NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
The benzodiazepines are a large class of medications that have multiple clinical uses including therapy of anxiety, insomnia, muscle spasm, alcohol withdrawal and seizures. As a class, the benzodiazepines do not cause significant serum enzyme elevations and have been linked to only very rare instances of acute, symptomatic liver disease.
The pharmacological effects of the benzodiazepines are a result of their interaction with the central nervous system, their effects being sedation, hypnosis, decreased anxiety, muscle relaxation, anterograde amnesia and anticonvulsant activity. At high doses, when given intravenously, the benzodiazepines may also cause coronary vasodilation and neuromuscular blockade. The CNS effects of benzodiazepines are believed to be mediated by activation of GABA A receptors and modulation of their inhibition of neurotransmission.
Benzodiazepines in current use, with their initial brand name and year of approval, include alprazolam (Xanax, 1981), chlordiazepoxide (Librium, 1960), clonazepam (Klonopin, 1997), clorazepate (Tranxene, 1972), diazepam (Valium, 1963), estazolam (ProSom, 1990), flurazepam (Dalmane, 1970), lorazepam (Ativan, 1977), midazolam (Versed, 1985), oxazepam (Serax, 1965), quazepam (Doral, 1985), temazepam (Restoril, 1981), triazolam (Halcion, 1982), and most recently clobazam (2011). They are variously indicated for treatment of anxiety, alcohol withdrawal symptoms, insomnia, muscle relaxation, panic disorders and seizure disorders. The benzodiazepines all share similar activity and clinical effects, but variability in dosing, pharmacokinetics, rapidity of uptake and half-life make them more suited for one or another of these indications. Thus, estazolam, flurazepam, quazepam, temazepam and triazolam are generally used as sleeping pills, whereas alprazolam, chlordiazepoxide, diazepam, and lorazepam are used largely in therapy of anxiety. Clobazam, clonazepam, and clorazepate are used as anticonvulsants, and high dose, parenteral diazepam and lorazepam are used for status epilepticus. Parenteral midazolam, diazepam and lorazepam are also used as anesthetics or anesthetic premedications.
Benzodiazepine therapy is uncommonly associated with serum enzyme elevations, and clinically apparent liver injury from the benzodiazepines is quite rare. Alprazolam, chlordiazepoxide, clonazepam, clorazepate, diazepam, flurazepam and triazolam have been linked to rare instances of cholestatic liver injury but the other benzodiazepines have not. The absence of reports of this rare adverse event, however, may be due to the fact that these other benzodiazepines are not as commonly or continuously used. Alternatively, the use of the sleeping aids in intermittent and low doses may favor their lack of hepatic injury.
References for drug induced liver injury for all of the benzodiazepines follow this introduction, and specific references are appended to the text for the specific agents. The individual agents discussed include the following:
The following benzodiazepine drugs are discussed in the record for Anticonvulsants Drugs:
Drug Class: Anticonvulsants, Benzodiazepines
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Alprazolam | 28981-97-7 | C17-H13-Cl-N4 |
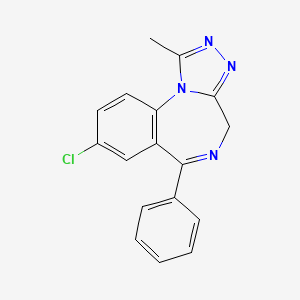
|
| Diazepam | 439-14-5 | C16-H13-Cl-N2-O |

|
| Flurazepam | 1172-18-5 | C21-H23-Cl-F-N3-O.2Cl-H |

|
| Lorazepam | 846-49-1 | C15-H10-Cl2-N2-O2 |
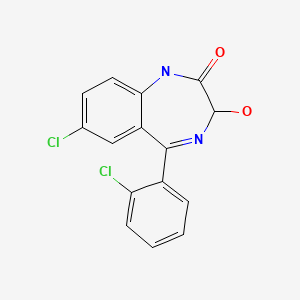
|
| Midazolam | 59467-70-8 | C18-H13-Cl-F-N3 |
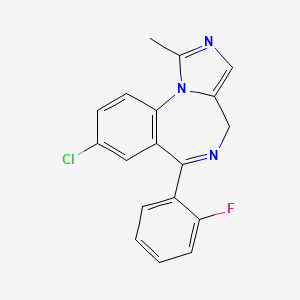
|
| Clobazam | 22316-47-8 | C16-H13-Cl-N2-O2 |

|
| Clonazepam | 1622-61-3 | C15-H10-Cl-N3-O3 |
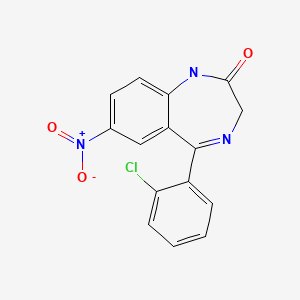
|
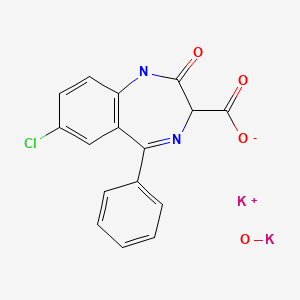
| Clorazepate | 57109-90-7 | C16-H10-Cl-N4-O3.K.H-K-O |
|
ANNOTATED BIBLIOGRAPHY
References updated: 24 January 2017
- Zimmerman HJ. Benzodiazepines. Psychotropic and anticonvulsant agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 491-3.(Expert review of benzodiazepines and liver injury published in 1999; mentions rare instances of cholestatic hepatitis have been reported due to alprazolam, chlordiazepoxide, diazepam, flurazepam, and triazolam, and hepatocellular injury with clorazepate and clotiazepam, but no reports of hepatic injury with lorazepam, oxazepam or temazepam).
- Larrey D, Ripault M-P. Benzodiazepines. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 455.(Review of drug induced liver injury mentions that there have been rare instances of acute liver injury [usually cholestatic] reported with alprazolam, chlordiazepoxide, clonazepam, clorazepate, clotiazepam, diazepam, flurazepam, triazolam and more recently bentazepam [which is not available in the US]; a hepatitis-like pattern was reported with alprazolam and diazepam).
- Mihic SJ, Harris RA. Hypnotics and sedatives. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 457-80.(Textbook of pharmacology and therapeutics).
- Cacioppo J, Merlis S. Chlordiazepoxide hydrochloride(Librium) and jaundice: report of a case. Am J Psychiatry 1961; 117: 1040-1. [PubMed: 13689710](39 year old man with schizophrenia and seizures on phenytoin developed jaundice 5 days after starting chlordiazepoxide [50 mg/day] [icterus index 49.6, Alk P 3 times ULN], resolving within 3 months of stopping).
- Cook GC, Sherlock S. Jaundice and its relation to therapeutic agents. Lancet 1965; 1: 175-9. [PubMed: 14238042](11 cases of drug induced liver disease, all due to agents no longer in use; 2 were receiving diazepam, but other agents were more likely the cause in both cases).
- Abbruzzese A, Swanson J. Jaundice after therapy with chlordiazepoxide hydrochloride. N Engl J Med 1965; 273; 321-2. Not in PubMed [PubMed: 21417069](51 year old man developed jaundice and pruritus 4-5 weeks after starting chlordiazepoxide [bilirubin ~4.0 mg/dL, AST 300 U/L, Alk P 2.5 times ULN], biopsy showed inflammation and cholestasis, recovery not mentioned).
- Cunningham ML. Acute hepatic necrosis following treatment with amitriptyline and diazepam. Brit J Psychiat 1965; 111: 1107-9. [PubMed: 5841222](54 year old woman with dementia-depression placed on amitriptyline and diazepam developed progressive confusion 5 months later followed by jaundice, obtundation and death [bilirubin 5.2 mg/dL, ALT 200 U/L, Alk P 9.5 unknown units], autopsy showed small liver, but no description of histology).
- Pickering D. Hepatic necrosis after chlordiazepoxide therapy. N Engl J Med 1966; 274: 1449. Not in PubMed(64 year old woman developed jaundice 3 weeks after 12 day course of chlordiazepoxide [bilirubin ~32 mg/dL, ALT 225 U/L, Alk P 38 U/L], prolonged course, transient ascites, prednisone therapy).
- Lo KJ, Eastwood IR, Eidelman S. Cholestatic jaundice associated with chlordiazepoxide hydrochloride (Librium) therapy. Report of a case and review of the literature. Am J Dig Dis 1967; 12: 845-9. [PubMed: 4952749](26 year old woman developed nausea after 2 and jaundice after 11 days of chlordiazepoxide therapy [bilirubin 5.0 mg/dL, AST 51 U/L, Alk K 69 KA ~5 times ULN], recovery over 8 weeks, marked pruritis).
- Buchanan N, Cane RD. Liver function tests and the prolonged use of high-dose diazepam with special reference to tetanus. S Afr Med J 1978 54: 768. [PubMed: 741305](After noting jaundice in a few patients receiving high doses of diazepam for tetanus, the authors prospectively tested 4 patients finding no consistent rises in serum ALT, Alk P or bilirubin on therapy).
- Kratzsch KH, Buttner W, Reinhardt G. [Intrahepatic cholestasis following chlordiazepoxide-contribution to the differential diagnosis of drug jaundice]. Z Gesamte Inn Med 1972; 27: 408-11. German. [PubMed: 5055057](3 cases of cholestatic hepatitis during chlordiazepoxide use, after 4 weeks, 6 years and 2 days [bilirubin 6.0, 1.0 and 6.4 mg/dL, ALT 245, 18 and 25 U/L, Alk P 1.6 1.0 and 20 times ULN], with rapid recovery in 1-2 months: all 3 had biopsies showing intrahepatic cholestasis).
- Stacher G. Intrahepatic cholostasis following combined diazepam-barbiturate therapy in patients with tetanus]. Wien Klin Wochenschr. 1973; 85: 401-6. German. [PubMed: 4705881]
- Franks E, Jacobs WH. Cholestatic jaundice possibly due to benzodiazepine-type drugs. Mo Med 1975; 72: 605-6. [PubMed: 1181510](40 year old woman on multiple drugs including chlorpromazine developed jaundice [bilirubin 2.0 rising to 9.7 mg/dL, ALT 280 U/L, Alk P 546 U/L, 16% eosinophils], seemed to worsen on benzodiazepines including chlordiazepoxide, diazepam and flurazepam, resolving rapidly when they were stopped, but attribution to benzodiazepines difficult).
- Fors B, Nilsson F. [Hepatitis probably induced by diazepam medication] Lakartidningen 1968; 65: 4528-31. Swedish. [PubMed: 5745628](24 and 34 year old women developed mild hepatitis with jaundice 1 and 4 months after starting diazepam [bilirubin 11 and 4.2 mg/dL, ALT 434 U/L Alk ~2 times ULN], with rapid resolution with stopping).
- Fang MH, Ginsberg AL, Dobbins WO 3rd. Cholestatic jaundice associated with flurazepam hydrochloride. Ann Intern Med 1978; 89: 363-4. [PubMed: 28685](70 year old man developed fatigue followed by pruritis 3 months after starting flurazepam [bilirubin 6.6 mg/dL, ALT 179 U/L, Alk P 232 U/L, no eosinophilia], improving only when the drug was withdrawn).
- Parker JLW. Potassium clorazepate(Tranxene)-induced jaundice. Postgrad Med J 1979; 55: 908-10. [PMC free article: PMC2425684] [PubMed: 44913](27 year old man developed jaundice, pruritus and fever, 2 months after starting clorazepate [bilirubin 7.6 mg/dL, ALT 880 U/L, Alk P 2.3 times ULN] and had 3 liver biopsies done over 5 months showing intrahepatic cholestasis with thin portal-portal fibrosis septae that persisted as cholestasis and inflammation resolved).
- Bonkowsky HL, Sinclair PR, Emery S, Sinclair JF. Seizure management in acute hepatic porphyria: risks of valproate and clonazepam. Neurology 1980; 30: 588-92. [PubMed: 6770287](38 year old man with acute intermittent porphyria and seizures did not respond to clonazepam, and testing in chicken embryos showed that it increased hepatic porphyrins and ALA synthase activity).
- Cobden I, Record CO, White RWB. Fatal intrahepatic cholestasis associated with triazolam. Postgrad Med J 1981; 57: 730-1. [PMC free article: PMC2426204] [PubMed: 6122205](44 year old man developed jaundice after 5 months of intermittent triazolam therapy for insomnia [bilirubin 10.6 mg/dL, AST 97 U/L, Alk P 104 U/L]; jaundice deepened and patient died, autopsy showing intrahepatic cholestasis without obstruction or cirrhosis).
- Reynolds R, Lloyd DA, Slinger RP. Cholestatic jaundice induced by flurazepam hydrochloride. Can Med Assoc J 1981; 124: 893-4. [PMC free article: PMC1705357] [PubMed: 7214289](44 year old woman taking flurazepam intermittently for 6 months developed abdominal pain [bilirubin 8.2 mg/dL, AST 106 U/L, Alk P 209 U/L], resolving rapidly upon stopping, biopsy showed intrahepatic cholestasis).
- Tedesco FJ, Mills LR. Diazepam(Valium) hepatitis. Dig Dis Sci 1982; 27: 470-2. [PubMed: 7075434](45 year old man developed elevations of ALT [130 U/L] while on isoniazid [for 1 day] and diazepam [3 days], which resolved upon stopping and recurred upon rechallenge with diazepam, but not isoniazid which was tolerated long term).
- Døssing M, Andreasen PB. Drug-induced liver disease in Denmark. An analysis of 572 cases of hepatotoxicity reported to the Danish Board of Adverse Reactions to Drugs. Scand J Gastroenterol 1982; 17: 205-11. [PubMed: 6982502](Among 572 cases of hepatotoxicity reported to a Danish registry between 1968 and 1978, 97 were due to psychotropic agents, but only two attributed to benzodiazepines).
- Jacobson AF, Goldstein BJ, Dominguez RA, Steinbook RM. A placebo-controlled, double-blind comparison of clobazam and diazepam in the treatment of anxiety. J Clin Psychiatry 1983; 44: 296-300. [PubMed: 6135690](Controlled trial of 4 weeks of diazepam vs clobazam vs placebo in 114 patients with anxiety found sedation similar with either drug, but dizziness more frequent with diazepam; no mention of ALT elevations or hepatotoxicity, although ALT was measured at the start and end of therapy).
- Roy-Byrne P, Vittone BJ, Uhde TW. Alprazolam-related hepatotoxicity. Lancet. 1983; 2: 786-7. [PubMed: 6137615](30 year old woman was found to have elevated ALT [96 U/L] 2 weeks after starting alprazolam, which promptly resolved when drug was stopped; rechallenge led to asymptomatic ALT rise within 9 days [28 to 70 U/L]).
- Keränen T, Sivenius J. Side effects of carbamazepine, valproate and clonazepam during long-term treatment of epilepsy. Acta Neurol Scand Suppl 1983; 97: 69-80. [PubMed: 6424398](Clonazepam has many dose related sedative side effects, but compared to carbamazepine and valproate, few serious or long term side effects; no mention of hepatic side effects of clonazepam).
- Davion T, Capron-Chivrac D, Andrejak M, Capron JP. [Hepatitis due to antiepileptic agents] Gastroenterol Clin Biol 1985; 9: 117-26. [PubMed: 3920108](Review of hepatotoxicity of anticonvulsants; among benzodiazepines, cases of cholestatic hepatitis have been linked to chlordiazepoxide and diazepam, but liver injury from this class of drugs is rare).
- Judd FK, Norman TR, Marriott PF, Burrows GD. A case of alprazolam-related hepatitis. Am J Psychiatry 1986; 143: 388-9. [PubMed: 2869702](59 year old woman developed lethargy 2 weeks after starting alprazolam followed by jaundice [bilirubin 2.6 mg/dL, AST 156 U/L, Alk P 241 U/L], resolving within 4 weeks of switching to diazepam).
- Noyes R, DuPont RL, Pecknold JC, Rifkin A, Rubin RT, Swinson RP, Ballenger JC, et al. Alprazolam in panic disorder and agoraphobia: results from a multicenter trial. Arch Gen Psychiatry 1988; 45: 423-8. [PubMed: 3358644](Controlled trial of alprazolam vs placebo in 525 patients with panic disorder; side effects were sedation, ataxia, fatigue, slurred speech, amnesia and increased appetite; 2 patients on alprazolam developed liver disease, one with jaundice at 4 weeks, resolving quickly on stopping and a second with abnormal laboratory values that returned to normal with lowering the dose; details not given).
- Olsson R, Zettergren L. Anticonvulsant-induced liver damage. Am J Gastroenterol 1988; 83: 576-7. [PubMed: 3364416](30 year old man developed fever, rash and jaundice 6 weeks after starting phenytoin; he was switched to carbamazepine and clonazepam, but redeveloped jaundice 3 months later; resolved with stopping, but recurred with restarting clonazepam alone [bilirubin 1.8 mg/dL, ALT 1380 U/L, Alk P 176 U/L] without rash, fever or eosinophilia; resolving in 6 weeks of stopping clonazepam).
- Habersetzer F, Larrey D, Babany G, Degott C, Corbic M, Pessayre D, Benhamou JP. Clotiazepam-induced acute hepatitis. J Hepatol 1989; 9: 256-9. [PubMed: 2572625](65 year old woman developed jaundice 6 months after starting clotiazepam [bilirubin 5.1 mg/dL, ALT 1028 U/L, GGT 143 U/L] with no rash or fever, resolving on stopping clotiazepam and no recurrence after taking triazolam and temazepam).
- Nahata MC, Murray RD, Zingarelli J, Li BU, McClung HJ, Lininger B. Efficacy and safety of a diazepam and meperidine combination for pediatric gastrointestinal procedures. J Pediatr Gastroenterol Nutr 1990; 10: 335-8. [PubMed: 2324894](Safety of use of intravenous diazepam in children undergoing endoscopy; no mention of ALT abnormalities or liver injury).
- Clobazam in treatment of refractory epilepsy: the Canadian experience. A retrospective study. Canadian Clobazam Cooperative Group. Epilepsia 1991; 32: 407-16. [PubMed: 2044502](Retrospective survey of Canadian neurologists with experience using clobazam in refractory epilepsy in a total of 877 patients; 32% had adverse events, most commonly somnolence, dizziness, ataxia, mood changes, hostility, blurred vision and weight gain; no mention of ALT elevations or hepatotoxicity).
- Suzuki A, Aso K, Ariyoshi C, Ishimaru M. Acute intermittent porphyria and epilepsy: safety of clonazepam. Epilepsia 1992; 33: 108-11. [PubMed: 1733741](13 year old girl with acute intermittent porphyria worsened on valproate and on phenytoin therapy, but clonazepam led to control of seizures and no worsening of porphyria).
- Hindmarch I, Fairweather DB, Rombaut N. Adverse events after triazolam substitution. Lancet 1993; 341: 55. [PubMed: 8093301](Survey of UK physicians, one year after withdrawal of triazolam in the UK, found higher rate of adverse reactions [largely CNS] with replacements such as temazepam, loprazolam, nitrazepam and lormetazepam, but no mention of hepatotoxicity).
- Andrade RJ, Lucena MI, Alcantara R, Fraile JM. Bentazepam-associated chronic liver disease. Lancet 1994; 343: 860. [PubMed: 7908109](65 year old man developed fatigue and weight loss 4 months after starting bentazepam [normal bilirubin, ALT 142 U/L, GGT 109 U/L, normal Alk P], biopsy showed chronic hepatitis, piecemeal necrosis and bridging fibrosis and abnormalities, resolved upon stopping).
- Andrade RJ, Lucena MI, Aguilar J, Lazo MD, Camargo R, Moreno P, García-Escaño MD, et al. Chronic liver injury related to use of bentazepam: an unusual instance of benzodiazepine hepatotoxicity. Dig Dis Sci 2000; 45: 1400-4. [PubMed: 10961721](3 cases of liver injury after taking bentazepam for 1, 3 and 5 months with elevated bilirubin in one [4.6 mg/dL]; variable ALT [117-1050 U/L], and Alk P elevations [154-346 U/L]; rapid resolution; some patients switched to other benzodiazepines without difficulty).
- Moulin CH, Rolachon A, Cohard M, Girard M, Bichard P, Pasquier D, Mallaret M, et al. [Fulminant hepatitis secondary to alprazolam] Therapie 1994; 49: 362-3. French. [PubMed: 7878609](64 year old man developed jaundice and confusion 3 weeks after starting alprazolam [bilirubin 4.1 mg/dL, ALT 100 times ULN, Alk P 3.4 times ULN and prothrombin index of 19%], resolution starting within one week and all tests returning to normal by 3 months).
- Wallace SJ. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf 1996; 15: 378-93. [PubMed: 8968693](Systematic review; ALT elevations occur in 4% of children on phenytoin, 6% on valproate, 1% on carbamazepine; “No child taking… benzodiazepines had raised liver enzyme levels,”).
- Lewis JH, Zimmerman HJ. Drug- and chemical-induced cholestasis. Clin Liver Dis 1999; 3: 433-64, vii. [PubMed: 11291233](Review of drug induced cholestatic syndromes, listing many causes including chlordiazepoxide and flurazepam; “Benzodiazepines may cause cholestatic injury, although this is rare”).
- Selim K, Kaplowitz N. Hepatotoxicity of psychotropic drugs. Hepatology 1999; 29: 1347-51. [PubMed: 10216114](Review of hepatotoxicity of phenothiazines, butyrophenones, tricyclics, MAO inhibitors, acetylcholinesterase inhibitors, and psychotropic drugs of abuse; “benzodiazepines…have a very low hepatotoxic potential, with only case reports in the literature, usually with a cholestatic pattern).
- de-la-Serna C, Gil-Grande LA, Sanromán AL, Gonzalez M, Ruiz-del-Arbol L, Garcia Plaza A. Bentazepam-induced hepatic bridging necrosis. J Clin Gastroenterol. 1997;25:710-1. [PubMed: 9451703](Bentazepam is a benzodiazepine used in Europe since 1981; 49 year old woman developed liver test abnormalities without symptoms 2 months after starting bentazepam [normal bilirubin, ALT 136 U/L, GGT 61 U/L], which persisted for 10 months and resolved rapidly when drug was stopped; biopsy showed chronic hepatitis with bridging necrosis).
- Kanda T, Yokosuka O, Fujiwara K, Saisho H, Shiga H, Oda S, Okuda K, et al. Fulminant hepatic failure associated with triazolam. Dig Dis Sci 2002; 47: 1111-4. [PubMed: 12018909](64 year old woman developed acute liver failure after taking triazolam intermittently for a year [bilirubin 6.1 rising to 18.6 mg/dL, ALT 1272 U/L] with progressive ascites and encephalopathy; successful living donor liver transplant 1 month after onset).
- Andrade RJ, López-Torres E, Lucena MI, Fernández Mdel C. [Acute hepatitis due to bentazepam]. Med Clin (Barc) 2003; 120: 678-9. Spanish. [PubMed: 12747823](Bentazepam is an oral benzodiazepine that was approved for use in anxiety in 1981 in Spain [but never in the US]; 55 year old woman developed fatigue within 2 weeks and jaundice with 4-5 months of starting bentazepam therapy [bilirubin 9.3 mg/dL, ALT 1932 U/L, Alk P 160 U/L], improving after stopping).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, of which 10 were attributed to phenytoin, 10 to valproate and 1 to carbamazepine, but none to benzodiazepines).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. [PubMed: 16165719](36 years of reporting to Swedish registry identified 103 cases of acute liver failure due to drugs, of which 1 was attributed to phenytoin, 1 to valproate and 1 to carbamazepine, but none to benzodiazepines).
- Gil-Martín A, Sáez-Royuela F, Arias L, Angulo ML, Nogal B. [Hepatic fibrosis after antidepressant treatment]. Rev Esp Enferm Dig. 2005;97:461-2. Spanish. [PubMed: 16048430](32 year old woman developed pruritis and jaundice 1-2 months after starting sertraline, alprazolam and clorazepate, resolving with stopping sertraline, but had persistent minor ALT elevations and biopsy showing mild bridging fibrosis 4 months later).
- Andrade RJ, Lucena MI, Kaplowitz N, García-Muņoz B, Borraz Y, Pachkoria K, García-Cortés M, et al. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology. 2006; 44: 1581-8. [PubMed: 17133470](28 of 493 [5.7%] cases of drug induced liver disease had evidence of chronicity, including 3 cases due to bentazepam and one with clorazepate, the latter with recovery at 25 months).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther 2007; 25: 1401-9. [PubMed: 17539979](Among 126 cases of drug induced liver injury seen in Spain between 1993-2000, 20 were attributed to benzodiazepines including 5 for clorazepate, 5 alprazolam, 6 lorazepam and 4 diazepam, but compared to controls, relative risk of injury was increased only for clorazepate [8.3: estimated frequency 3.4 per 100,000 person-year exposures]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to a benzodiazepine).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand 2008; 118: 281-90. [PubMed: 18341684](Review of hepatotoxicity of all anticonvulsants focusing upon phenytoin, valproate, carbamazepine; “Furthermore, hepatoxicity has not been convincingly shown to be associated with the use of benzodiazepines”).
- Conry JA, Ng YT, Paolicchi JM, Kernitsky L, Mitchell WG, Ritter FJ, Collins SD, Tracy K, et al. Clobazam in the treatment of Lennox-Gastaut syndrome. Epilepsia 2009; 50: 1158-66. [PubMed: 19170737](Among 68 children with Lennox-Gastaut syndrome who were treated with either low or high dose clobazam for 7 weeks, seizure reduction was more frequent with the higher dose as were side effects, which included somnolence, sedation, constipation, salivation, insomnia, irritability and lability; no mention of ALT elevations or hepatotoxicity).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children; benzodiazepines were not among the top 40 agents implicated).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were linked to benzodiazepine use).
- Ng YT, Conry JA, Drummond R, Stolle J, Weinberg MA; OV-1012 Study Investigators. Randomized, phase III study results of clobazam in Lennox-Gastaut syndrome. Neurology. 2011; 77: 1473-81. [PubMed: 21956725](Among 238 patients with Lennox-Gastaut syndrome treated with one of 3 doses of clobazam or placebo for at least 18 weeks, no patient developed serious hepatic adverse events and there were no abnormal clinical laboratory results linked to clobazam therapy).
- Clobazam (Onfi) for Lennox-Gastaut syndrome. Med Lett Drugs Ther 2012; 54 (1385): 18-9. [PubMed: 22382580](Concise review of the pharmacology, clinical efficacy, adverse events, drug interactions and costs of clobazam for Lennox-Gastaut syndrome shortly after its approval for this indication in the US; no mention of ALT elevations or hepatotoxicity).
- Yang LP, Scott LJ. Clobazam : in patients with Lennox-Gastaut syndrome. CNS Drugs 2012; 26: 983-91. [PubMed: 23034582](Summary of the structure, mechanism of action, pharmacology, clinical efficacy and safety of clobazam for Lennox Gastaut syndrome; no mention of hepatotoxicity or ALT elevations).
- Ng YT, Conry J, Paolicchi J, Kernitsky L, Mitchell W, Drummond R, Isojarvi J, Lee D, Owen R; OV-1004 study investigators. Long-term safety and efficacy of clobazam for Lennox-Gastaut syndrome: interim results of an open-label extension study. Epilepsy Behav 2012; 25: 687-94. [PubMed: 23141144](Among 267 children and adults who participated in controlled trials of clobazam and were enrolled in a long term extension study, 13 discontinued therapy because of side effects and six died, but none were attributed to hepatotoxicity).
- Wick JY. The history of benzodiazepines. Consult Pharm 2013; 28: 538-48. [PubMed: 24007886](History of the development, approval, widescale use and eventual restriction and decrease in use of the benzodiazepines).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were linked to benzodiazepines).
- Wheless JW, Phelps SJ. Clobazam: a newly approved but well-established drug for the treatment of intractable epilepsy syndromes. J Child Neurol 2013; 28: 219-29. [PubMed: 23112237](Review of the pharmacology, safety and efficacy of clobazam in Lennox Gastaut syndrome; no mention of hepatotoxicity).
- Drugs for epilepsy. Treat Guidel Med Lett. 2013; 11: 9-18. Erratum in: Treat Guidel Med Lett. 2013; 11: 112. [PubMed: 23348233](Concise review of indications and side effects of anticonvulsants; clobazam is approved only for treatment of severe seizures associated with Lennox-Gastaut syndrome; no mention of hepatotoxicity).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none of the 96, none were attributed to clobazam or other benzodiazepines).
- Klehm J, Thome-Souza S, Sánchez Fernández I, Bergin AM, Bolton J, Harini C, Kadish NE, et al. Clobazam: effect on frequency of seizures and safety profile in different subgroups of children with epilepsy. Pediatr Neurol 2014; 51: 60-6. [PubMed: 24830765](Among 300 children with seizures treated with clobazam for more than 1 month at a single referral center, 28% became seizure free and the most common side effects were tiredness [15%] and mood or behavioral changes [8%]; no mention of ALT elevations or hepatotoxicity).
- Conry JA, Ng YT, Kernitsky L, Mitchell WG, Veidemanis R, Drummond R, Isojarvi J, Lee D, et al.; OV-1004 Study Investigators. Stable dosages of clobazam for Lennox-Gastaut syndrome are associated with sustained drop-seizure and total-seizure improvements over 3 years. Epilepsia 2014; 55: 558-67. [PMC free article: PMC4303987] [PubMed: 24580023](Among 267 patients with Lennox-Gastaut syndrome enrolled in a long term, open label, observational study of clobazam, adverse events were similar to those in controlled trials and none of the serious adverse events or the 18 discontinuations because of side effects or the 10 deaths were attributed to liver injury).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, but none were attributed to a benzodiazepine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, no cases were attributed to a benzodiazepine).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- In Brief: New benzodiazepine warnings.[Med Lett Drugs Ther. 2020]In Brief: New benzodiazepine warnings.. Med Lett Drugs Ther. 2020 Nov 2; 62(1610):175.
- Review Muscle relaxants for pain management in rheumatoid arthritis.[Cochrane Database Syst Rev. 2012]Review Muscle relaxants for pain management in rheumatoid arthritis.Richards BL, Whittle SL, Buchbinder R. Cochrane Database Syst Rev. 2012 Jan 18; 1:CD008922. Epub 2012 Jan 18.
- Systematic review of the benzodiazepines. Guidelines for data sheets on diazepam, chlordiazepoxide, medazepam, clorazepate, lorazepam, oxazepam, temazepam, triazolam, nitrazepam, and flurazepam. Committee on the Review of Medicines.[Br Med J. 1980]Systematic review of the benzodiazepines. Guidelines for data sheets on diazepam, chlordiazepoxide, medazepam, clorazepate, lorazepam, oxazepam, temazepam, triazolam, nitrazepam, and flurazepam. Committee on the Review of Medicines.. Br Med J. 1980 Mar 29; 280(6218):910-2.
- Review Midazolam and other benzodiazepines.[Handb Exp Pharmacol. 2008]Review Midazolam and other benzodiazepines.Olkkola KT, Ahonen J. Handb Exp Pharmacol. 2008; (182):335-60.
- Vasorelaxant effects of benzodiazepines, non-benzodiazepine sedative-hypnotics, and tandospirone on isolated rat arteries.[Eur J Pharmacol. 2021]Vasorelaxant effects of benzodiazepines, non-benzodiazepine sedative-hypnotics, and tandospirone on isolated rat arteries.Kagota S, Morikawa K, Ishida H, Chimoto J, Maruyama-Fumoto K, Yamada S, Shinozuka K. Eur J Pharmacol. 2021 Feb 5; 892:173744. Epub 2020 Nov 19.
- Benzodiazepines - LiverToxBenzodiazepines - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...