NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Central nervous system stimulants used for attention deficit disorder, narcolepsy or excessive sleepiness include the amphetamines, methylphenidate, atomoxetine, modafinil, armodafinil, pitolisant and solriamfetol. Stimulants that are no longer used for medical conditions, but that are abused, include cocaine and ecstasy or methylenedioxymethamphetamine (MDMA). The individual agents discussed include the following:
The following links are to individual drug records.
- Amphetamines (including ecstasy or methylenedioxymethamphetamine)
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Armodafinil – Generic, Nuvigil®
Atomoxetine – Generic, Strattera®
Dextroamphetamine – Generic, Adderall®, Dexedrine®
Dextroamphetamine and Amphetamine – Generic, Adderall®
Lisdexamfetamine – Vyvanse®
Methamphetamine – Generic, Desoxyn®
Modafinil – Generic, Provigil®
Oxybate – Xyrem®
Pitolisant – Wakix®
Solriamfetol – Sunosi®
COMPLETE LABELING [Dextroamphetamine]
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Armodafinil | 112111-43-0 | C15-H15-N-O2-S |
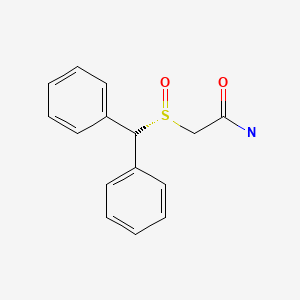
|
| Atomoxetine | 83015-26-3 | C17-H21-N-O |
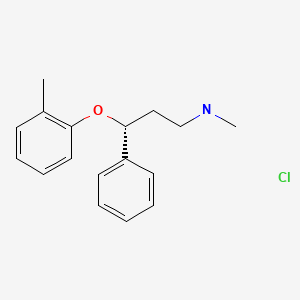
|
| Dextroamphetamine | 51-64-9 | C9-H13-N |
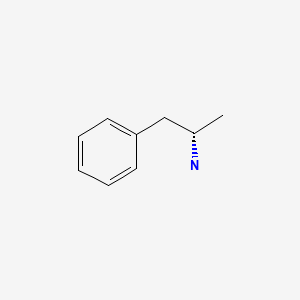
|
| Lisdexamfetamine | 608137-32-2 | C15-H25-N3-O |
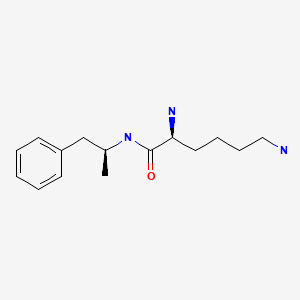
|
| Methamphetamine | 537-46-2 | C10-H15-N |
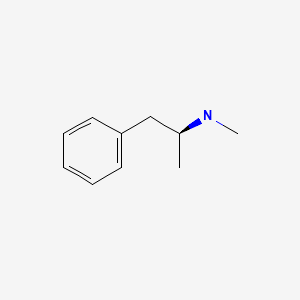
|
| Methylphenidate | 113-45-1 | C14-H19-N-O2 |
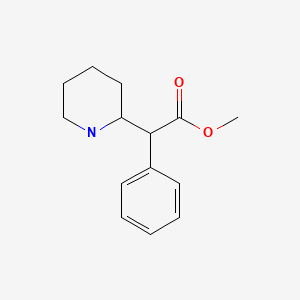
|
| Modafinil | 68693-11-8 | C15-H15-N-O2-S |
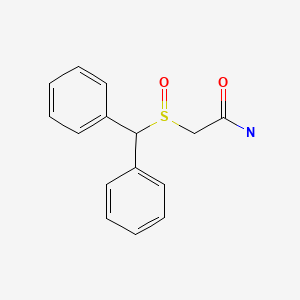
|
| Sodium Oxybate | 502-85-2 | C4-H7-Na-O3 |
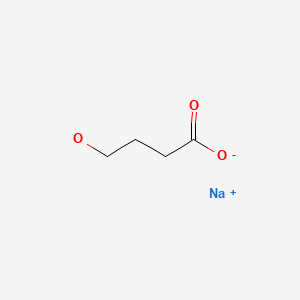
|
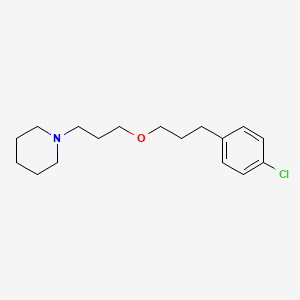
| Pitolisant | 362665-56-3 | C17-H26-Cl-N-O |
|
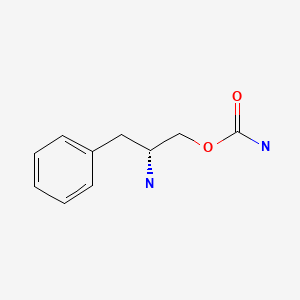
| Solriamfetol | 178429-62-4 | C10-H14-N2-O2 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 12 August 2021
- Larrey D, Ripault MP. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 443-62.(Review of hepatotoxicity of psychotropic agents does not discuss modafinil and armodafinil).
- Westfall TC, Macarthur H, Westfall DP. Narcolepsy and sleep/wake imbalance. Adrenergic agonists and antagonists. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 207.(Textbook of pharmacology and therapeutics).
- Randomized trial of modafinil for the treatment of pathological somnolence in narcolepsy. US Modafinil in Narcolepsy Multicenter Study Group. Ann Neurol. 1998;43:88–97. [PubMed: 9450772](Controlled trial of modafinil [200 or 400 mg] vs placebo for 9 weeks; modafinil demonstrated an excellent safety profile for up to 40 weeks of open label treatment; “there were few clinically meaningful changes in laboratory values”).
- Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy. US Modafinil in Narcolepsy Multicenter Study Group. Neurology. 2000;54:1166–75. [PubMed: 10720292](Controlled 9 week trial of two doses of modafinil [200 and 400 mg] vs placebo in 271 patients with narcolepsy: “There were no meaningful differences among treatment groups at week 9 in clinical laboratory test results”).
- Mitler MM, Harsh J, Hirshkowitz M, Guilleminault C. Long-term efficacy and safety of modafinil (PROVIGIL((R))) for the treatment of excessive daytime sleepiness associated with narcolepsy. Sleep Med. 2000;1:231–43. [PubMed: 10828434](478 adults with narcolepsy were enrolled in two 40-week, open label extension studies using 200-400 mg of modafinil daily; common side effects were headache, nervousness, and nausea; clinically significant elevations in ALT occurred in 6 [1.5%] and total bilirubin in 1 patient, but no other details given).
- A new indication for gamma hydroxybutyrate (Xyrem) in narcolepsy. Med Lett Drugs Ther. 2006;48(1227):11–2. [PubMed: 16444137](Concise review of narcolepsy and the mechanism of action, clinical efficacy, and adverse effects of oxybate, shortly after the broadening of its indications to include excessive daytime sleepiness in patients with narcolepsy; no mention of serum enzyme elevations or hepatotoxicity).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, including one attributed to cocaine and one to ecstasy but none to methylphenidate, modafinil, or oxybate).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one was attributed to methylphenidate, but none to amphetamines, modafinil, armodafinil, or oxybate).
- Kaplan S, Goehring EL Jr, Melamed-Gal S, Nguyen-Khoa BA, Knebel H, Jones JK. Modafinil and the risk of cardiovascular events: Findings from three US claims databases. Pharmacoepidemiol Drug Saf. 2018;27:1182–90. [PubMed: 30106194](Analysis of 3 large US health care claims database systems [2006-2008] found no increased risk for myocardial infarction or cardiovascular hospitalization among modafinil users vs non-users).
- Solriamfetol (Sunosi) for excessive daytime sleepiness. Med Lett Drugs Ther. 2019;61(1579):132–4. [PubMed: 31581157](Concise review of the mechanism of action, clinical efficacy, safety and cost of solriamfetol for excess sleepiness associated with narcolepsy and obstructive sleep apnea mentions adverse events of headache, nausea, anorexia, dry mouth, anxiety, and insomnia, but does not discuss ALT elevations of hepatotoxicity).
- Thorpy MJ. Recently approved and upcoming treatments for narcolepsy. CNS Drugs. 2020;34:9–27. [PMC free article: PMC6982634] [PubMed: 31953791](Review of the mechanism of action, pharmacology, drug-drug interactions, clinical efficacy and safety of newly approved medications for narcolepsy including pitolisant and solriamfetol; no mention of ALT elevations or hepatotoxicity).
- Drugs for ADHD. Med Lett Drugs Ther. 2020;62(1590):9–15. [PubMed: 31999670](Concise review of drugs for attention deficit/hyperactivity disorder mentions that methylphenidate and the CNS stimulants are the first line agents for school age children and adolescents and that adverse events can include decreased appetite, abdominal pain, headache and insomnia, but does not mention ALT elevations or hepatotoxicity).
- Nourbakhsh B, Revirajan N, Morris B, Cordano C, Creasman J, Manguinao M, Krysko K, et al. Safety and efficacy of amantadine, modafinil, and methylphenidate for fatigue in multiple sclerosis: a randomised, placebo-controlled, crossover, double-blind trial. Lancet Neurol. 2021;20:38–48. [PMC free article: PMC7772747] [PubMed: 33242419](In a placebo controlled, 4-period, crossover trial of amantadine, modafinil and methylphenidate for up to 6 weeks, none of the drugs were superior to placebo in improving fatigue, but all three were associated with more side effects than placebo; ALT elevations and hepatotoxicity were not mentioned).
- Pitolisant (Wakix) for narcolepsy. Med Lett Drugs Ther. 2021;63(1617):19–21. [PubMed: 33647004](Concise review of the mechanism of action, and relative efficacy, safety and costs of pitolisant in relation to other medications for narcolepsy shortly after its approval for use in the US, mentions side effects of headache, insomnia, nausea and prolongation of the QTc interval).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Update on the pharmacologic management of narcolepsy: mechanisms of action and clinical implications.[Sleep Med. 2020]Review Update on the pharmacologic management of narcolepsy: mechanisms of action and clinical implications.Thorpy MJ, Bogan RK. Sleep Med. 2020 Apr; 68:97-109. Epub 2019 Sep 11.
- Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine clinical practice guideline.[J Clin Sleep Med. 2021]Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine clinical practice guideline.Maski K, Trotti LM, Kotagal S, Robert Auger R, Rowley JA, Hashmi SD, Watson NF. J Clin Sleep Med. 2021 Sep 1; 17(9):1881-1893.
- Review Treatment of Excessive Daytime Sleepiness in Patients with Narcolepsy.[Curr Treat Options Neurol. 2019]Review Treatment of Excessive Daytime Sleepiness in Patients with Narcolepsy.Pérez-Carbonell L. Curr Treat Options Neurol. 2019 Nov 12; 21(11):57. Epub 2019 Nov 12.
- Review Pharmacologic Management of Excessive Daytime Sleepiness.[Sleep Med Clin. 2020]Review Pharmacologic Management of Excessive Daytime Sleepiness.Takenoshita S, Nishino S. Sleep Med Clin. 2020 Jun; 15(2):177-194.
- Review Drugs for patients with epilepsy and excessive daytime sleepiness.[Epilepsy Behav. 2021]Review Drugs for patients with epilepsy and excessive daytime sleepiness.Zaccara G, Bartolini E, Tramacere L, Lattanzi S. Epilepsy Behav. 2021 Sep 14; 124:108311. Epub 2021 Sep 14.
- Central Nervous System (CNS) Stimulants - LiverToxCentral Nervous System (CNS) Stimulants - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...