; from 2003 to 2010 in 106 patients and from 1996 to 2003 in 262 patients, respectively. Both also compared their own results to others reported in the literature.
Both of those papers considered ICHs to be important and serious adverse events associated with DBS, reporting probabilities in their own departments of 1.9% (2 of 106 patients) and 0.4% (1 of 262 patients) and higher results in the literature they reviewed (Beric 20013.3%; Kondziolka 2002, 1.5%; Oh 2002, 3.6%; Umemura 2003, 3.6%; Limousin 1999, 2.7%; Lyons 2004, 1.2%). However, details on the event, such as the severity, were not reported. As a result, the committee sought the paper by Binder 2005 who examined symptomatic and asymptomatic haemorrhages across all 280 DBS procedures performed for movement disorders between June 1998 and May 2004.
Skin infection may be caused by both DBS surgery and implanted hardware components. Consequently, the definition of infection in the literature was not unanimous; some restricted the definition to hardware-involving infections with positive only cultures, whereas others also included superficial infections over the implanted hardware. As a result, the model considered early infections in the first cycle, later infections in the second cycle and antibiotic treatment to remain on DBS, or removal of the system.
Table 23 below presents the probability of perioperative DBS-related complications used in the model.
Table 23. Probability of perioperative DBS-related complications
According to the committee, hardware-related failures can occur at any time during or after the procedure. Bovistis 2010 defined hardware failures as an electrode breakage, lead or extension fracture or migration or misplacement and found those to be experienced by 4 of 106 patients (3.8%) in their department. Voges 2006 reviewed the literature and found lead fractures to range from 1.7% (Voges 2006) to 15.2% (Kondziolka 2002), lead migrations from 1.5% (Kondziolka 2002) to 6.3% (Lyons 2004) and extension wires from 1.1% (Voges 2006) to 3.5% (Beric 2001). However, they also found zero cases reported for each type of hardware failure. In their own study, Voges 2006 reported hardware-related problems in 25 of 180 (13.9%) of patients during their long-term observation. In the model an annual probability of 4.0% was used to reflect a weighted average of those papers. The methods and data used to obtain this value is provided in Table 24.
Health-related quality of life
The QALY is NICE’s preferred measure of benefit for economic evaluation. This is because it can be seen as a generic measure of health which allows a comparison across treatments which affect different dimensions of health.
The QALY reflects the 2 principle objectives of health care:
- increase longevity;
- increase quality of life.
Estimating a QALY involves placing a quality of life weight on a particular health state. This quality weight lies between 0 and 1, where 1 denotes full or ‘perfect health’ and 0 denotes death. Based on a need for consistency across appraisals and guidelines, NICE favours the EQ-5D to value health states - a generic, preference based measure which comes with preexisting utility values obtained from a representative sample of the UK general population, although others measures and value sets are available.
Clinical effectiveness data (specifically health-related quality of life data) was taken from 2 before and after type studies (Vidailhet 2009 and Romito 2015) that reported the results for each of the 8 domains of the SF-36, pre- and post- DBS treatment. To allow for subsequent use in the health economic analyses, the SF-36 was mapped on to the EQ-5D using the mapping regression coefficients produced by Ara and Brazier 2008 (Table 25).
Table 25. EQ-5D regression coefficients
Given that no comparative data was identified, it was assumed the utility pre-DBS is equivalent to the utility associated with “usual care”. It was also assumed that the utility post-DBS holds when patients remain on DBS care.
When Romito 2015 was chosen to inform the model, the values 1-year and 2-years post-DBS were applied in the first and second year, whilst the value associated with the last visit was carried to a lifetime horizon.
There is a clear difference in pre-treatment utility between Vidailhet 2009 and Romito 2015, with participants in Romito 2015 entering the study with a much lower quality of life (0.35 vs. 0.61) (Table 27) most likely due to Romito 2015 only including patients with acquired dystonia (who may be less accustomed or have adapted to their condition) compared to Vidailhet which only included patients with idiopathic or inherited dystonia. For example, Vidailhet 2009 required optimum pharmacological treatments to be ineffective, whereas Romito 2015 did not specify this. In addition, it has been shown that mapping functions tend to overestimate utilities associated with severe health states and underestimate utilities associated with good health (Rowen 2009). For these reasons, the studies were not pooled and used separately in the model. However, it is evident that if DBS is not found to be cost-effective when Romito 2015 is used to inform the model, it will not be cost-effective according to Vidailhet 2009 who provides lower incremental QALY gains post- vs. pre- DBS.
People who undergo DBS experience some level of disutility due to the length and intensity of their inpatient stay, as DBS is an invasive and complex procedure. Despite this, no utility values in relation to the procedure were identified from the literature. Instead, the disutility was imputed using the EQ-5D health state valuation equation for the UK reported by Dolan 1997 which allows estimation of a person’s utility based on their responses to the EQ-5D classification system. The system has 5 dimensions (mobility, self-care, ability to perform usual activities, pain/discomfort, and anxiety/depression) and in the version used by Dolan 1997, each dimension had 3 levels of response (no problems, moderate problems, and severe problems).
Only the utility decrement due to usual activities was applied as this was considered to be the most dependable dimension on the neurosurgical procedure. This disutility is expressed by the following equation:
Where:
- α = 0.081 (constant applied to any level of disutility in any of the 5 EQ-5D dimensions)
- UA = −0.036 (for each level of disutility associated with usual activities)
- U2 = −0.022 (for being unable to perform usual activities)
- N3 = −0.269 (when any of the 5 dimensions of EQ-5D is severe)
As the baseline utility for people with cerebral palsy in the model is less than 1 (perfect health) for both Romito 2015 (0.35) and Vidailheit 2009 (0.61) the α value was not applied at the estimation of the utility decrement. and they moved from a state of moderate problems to being unable to perform them. Also assuming that at least one other dimension was severe, the N3 value is not added again, resulting in a disutility of −0.094 (−0.036–0.036–0.022).
To reflect the length of the procedure, the disutility was applied for 2 weeks in the model - a QALY loss of −0.004 (−0.094*(2/52)).
Hardware-related failures also require surgery to correct. For this reason, a 1 week QALY loss −0.002 (−0.094*(1/52)) was applied to patients receiving surgical treatment to correct hardware-related failures.
DBS-related complications
Infection
The committee agreed that an infection would negatively impact a patient’s quality of life, namely from the pain/discomfort infections can cause. As a result, a source for a disutility was sought, but in the absence of a relevant source, the method to estimate the disutility associated with the procedure was used by applying the dimension for pain/discomfort.
Where:
- α = 0.081 (constant applied to any level of disutility in any of the 5 EQ-5D dimensions)
- PD = −0.123 (for each level of disutility associated with pain/discomfort)
- P2 = −0.140 (for severe pain/discomfort)
- N3 = −0.269 (when any of the 5 dimensions of EQ-5D is severe)
As before, people with cerebral palsy already have a utility less than 1. Assuming that they moved from a state of no pain/discomfort to moderate pain/discomfort the resulting disutility is −0.123.
This disutility of −0.123 was applied for 2-weeks in the model, as pain/discomfort from an infection would be unlikely to last longer. This gave a 2-week QALY loss of −0.0047 (−0.123*(2/52)) attributed to pain/discomfort from an infection.
Seizure
A loss of −0.0014 was reported by Lee 2013 for a seizure (>10 minutes or repeated but not admitted). This value was estimated from the parents of children with epilepsy and a Delphi panel audit of clinicians in Wales for the treatment of prolonged acute convulsive seizures in children and adolescents.
Lee 2013 was the only relevant source identified to inform this input.
ICH
Lip 2015 estimated utilities for mild, moderate and severe ischemic or haemorrhagic strokes from a UK based utility catalogue of EQ-5D sores for the UK (Sullivan 2011). However, patients entered their cost-utility model at 70 years of age. To account for this, the health state utility decrement for ICH was estimated using the percentage reduction in utility when the utilities estimated by Lip 2015 are compared with EQ-5D population event-free norms (Kind 1999).
The percentage utility for a minor ICH was estimated by calculating the percentage change from the patient in Lip 2015 with a minor ICH (utility 0.6151) to a patient aged 65–74 years without a minor ICH (Kind 1999 utility 0.7800): 0.6151/0.7800 = 78.9%.
Similarly, the percentage utility for a major ICH was estimated by calculating the percentage change from the patient in Lip 2015 with a major ICH (utility 0.5142) to a patient aged 65–74 years without a minor ICH (Kind 1999 utility 0.7800): 0.5142/0.7800 = 65.9%.
Long-term ICH care
Begum 2015 considered the long-term effects of a haemorrhagic stroke/ICH in their cost-utility analysis by including a disutility for the subsequent cycles following a haemorrhagic stroke/ICH. Begum 2015 added, that their utility values taken from the Platelet inhibition and patient Outcomes (PLATO) trial were elicited from a large number of patients and had been applied in many recent heath technology appraisal submissions as a robust source.
The relative percentage utility for long-term ICH care in the model was estimated by calculating the percentage change from the patient in Begum 2015 with a long-term ICH (utility 0.792) to the baseline (event-free) utility they reported (0.842): 0.792/0.842 = 94.1%.
Table 28 summarises the disutilities applied in the model.
Table 28. Disutility from DBS-related complications
A sensitivity analysis assuming no utility decrements was explored in the model as DBS-related complications can be minor. In addition, the methods used to estimate the disutility may overestimate the impact of the event given the lack of relevant quality of life data reported in the literature.
Mortality
Cerebral palsy-related
The committee considered Brook 2014 to provide up-to-date survival estimates for people with cerebral palsy living in California that would be generalisable to adults living in England and Wales.
Brook 2014 reported survival estimates for 5 levels of severity which enabled the model to select those levels appropriate for people with dyskinetic cerebral palsy. To select the appropriate levels, the committee agreed it would be reasonable to assume that GMFCS is stable and can be informed by paediatric data that assess GMFCS and learning disability in older children with dyskinetic cerebral palsy. Himmelmann 2007 described 48 participants with dyskinetic cerebral palsy in Western Sweden. Their gross motor function was classified according to the GMFCS and was subsequently transformed into the limitations by Brook 2014 to create a weighted average (Table 29).
Table 29. GMFCS levels used to inform dystonic limitations
The probability a child with cerebral palsy will survive is reported up to the ages of 10, 15, 20, 25, and 30 years by Brook 2014. However, given that people with cerebral palsy are expected to live up to 70 years, the data beyond 30 years was extrapolated using a polynomial trend (Figure 17).
The committee agreed there was no evidence to suggest DBS treatment impacts survival following the procedure; hence, the same trend was applied to both treatment arms in the model.
DBS procedure
DBS is a risky and invasive procedure. The committee agreed that procedure-related mortalities reported in the literature were low, but concluded procedure-related mortality was an important possibility to capture in the model.
Boviastis 2010 reported a perioperative mortality of 0.94% in their study, whilst the literature review by Voges 2006 identified 1 study that reported mortality (Umemura 2003, 1.8%) with a similar rate. However, the remaining studies reviewed by Voges 2006 did not report mortality.
Major ICH
Gonzalez 2013 investigated short-term case fatality and long-term mortality after ICH using data from The Health Improvement Network (THIN) database over the years 2000 to 2008. A total of 1,733 individuals with an ICH and 9,583 controls were available with follow-up data.
Using logistic regression, event fatalities were stratified by age. For people aged 20 to 49 years Gonzalez 2013 estimated a 30-day case fatality of 29.7% for an ICH.
Cox proportional hazards regression analyses were used to determine whether patients were at increased risk of death in the first year (excluding the first 30 days immediately after the event) and after 1 year compared with the general population (controls) in THIN.
They found that the risk of death was significantly higher among stroke patients during the first year of follow-up compared with controls (HR 2.60, 95% CI 2.09–3.24) and remained elevated among survivors at 1 year (HR 2.02, 95% CI 1.75–2.32).
Resource and cost use
Deep brain stimulation (DBS)
Yianni 2005 provided a detailed cost-analysis of DBS surgery, including the preoperative assessment, surgery, equipment, postoperative management/follow-up and complications when they estimated cost-effectiveness. Costs were examined over a period of 2 years on 26 patients with primary dystonia. The effectiveness of DBS between primary dystonia and dystonic cerebral palsy will differ; hence, their estimate of cost-effectiveness was not considered to be relevant for this guideline.
However, the Committee agreed that the resources reported by Yianni 2005 would be very similar to those for dystonic cerebral palsy. Those costs are reproduced here in 2002/3 prices and 2015/16 prices using the hospital and community health services pay and prices index uplift (Curtis 2015) (Table 30).
Table 30. Cost of DBS reproduced from Yianni 2005
The committee suspected that Yianni 2005 may not reflect the latest innovations in equipment, particularly with regards to the type of rechargeable battery now available. To account for this uncertainty, a tornado diagram was presented, varying the cost inputs by +/−50%.
DBS-related complications
Replacement IPG
Yianni 2005 reported a cost of £8,356 (2015/16 cost: £11,613) to replace an IPG. According to the committee IPGs are usually replaced every 5 years. However, the committee also noted that the lifespan of an IPG is variable and could improve with innovations. To account for this, a replacement every 2 and 8 years was explored in sensitivity analysis.
Hardware-related
Yianni 2005 reported a cost of £11,169 (2015/16 cost: £15,523) to correct hardware failures. No further details on this estimate were provided.
Infection-related
Yianni 2005 reported a cost of £17,319 (2015/16 cost: £24,070) to manage infections. No further details on this estimate were provided, but the committee agreed that the high cost may include the cost to remove the DBS system.
Patients who remain on DBS following an infection, received antibiotics via intravenous infusion for 2 weeks at a cost of £560 (BNF August 2017: Ciprofloxacin 400 mg every 8–12 hours, £20.00 per infusion).
Seizure
A seizure would require a CT scan (NHS Reference Costs 2015/16: RD20A direct access, £99) and intravenous anticonvulsants such as diazepam (NHS Electronic Drug Tariff: 10mg/2ml solution for injection ampoules, £0.55/ampoule) to assess and manage.
Minor ICH
The cost of a minor ICH (£2,745) was taken from NHS Reference Costs 2015/16 using the code associated the lowest complications and comorbidity (CC) score (currency code: AA35F; currency description: stroke with CC score 0–3).
If patients experienced the ICH before their surgery was completed, the surgery would be abandoned and reversed at a 2015/16 cost of £8,499 (Yianni 2005).
Major ICH
The cost of a major ICH (£4,854) was taken from a weighted average of NHS Reference Costs 2015/15 that related to a stroke (currency codes AA35A:AA35F) to incorporate complex and costly strokes associated with complications and comorbidities.
DBS equipment would be removed following a major ICH at a cost of £8,499 (Yianni 2005) based on the assumption that all major ICHs occurred near or after complete surgery.
According to NICE CG92 and NICE CG68 long-term ICH care would cost £4,826 per year to manage (£4,826 × [2015/16 PPI 297.0/2009/10 PPI 268.6] = £5,336).
Usual care
In the base case, patients received trihexyphenidyl (5mg daily) to align with the type of pre-treatment participants received in Vidailhet 2009 and Romito 2015 and the type of pharmacological treatment used in clinical practice today (Table 31). Patients receiving trihexyphenidyl visit a neurologist each year to monitor their response at a cost of £161 (NHS Reference Costs 2015/16, currency code WF01A, service code 400, non-admitted face-to-face attendance follow-up, neurology).
Table 31. Cost of usual care (trihexyphenidyl)
A sensitivity analysis administering botulinum toxin every 6 months was also explored as a sensitivity analysis. Botulinum toxin involves a day–case admission performed by a neurologist, rehabilitation medicine doctor, or a specially trained physiotherapist or nurse in a specialist clinic. Adults with cerebral palsy are unlikely to be sedated, but ultrasound or electromyography may be used for guidance. However, given that recommendations were not included on ultrasound or electromyography guidance, they are not added here for consistency.
The appointment for the injection of botulinum has a NHS reference cost assigned – Torsion dystonia and other involuntary movements drugs band 1 (code XD09Z). This reference cost (£324) will include all costs related to the procedure, the day case admission, drug costs and staff costs.
Following the injections, patients would be monitored every 3 to 4 months by the specialist clinic at a cost of £161 (NHS Reference Costs 2015/16, currency code WF01A, service code 400, non-admitted face-to-face attendance follow-up, neurology) to assess their response and need for repeat injections.
Sensitivity analysis
A series of sensitivity analyses were undertaken in order to test how sensitive the results were to uncertainty in individual parameters. Parameters varied in sensitivity analysis were chosen on the basis of uncertainty in their estimation or the potential impact that they had on the results. Extreme analysis were reported when smaller changes in those analysis led to negligible differences in the results. For example, changing all utility decrements to zero instead of a single utility decrement. The values varied, along with their rationale are shown in Table 32.
Table 32. Description of sensitivity analysis
Probabilistic sensitivity analysis (PSA) was conducted in the model to take account of the simultaneous effect of uncertainty relating to model parameter values. Key parameters in the model relating to clinic effectiveness were varied by sampling from probability distributions. The model was run for 1,000 simulations to generate estimates of total costs and total QALYs by varying those parameters simultaneously. The model structure and model settings were kept constant. Cost parameters were not varied in PSA as the cost of equipment, drugs and monitoring related to the interventions were expected to be fixed. Disutility values associated with complications were not varied as their distributions around the mean could not be calculated for all complications, and given the small decrement associated with those complications, this was a minor omission. As previously stated, cost inputs were varied in sensitivity analysis using +/−50% of the base case value.
Results
As discussed previously, the results for Vidailhet 2009 and Romito 2015 are presented separately due to study heterogeneity. The total costs for each intervention are the same for each study as the studies only vary in the utility data they provide.
Study participants in Vidailhet 2009 had a greater utility pre- and post- DBS treatment compared to Romito 2015 (pre-DBS: 0.61 vs. 0.35; post-DBS: 0.72 vs. 0.66). As a result, the total QALYs are much higher when Vidailhet 2009 is used to inform the model. Moreover, study participants in Romito 2015 had more potential to benefit from DBS treatment with a much greater improvement in their utility value pre- vs. post-DBS treatment (0.66 − 0.35 = 0.31). Therefore, if DBS is not found to be cost-effective when Romito 2015 is used to inform the model, DBS will not be cost-effective according to Vidailhet 2009 who has less favourable incremental utility data pre- vs. post- DBS.
Base case results
When Romito 2015 was used to inform the model, DBS was more costly and more effective than usual care, with an ICER on NICE’s lower threshold (Table 34). This is illustrated in Figure 18 with an ICER in the north-east quadrant.
DBS was also more costly than usual care according to Vidailhet 2009, but relatively less effective than Romito 2015. As a result, the ICER was higher when Vidailhet 2009 was used to inform the model.
It is important to note that Vidailhet 2009 produced more QALYs than Romito 2015 because Vidailhet 2009 reported higher utility values pre- and post-DBS treatment (Table 34).
However, as stated above, the range is greater for Romito 2015. The total costs do not differ between the studies as they only differ in utility values.
Sensitivity analysis results
The total QALYs increased for DBS when utility decrements were removed and when the risk of complications were removed. This reduced the ICER for Vidailhet 2009 and Romito 2015, but the ICER for Vidailhet 2009 remained above NICE’s upper threshold of £30,000 per QALY.
Reducing the time horizon reduced the number of QALYs that could be accrued and amplified the cost of the DBS procedure. This analysis increased the ICER above NICE’s upper threshold in both studies.
When usual care consisted of botulinum toxin (a more costly treatment than trihexyphenidyl) the incremental cost reduced. This reduced the ICER for Vidailhet 2009 and Romito 2015, but the ICER for Vidailhet 2009 remained above NICE’s upper threshold of £30,000 per QALY.
The results of each analysis are provided in Table 35 for Romito 2015 and Table 36 for Vidailhet 2009.
Table 35. Results of sensitivity analysis (Romito 2015)
Table 36. Results of sensitivity analysis (Vidailhet 2009)
Figure 19 illustrates the ICERs for Romito 2015 when each component of the procedure was varied using half the cost of the base case (−50%) and 150% of the base case (+50%). In the worst case scenario, increasing the cost of the total procedure by 50% increased the ICER to £23,918. In the best case scenario, reducing the total cost of the procedure by 50% reduced the ICER to £16,420.
Figure 20 also used this method to show the variability in ICERs to treat the complications of DBS for Romito 2015. The most influential parameters were related to the replacement of the IPG. When the cost to replace the IPG was varied by 50% the ICER ranged from £16,456 to £23,873. When the frequency of replacements was changed from every 5 years to every 2 or 8 years, the ICER ranged from £16,884 to £33,474.
Figure 19. Tornado diagram of the costs associated with the procedure and monitoring (Romito 2015)
Figure 20. Tornado diagram of the costs to treat the complications of DBS (Romito 2015)
Figure 21 illustrates the ICERs for Vidailhet 2009 when each component of the procedure was varied using half the cost of the base case (−50%) and 150% of the base case (+50%). Figure 22 also used this method to show the variability in ICERs to treat the complications of DBS for Vidailhet 2009. All ICERs remained above NICE’s upper threshold when those parameters were varied. Similarly to Romito 2015, the most influential parameters included the total cost of the procedure (namely stimulation equipment) and IPG replacements.
Figure 22. Tornado diagram of the costs to treat the complications of DBS (Vidailhet 2009)
Probabilistic results
For Romito 2015, all simulations found DBS to be more effective and more expensive than usual care with a mean probabilistic ICER of £20,077. Furthermore, 739 of 1,000 simulations had ICER’s below £20,000 and 927 below £30,000. This is illustrated in Figure 23 where simulations cross the WTP threshold in the north-east quadrant. The simulations do not fall below an incremental cost of £60,000 as the cost inputs were not sampled. For this reason, the incremental cost cannot fall below the cost to provide DBS.
The cost-effectiveness acceptability curve (CEAC) also illustrated that DBS would be considered as the most optimal treatment for WTP thresholds over £17,000 (Figure 24).
Figure 23. PSA simulations (Romito 2015)
When Vidailhet 2009 was used to inform the model, the mean ICER was £72,323 with almost all simulations (996 of 1,000) in the north-east quadrant above NICE’s threshold (Figure 25). The CEAC also illustrated that usual care would be considered as the most optimal treatment for WTP thresholds up to £65,000 per QALY (Figure 26).
Discussion
This is the first cost-effectiveness analysis of DBS to manage dystonia in adults with cerebral palsy. Using QALYs, as the measure of effectiveness, incorporates changes in morbidity and mortality and allows broad comparisons across all health care interventions provided by the NHS. In addition, undertaking cost-utility analysis was of upmost importance, given the need to assess the trade-offs from various treatment related adverse events, complications and failures.
The economic model developed for this review was based on committee opinion regarding current treatment pathways and the plausibility of relationships between complications and their subsequent consequences.
The model was informed by 2 before-and-after type studies in the absence of higher quality studies such as randomised controlled trials. The utility pre-DBS in those 2 studies that were included was used as a proxy for usual care based on the assumption that if DBS was not available, participants would stay on the same treatment schedule. It is also important to note that the utility post-DBS may double count adverse events if a number of participants experienced them. However, the adverse events reported by the studies were relatively minor and potentially unrelated to DBS.
In clinical practice, a patient would not undergo an expensive, invasive and risky procedure such as DBS if pharmacological treatment effectively managed their dystonia. As a result, it is those patients for whom pharmacological treatment is ineffective where DBS would be considered. For these reasons, it is important for studies to state their inclusion criteria and the aims of treatment to know if we are comparing successful pharmacological treatment with DBS, or failed pharmacological treatment with DBS as the QALY gain would be very different for a pharmacologically successfully treated patient and one for which pharmacological treatment failed.
Some participants remained on pharmacological treatment after DBS at a lower dose in Vidailhet 2009 and Romito 2015. A simplifying assumption was made in the model that people would discontinue pharmacological treatment for dystonia after DBS. Drugs for dystonia are relatively cheap and could be monitored during routine reviews for DBS. So whilst this assumption may underestimate the cost of DBS even if these treatments were used over the lifetime of the patient the impact would be negligible and very unlikely to change conclusions.
The trials included in the clinical evidence review began DBS much later than the 19 years of age assumed by this economic evaluation. Given the models assumptions around survival it would overestimate the QALYs gained from treatment if it was initiated at a later age. However, the committee considered the inclusion criteria in the trials and concluded that age was independent of eligibility.
There was concern that the outcomes of DBS may be misrepresented by the studies, since the data was based on small numbers of participants. Due to such sparse evidence, it is clear more research on DBS is needed to increase confidence in its effects.
There is a potential publication bias, in that most studies are led by neurosurgeons and therefore, neuropsychiatric adverse events such as suicidal ideation, cognitive impairment or hallucinations, and more subtle physical adverse events may not be looked for. As a result the committee may want to consider a recommendation for specialists offering DBS to collect information on those short- and long-term outcomes with agreed consistent definitions.
The probability of failure and cost of DBS will be impacted on by whether the DBS implantation is image guided versus microelectrode recording guided, awake versus asleep, the programme used and bipolar versus monopolar stimulation, which also affects IPG battery life. Unfortunately, the studies on DBS used to inform the inputs in this model varied in this level of detail. As a result, the exact method of surgery is not defined in the model. However, this is not considered to be a severe limitation as the model was informed by a number of studies that reported the probability of DBS-related complications. Additional analysis varying the cost of treatment by +/−50% also assessed the impact on costs.
An important assumption in the model included extrapolation of the trial data to a lifetime horizon. On the one hand, this was useful to assess all important differences in costs and outcomes that would be possible from lifetime treatment and any potential complications. However on the other, it could potentially be misleading if the treatment effect is time dependent and could reduce the cost-effectiveness of DBS if effects reduced with time. To account for this uncertainty, the time horizon in the model could be varied.
Conclusion
DBS is more effective but also more costly than usual care according to Romito 2015 and Vidalhet 2009. When the ICER is considered the 2 studies lead to conflicting decisions around cost effectiveness. DBS could be considered cost effective according Romito 2015, which produces an ICER below NICE’s advisory threshold of £20,000. Conversely, Vidailhet 2009 produces ICERs above £30,000 and would not be considered cost effective under conventional criteria.
References
- J Voges, Y Waerzeggers, M Maarouf, R Lehrke, A Koulousakis, D Lenartz, V Sturm. Deep-brain stimulation: long-term analysis of complications caused by hardware and surgery—experiences from a single centre. J Neurol Neurosurg Psychiatry 2006;77:868–872 [PMC free article: PMC2117492] [PubMed: 16574733]
- Binder DK, Rau GM, Starr PARisk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery 56:722–732, 2005 [PubMed: 15792511]
- Boviatsis EJ, Stavrinou LC, Themistocleous M, Kouyialis AT, Sakas DE. Surgical and hardware complications of deep brain stimulation. A seven-year experience and review of the literature. Acta Neurochir (2010) 152:2053–2062 [PubMed: 20658301]
- Beric A, Kelly PJ, Rezai A, Sterio D, Mogilner A, Zonenshayn M, Kopell B (2001) Complications of deep brain stimulation surgery. Stereotact Funct Neurosurg 77:73–78 [PubMed: 12378060]
- Kondziolka D, Whiting D, Germanwala A, Oh M (2002) Hardware-related complications after placement of thalamic deep brain stimulator systems. Stereotact Funct Neurosurg 79:228–233 [PubMed: 12890981]
- Lyons KE, Wilkinson SB, Overman J, Pahwa R (2004) Surgical and hardware complications of subthalamic stimulation: a series of 160 procedures. Neurology 63:612–616 [PubMed: 15326230]
- Oh MY, Abosch A, Kim SH, Lang AE, Lozano AM (2002) Longterm hardware-related complications of deep brain stimulation. Neurosurgery 50:1268–1274, discussion 1274–1266 [PubMed: 12015845]
- Sampson, F. C., Hayward, A., Evans, G., Morton, R., Collett, B., Functional benefits and cost/benefit analysis of continuous intrathecal baclofen infusion for the management of severe spasticity, Journal of Neurosurgery, 96, 1052–1057, 2002 [PubMed: 12066906]
- Romito, L. M., Zorzi, G., Marras, C. E. Pallidal stimulation for acquired dystonia due to cerebral palsy: beyond 5 years, European Journal of Neurology, 22, 426–e32, 2015 [PubMed: 25382808]
- Vidailhet, M., Yelnik, J., Lagrange, C. Bilateral pallidal deep brain stimulation for the treatment of patients with dystonia-choreoathetosis cerebral palsy: a prospective pilot study, The Lancet Neurology, 8, 709–717, 2009 [PubMed: 19576854]
- Brooks JC, Strauss DJ, Shavelle RM, Tran LM, Rosenbloom L, Wu YW. Recent trends in cerebral palsy survival. Part II: individual survival prognosis. Dev Med Child Neurol. 2014 Nov;56(11):1065–71 [PubMed: 25041081]
- Himmelmann K, Hagberg G, Wiklund LM, Eek MN, Uvebrant P. Dyskinetic cerebral palsy: a population-based study of children born between 1991 and 1998. Dev Med Child Neurol. 2007 Apr;49(4):246–51. [PubMed: 17376133]
- González-Pérez A1, Gaist D, Wallander MA, McFeat G, García-Rodríguez LA. Mortality after hemorrhagic stroke: data from general practice (The Health Improvement Network). Neurology. 2013 Aug 6;81(6):559–65. [PubMed: 23843467]
- Ara R, Brazier J. Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available). Value Health. 2008 Dec;11(7):1131–43 [PubMed: 18489495]
- Lip GY, Kongnakorn T, Phatak H, Kuznik A, Lanitis T, Liu LZ, Iloeje U, Hernandez L, Dorian P. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther. 2014 Feb 1;36(2):192–210.e20. [PubMed: 24508420]
- Lee D, Gladwell D, Batty AJ, Brereton N, Tate E. The cost effectiveness of licensed oromucosal midazolam (Buccolam(®)) for the treatment of children experiencing acute epileptic seizures: an approach when trial evidence is limited. Paediatr Drugs. 2013 Apr;15(2):151–62 [PubMed: 23512129]
- Begum N, Stephens S, Schoeman O, Fraschke A, Kirsch B, Briere JB, Verheugt FW, van Hout BA. Cost-effectiveness Analysis of Rivaroxaban in the Secondary Prevention of Acute Coronary Syndromes in Sweden. Cardiol Ther. 2015 Dec;4(2):131–53 [PMC free article: PMC4675751] [PubMed: 26099515]
- Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997 Nov;35(11):1095–108. [PubMed: 9366889]
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. The University of York. Centre for health economics 1999. Discussion paper 172.
- Health Improvement Scotland. What is the clinical and cost effectiveness of dynamic elastomeric fabric orthoses (DEFOs) for cerebral palsy? 2013 Technologies scoping report 14
- Rowen, D., Brazier, J., Roberts, J., Rowen, D., Brazier, J., Roberts, J. Mapping SF-36 onto the EQ-5D index: how reliable is the relationship? Health & Quality of Life Outcomes 2009; 7:27 [PMC free article: PMC2683169] [PubMed: 19335878]
Appendix K. Excluded studies
Clinical and economic lists of excluded studies for review question A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
Clinical studies
Table 37. Excluded clinical studies for interventions for dystonia (PDF, 372K)
Economic studies
No economic evidence was identified for this review.
Appendix L. Research recommendations
Research recommendations for review question A3: Which treatments (pharmacological treatment (levodopa, anticholinergic drugs, and botulinum toxin injections), neurosurgical procedure (deep brain stimulation, ITB) are most effective for managing dystonia in adults with cerebral palsy where dystonia is the predominant abnormality of tone?
No research recommendation was made for this review.
Final
Evidence reviews
These evidence reviews were developed by the National Guideline Alliance, hosted by the Royal College of Obstetricians and Gynaecologists
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Management of abnormal muscle tone: treatments to reduce dystoniaManagement of abnormal muscle tone: treatments to reduce dystonia
Your browsing activity is empty.
Activity recording is turned off.
See more...
