NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Midazolam is an intravenously administered benzodiazepine used as an anesthetic for conscious sedation for minor procedures and as an adjunct for general anesthesia. Midazolam has not been associated with serum aminotransferase elevations during therapy and has not been linked to cases of clinically apparent liver injury.
Background
Midazolam (mi daz' oh lam) is a benzodiazepine with particularly potent sedative activity. The sedative activity of the benzodiazepines is mediated by their ability to enhance gamma-aminobutyric acid (GABA) mediated inhibition of synaptic transmission through binding to the GABA A receptor. The use of midazolam has been largely as an intravenous anesthetic agent. Midazolam is used for conscious sedation for short-term, outpatient procedures such as upper and lower endoscopy, bronchoscopy, liver biopsy and cardiac catheterization. It is also used for induction of general anesthesia and for preoperative sedation. Midazolam is associated with anterograde amnesia which is often convenient for uncomfortable, minimally invasive procedures. Midazolam is available generically (and formerly under the brand name Versed) in parenteral forms for injection [1 mg/mL in 1-, 2-, 5-, and 10-mL vials], in disposal syringes and as an oral solution for pediatric use perioperatively. The typical dose for conscious sedation in adults is 1 to 4 mg intravenously over 2 to 5 minutes. Common side effects of the use of intravenous midazolam include nausea, hypotension, confusion, acute agitation, and respiratory depression. Acute overdose of midazolam can cause respiratory arrest and death. Midazolam has a boxed warning in its product label about the risks of severe respiratory depression with its use, a warning against combined use with opiates, and need to individualize the dose, to monitor respiratory and cardiac status, and have resuscitative drugs and equipment available.
Hepatotoxicity
Midazolam, like other benzodiazepines, is rarely associated with serum ALT or alkaline phosphatase elevations. Clinically apparent liver injury from midazolam has not been reported and must be extremely rare, if it occurs at all. Isolated single cases of clinically apparent liver injury have been reported with other benzodiazepines including alprazolam, chlordiazepoxide, clonazepam, diazepam, flurazepam, lorazepam, and triazolam. The clinical pattern of acute liver injury from benzodiazepines is typically cholestatic, but hepatocellular patterns of injury have been reported with clorazepate and clotiazepam. The injury is usually mild to moderate in severity with a time to onset of 1 to 6 months and rapid recovery once the benzodiazepine is stopped. Fever and rash are uncommon as is autoantibody formation.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Midazolam is extensively metabolized by the liver to inactive metabolites which are excreted in the urine. The liver injury from benzodiazepines is probably due to a rarely produced intermediate metabolite. The absence of liver injury is perhaps due to the short duration of therapy and low doses used.
Outcome and Management
In the isolated case reports of hepatic injury due to benzodiazepines recovery was rapid and complete after stopping the medication, without evidence of residual or chronic injury. No cases of acute hepatitis, liver failure, or chronic liver injury due to midazolam have been described. There is no information about cross reactivity with other benzodiazepines, but some degree of cross sensitivity may occur.
Drug Class: Sedatives and Hypnotics, Benzodiazepines, Antianxiety Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Midazolam – Generic, Versed® (Trade name discontinued)
DRUG CLASS
Benzodiazepines
Product labeling at DailyMed, National Library of Medicine, NIH
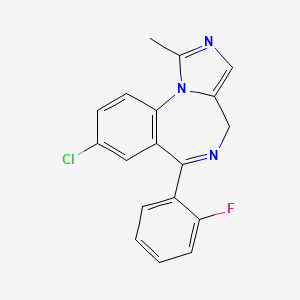
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Midazolam | 59467-70-8 | C18-H13-Cl-F-N3 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 22 June 2023
- Zimmerman HJ. Benzodiazepines. Psychotropic and anticonvulsant agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 491-3.(Expert review of benzodiazepines and liver injury published in 1999; mentions rare instances of cholestatic hepatitis have been reported due to alprazolam, chlordiazepoxide, diazepam, flurazepam, and triazolam, and hepatocellular injury with clorazepate and clotiazepam, but no reports of hepatic injury with lorazepam, oxazepam, or temazepam).
- Larrey D, Ripault MP. Benzodiazepines. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 455.(Review of benzodiazepine induced liver injury mentions that increases in liver enzymes during therapy are rare and significant hepatotoxicity uncommon; only a few cases [usually cholestatic] have been reported with alprazolam, chlordiazepoxide, diazepam, flurazepam, and triazolam).
- Mihic SJ, Mayfield J, Harris RA. Hypnotics and sedatives. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 339-53.(Textbook of pharmacology and therapeutics).
- Wallace SJ. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf. 1996;15:378–93. [PubMed: 8968693](Systematic review; ALT elevations occur in 4% of children on phenytoin, 6% on valproate, 1% on carbamazepine; but “no child taking… benzodiazepines had raised liver enzyme levels”).
- Lewis JH, Zimmerman HJ. Drug- and chemical-induced cholestasis. Clin Liver Dis. 1999;3:433–64. [PubMed: 11291233](Review of drug induced cholestatic syndromes, listing many causes including chlordiazepoxide and flurazepam; “benzodiazepines may cause cholestatic injury, although this is rare”).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Among 126 cases of drug induced liver injury seen in Spain between 1993-2000, 20 were attributed to benzodiazepines including 5 for clorazepate, 5 alprazolam, 6 lorazepam, and 4 diazepam, but compared to controls, relative risk of injury was increased only for clorazepate [8.3: estimated frequency 3.4 per 100,000 person-year exposures]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none attributed to a benzodiazepine).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand. 2008;118:281–90. [PubMed: 18341684](Review of hepatotoxicity of all anticonvulsants focusing upon phenytoin, valproate, carbamazepine; “furthermore, hepatoxicity has not been convincingly shown to be associated with the use of benzodiazepines”).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population-based study of drug induced liver injury from Iceland, 96 cases were identified over a 2-year period, but none were attributed to midazolam or any other benzodiazepine, despite the fact millions of prescriptions for them are filled yearly).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature on drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to midazolam or any other benzodiazepine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–1352.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, no cases were attributed to midazolam or any other benzodiazepine).
- Rex DK, Bhandari R, Desta T, DeMicco MP, Schaeffer C, Etzkorn K, Barish CF, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88:427–437.e6. [PubMed: 29723512](Among 461 adults undergoing colonoscopy who received remimazolam, midazolam, or placebo as a procedural sedative after a single dose of fentanyl, adequate sedation was achieved more frequently with remimazolam and onset after starting and recovery of full alertness after stopping was more rapid with remimazolam, with similar rates of adverse events and no serious adverse event).
- Pastis NJ, Yarmus LB, Schippers F, Ostroff R, Chen A, Akulian J, Wahidi M, et al. PAION Investigators. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155:137–146. [PubMed: 30292760](Among 446 patients undergoing bronchoscopy who received procedural sedation with remimazolam, midazolam or placebo, the onset of adequate sedation was faster with remimazolam than midazolam and time to being fully alert was more rapid after stopping while total and severe adverse event rates were similar in the three groups and success rates in completing the procedure were similar; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- A comparison of fospropofol to midazolam for moderate sedation during outpatient dental procedures.[Anesth Prog. 2013]A comparison of fospropofol to midazolam for moderate sedation during outpatient dental procedures.Yen P, Prior S, Riley C, Johnston W, Smiley M, Thikkurissy S. Anesth Prog. 2013 Winter; 60(4):162-77.
- Hypnosis as adjunct therapy in conscious sedation for plastic surgery.[Reg Anesth. 1995]Hypnosis as adjunct therapy in conscious sedation for plastic surgery.Faymonville ME, Fissette J, Mambourg PH, Roediger L, Joris J, Lamy M. Reg Anesth. 1995 Mar-Apr; 20(2):145-51.
- Review Remimazolam.[LiverTox: Clinical and Researc...]Review Remimazolam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Propofol Compared to Midazolam Sedation and to General Anesthesia for Percutaneous Microwave Ablation in Patients with Hepatic Malignancies: A Single-Center Comparative Analysis of Three Historical Cohorts.[Cardiovasc Intervent Radiol. 2...]Propofol Compared to Midazolam Sedation and to General Anesthesia for Percutaneous Microwave Ablation in Patients with Hepatic Malignancies: A Single-Center Comparative Analysis of Three Historical Cohorts.Puijk RS, Ziedses des Plantes V, Nieuwenhuizen S, Ruarus AH, Vroomen LGPH, de Jong MC, Geboers B, Hoedemaker-Boon CJ, Thöne-Passchier DH, Gerçek CC, et al. Cardiovasc Intervent Radiol. 2019 Nov; 42(11):1597-1608. Epub 2019 Jun 26.
- Review Remimazolam - current knowledge on a new intravenous benzodiazepine anesthetic agent.[Korean J Anesthesiol. 2022]Review Remimazolam - current knowledge on a new intravenous benzodiazepine anesthetic agent.Kim SH, Fechner J. Korean J Anesthesiol. 2022 Aug; 75(4):307-315. Epub 2022 May 19.
- Midazolam - LiverToxMidazolam - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...