NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Mexiletine is an oral antiarrhythmic agent that is used for suppression of ventricular arrhythmias. Long term mexiletine therapy is associated with a low rate of serum enzyme elevations and is a rare cause of clinically apparent acute liver injury.
Background
Mexiletine (mex il' e teen) is an analogue of the local anesthetic lidocaine and has electrophysiological effects that resemble quinidine (antiarrhythmic Class IB). Mexiletine appears to act by blocking open sodium channels and outward potassium channels. As a consequence, it decreases cardiac automaticity, increases refractory periods and slows conduction. Mexiletine was approved for use in the United States in 1985, and current indications include suppression of life threatening ventricular arrhythmias including ventricular tachycardia. It is also used off-label to treat neuropathy. Mexiletine is available in capsules of 150, 200 and 250 mg generically and under the brand name Mexitil. The usual maintenance dose in adults is 200 to 300 mg every 6 hours. The dosage used to treat neuropathy is typically 300 mg daily. The most common side effects include gastrointestinal upset, nausea, dizziness, anxiety, tremor, blurred vision and skin rash. Uncommon but potentially severe adverse events include worsening of arrhythmias and congestive heart failure, blood dyscrasias and hypersensitivity reactions, including Stevens Johnson syndrome.
Hepatotoxicity
In clinical trials, mexiletine was associated with a low rate of serum aminotransferase and alkaline phosphatase elevations (~0.5%). Despite wide scale use, mexiletine has only rarely been linked to cases of clinically apparent liver injury. Most cases are associated with a hypersensitivity reaction. Typically, patients present with fever, rash, eosinophilia and systemic systemics (DRESS syndrome) after 2 to 6 weeks of mexiletine therapy. Eosinophilia or atypical lymphocytosis usually accompany the liver test abnormalities but may arise or continue to worsen days or weeks after onset. The pattern of serum enzyme elevations ranges from cholestatic to hepatocellular, but jaundice is usually mild or absent. Autoimmune features are uncommon. Cases of Stevens Johnson and DRESS syndrome due to mexiletine have also been reported. Corticosteroids were often used, but largely for the fever and skin rash rather than the liver disease which was typically mild. Rare instances of cholestatic hepatitis with mexiletine therapy have also been reported and may represent a different form of liver injury.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The liver injury from mexiletine appears to be mediated by a hypersensitivity reaction. Mexiletine is metabolized in the liver by the cytochrome P450 system and an immunoallergic metabolite may be produced. The hypersensitivity reaction to mexiletine may be more common in Asian than other populations.
Outcome and Management
The liver injury due to mexiletine is usually overshadowed by the signs and symptoms of hypersensitivity. No cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome due to mexiletine have been reported. The signs and symptoms of hypersensitivity typically respond rapidly to corticosteroid therapy, but it is unclear whether the liver injury is similarly affected by the immunosuppression and many cases resolve rapidly without systemic corticosteroid therapy. There have been no reports of cross sensitivity to the liver injury between mexiletine and other oral antiarrhythmics, but shared sensitivity is unlikely.
Drug Class: Antiarrhythmic Agents
CASE REPORT
Case 1. Drug rash with eosinophilia and liver injury attributed to mexiletine.(1)
A 64 year old man developed rash and fever 30 days after starting mexiletine (100 mg three times daily) for a painful, postherpetic neuropathy. He had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. His other medications included amitriptyline (125 mg daily) and alprazolam (0.4 mg three times daily) which he had taken for the previous 2 years. On examination, the temperature was 38.3 oC. He had a morbilliform skin rash involving the face and trunk, but was not jaundiced. Laboratory tests showed a normal white count (7,300/μL) with 3% eosinophils and 1% atypical lymphocytes. Serum aminotransferase (ALT 252 U/L and AST 235 U/L) and alkaline phosphatase levels (574 U/L) were both elevated, but bilirubin was normal (0.7 mg/dL) (Table). His medications were stopped, but during the next week the rash worsened and he developed a marked eosinophilia (10.5%), atypical lymphocytosis (9.5%) and jaundice (Table). He was treated with dexamethasone (8 mg daily), followed by rapidly tapering doses of oral prednisolone. The fever and the rash improved, disappearing within 4 weeks. Jaundice also resolved rapidly, but serum alkaline phosphatase decreased more slowly and was still slightly elevated 7 weeks after stopping mexiletine. Amitriptyline was resumed at a higher dose without recurrence of jaundice or rash.
Key Points
| Medication: | Mexiletine (100 mg three times a day) |
|---|---|
| Pattern: | Mixed-cholestatic (R=2.2, falling to 1.8) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 30 days to rash, 38 to jaundice |
| Recovery: | Incomplete at 6 weeks |
| Other medications: | Amitriptyline, alprazolam |
Laboratory Values
Comment
The clinical presentation was typical of DRESS syndrome (drug rash with eosinophilia and systemic symptoms) such as occurs with the aromatic anticonvulsants and allopurinol. Rash is not an uncommon side effect of mexiletine, but liver injury and jaundice are rare. The cause is likely hypersensitivity, and corticosteroids are often used with an immediate effect on fever and skin rash. Whether the liver injury is improved by corticosteroid therapy is unclear. In this publication, long term follow up information was not provided.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Mexiletine – Generic, Mexitil®
DRUG CLASS
Antiarrhythmic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
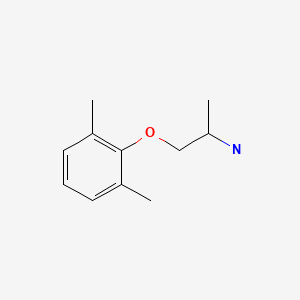
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Mexiletine | 31828-71-4 | C11-H17-N-O |
|
CITED REFERENCE
- 1.
- Higa K, Hirata K, Dan K. Mexiletine-induced severe skin eruption, fever, eosinophilia, atypical lymphocytosis, and liver dysfunction. Pain. 1997;73:97–9. [PubMed: 9414061]
ANNOTATED BIBLIOGRAPHY
References updated: 06 February 2020
- Zimmerman HJ. Antiarrhythmics. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 642-4.(Expert review of hepatotoxicity of antiarrhythmics published in 1999; mentions that mexiletine has been linked to 6 cases of clinically apparent liver injury).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 524.(Review of hepatotoxicity of cardiovascular drugs including antiarrhythmics mentions that mexiletine has been linked to cases of cholestatic hepatitis).
- Knollmann BC, Roden DM. Antiarrhythmic drugs. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 547-72.(Textbook of pharmacology and therapeutics).
- Campbell NP, Pantridge JF, Adgey AA. Long-term oral antiarrhythmic therapy with mexiletine. Br Heart J. 1978;40:796–801. [PMC free article: PMC483486] [PubMed: 687477](48 patients treated with mexiletine long term, found side effects were common but no mention of liver injury or ALT elevations).
- Pernot C, Netter P, Marcon F, Weber JL. Presse Med. 1983;12:1938. [Reversible hepatic anomalies during treatment with mexiletine] French. [PubMed: 6226010](48 year old man developed asymptomatic ALT elevations 15 days after starting mexiletine, but they decreased and drug was continued to 3 months when ALT rose to 1030 U/L, GGT 265 U/L, bilirubin 1.1 mg/dL, falling towards normal over next 4 months after stopping).
- Geisler C, Keiding S. Ugeskr Laeger. 1984;146:1212–3. [Cholestatic hepatitis during treatment with mexiletine/lidocaine] Danish. [PubMed: 6730049]
- Dejgard A, Petersen P, Kastrup J. Mexiletine for treatment of chronic painful diabetic neuropathy. Lancet. 1988 Jan;1:9–11. [PubMed: 2891940](Cross over, double blind trial of 26 weeks of mexiletine for diabetic neuropathy; no mention of ALT levels).
- Higa K, Hirata K, Dan K. Mexiletine-induced severe skin eruption, fever, eosinophilia, atypical lymphocytosis, and liver dysfunction. Pain. 1997;73:97–9. [PubMed: 9414061](64 year old man developed jaundice, fever and rash 30 days after starting mexiletine [bilirubin 3.7 mg/dL, ALT 242 U/L, Alk P 1280 U/L, 51% eosinophilia, 9% atypical lymphocytes], resolving with corticosteroid therapy in 4 weeks: Case 1).
- Roden DM. Antiarrhythmic drugs: from mechanisms to clinical practice. Heart. 2000;84:339–46. [PMC free article: PMC1760959] [PubMed: 10956304](Overview of antiarrhythmic drugs which are separated in four classes based upon molecular target: I being sodium channel blockers; II beta blockers; III potassium channel blockers; and, IV calcium channel blockers; and, some agents having multiple targets).
- Sasaki K, Yamamoto T, Kishi M, Yokozeki H, Nishioka K. Acute exanthematous pustular drug eruption induced by mexiletine. Eur J Dermatol. 2001;11:469–71. [PubMed: 11525960](56 year old man developed rash 1 month after starting mexiletine [ALT 129 U/L, bilirubin and Alk P not given, eosinophils 20%], resolving within 3 months of stopping).
- Seino Y, Yamauchi M, Hirai C, Okumura A, Kondo K, Yamamoto M, Okazaki Y. A case of fulminant Type 1 diabetes associated with mexiletine hypersensitivity syndrome. Diabet Med. 2004;21:1156–7. [PubMed: 15384968](46 year old man developed rash 41 days after starting mexiletine for diabetic neuropathy [ALT 66 rising to ~720 U/L, bilirubin and Alk P not given], resolving with corticosteroid therapy with worsening of diabetes).
- Sekiguchi A, Kashiwagi T, Ishida-Yamamoto A, Takahashi H, Hashimoto Y, Kimura H, Tohyama M, et al. Drug-induced hypersensitivity syndrome due to mexiletine associated with human herpes virus 6 and cytomegalovirus reactivation. J Dermatol. 2005;32:278–81. [PubMed: 15863850](66 year old man developed fever and rash with ALT elevations one month after starting mexiletine with progressive course, and HHV-6 and CMV present in serum, and death from myocarditis).
- Yagami A, Yoshikawa T, Asano Y, Koie S, Shiohara T, Matsunaga K. Drug-induced hypersensitivity syndrome due to mexiletine hydrochloride associated with reactivation of human herpesvirus 7. Dermatology. 2006;213:341–4. [PubMed: 17135743](44 year old developed rash, fever and mononucleosis-like syndrome 6 months after starting mexiletine [ALT 144 U/L, atypical lymphocytes 15%, bilirubin and Alk P not given], with presence of human herpesvirus-7 DNA in white cells and skin; treated with prednisolone and resolved in 2-3 months).
- Drugs for cardiac arrhythmias. Treat Guidel Med Lett. 2007;5:51–8. [PubMed: 17505408](Concise review of drugs for arrhythmias; mexiletine is an orally effective congener of lidocaine and is used as an alternative to amiodarone for ventricular arrhythmias; mentions hepatitis as an adverse event).
- Lee SP, Kim SH, Kim TH, Sohn JW, Shin DH, Park SS, Yoon HJ. A case of mexiletine-induced hypersensitivity syndrome presenting as eosinophilic pneumonia. J Korean Med Sci. 2010;25:148–51. [PMC free article: PMC2800002] [PubMed: 20052362](82 year old man developed cough, fever and rash 6 months after starting mexiletine [ALT 321 U/L, bilirubin and Alk P not given, eosinophils 24%], eosinophilic pneumonitis on lung biopsy, resolving with prednisone therapy within 2 months of stopping).
- Statland JM, Bundy BN, Wang Y, Rayan DR, Trivedi JR, Sansone VA, Salajegheh MK, et al. Consortium for Clinical Investigation of Neurologic Channelopathies. Mexiletine for symptoms and signs of myotonia in nondystrophic myotonia: a randomized controlled trial. JAMA. 2012;308:1357–65. [PMC free article: PMC3564227] [PubMed: 23032552](Among 59 patients with nondystrophic myotonia treated with mexiletine [200 mg] or placebo 3 times daily for 4 weeks followed by cross-over, side effects included gastrointestinal discomfort and there were no serious adverse events and no mention of ALT elevations or hepatotoxicity).
- Uhara H, Saiki M, Kawachi S, Ashida A, Oguchi S, Okuyama R. Clinical course of drug-induced hypersensitivity syndrome treated without systemic corticosteroids. J Eur Acad Dermatol Venereol. 2013;27:722–6. [PubMed: 22540194](Among 12 patients with drug induced hypersensitivity syndrome, all recovered including one case due to mexiletine; a 70 year old man with post-herpetic neuropathy who developed fever, rash, eosinophilia and ALT elevations [239 U/L] 34 days after starting mexiletine, and recovered without corticosteroid therapy in 37 days).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to mexiletine).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none of which were attributed to mexiletine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 7 [0.8%] were attributed to antiarrhythmics, but none to mexiletine).
- Suetterlin KJ, Bugiardini E, Kaski JP, Morrow JM, Matthews E, Hanna MG, Fialho D. Long-term safety and efficacy of mexiletine for patients with skeletal muscle channelopathies. JAMA Neurol. 2015;72:1531–3. [PubMed: 26658970](Among 63 patients with myotonia treated with mexiletine for up to 17.8 years [mean 4.8 years], there were no serious adverse events but dyspepsia was common and led to discontinuation in 4 patients; no mention of ALT elevations or hepatotoxicity).
- Shibuya K, Misawa S, Kimura H, Noto Y, Sato Y, Sekiguchi Y, Iwai Y, et al. A single blind randomized controlled clinical trial of mexiletine in amyotrophic lateral sclerosis: efficacy and safety of sodium channel blocker phase II trial. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:353–8. [PubMed: 25960085](Among 60 patients with amyotrophic lateral sclerosis treated with riluzole with or without mexiletine [300 mg daily] for 6 months, adverse events were more frequent in those receiving mexiletine but there were no serious adverse events and only one case of “liver dysfunction” which occurred in a riluzole only treated subject).
- Weiss MD, Macklin EA, Simmons Z, Knox AS, Greenblatt DJ, Atassi N, Graves M, et al. Mexiletine ALS Study Group. A randomized trial of mexiletine in ALS: Safety and effects on muscle cramps and progression. Neurology. 2016;86:1474–81. [PMC free article: PMC4836879] [PubMed: 26911633](Among 60 patients with amyotrophic lateral sclerosis treated with mexiletine [300 or 900 mg daily] or placebo for 12 weeks, adverse events and discontinuations were common with the higher dose of mexiletine and muscle cramps were less; there were no serious adverse events and no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Mexiletine: an effective antiarrhythmic drug for treatment of ventricular arrhythmias in congenital heart disease.[J Am Coll Cardiol. 1987]Mexiletine: an effective antiarrhythmic drug for treatment of ventricular arrhythmias in congenital heart disease.Moak JP, Smith RT, Garson A Jr. J Am Coll Cardiol. 1987 Oct; 10(4):824-9.
- Review Oral mexiletine in the treatment of refractory ventricular arrhythmias: the role of electrophysiologic techniques.[Am Heart J. 1984]Review Oral mexiletine in the treatment of refractory ventricular arrhythmias: the role of electrophysiologic techniques.Schoenfeld MH, Whitford E, McGovern B, Garan H, Ruskin JN. Am Heart J. 1984 May; 107(5 Pt 2):1071-8.
- Review Mexiletine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in the treatment of arrhythmias.[Drugs. 1990]Review Mexiletine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in the treatment of arrhythmias.Monk JP, Brogden RN. Drugs. 1990 Sep; 40(3):374-411.
- Mexiletine. Long-term efficacy and side effects in patients with chronic drug-resistant potentially lethal ventricular arrhythmias.[Arch Intern Med. 1990]Mexiletine. Long-term efficacy and side effects in patients with chronic drug-resistant potentially lethal ventricular arrhythmias.Kerin NZ, Aragon E, Marinescu G, Faitel K, Frumin H, Rubenfire M. Arch Intern Med. 1990 Feb; 150(2):381-4.
- Review Mexiletine: pharmacology and therapeutic use.[Clin Cardiol. 1990]Review Mexiletine: pharmacology and therapeutic use.Manolis AS, Deering TF, Cameron J, Estes NA 3rd. Clin Cardiol. 1990 May; 13(5):349-59.
- Mexiletine - LiverToxMexiletine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...