NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsVINBLASTINE, VINCRISTINE, VINORELBINE
OVERVIEW
Introduction
The vinca alkaloids include vincristine, vinblastine and vinorelbine which are important antineoplastic agents used in many chemotherapeutic regimens for a wide variety of cancers. Despite their cytotoxic activity against cancer cells, the vinca alkaloids have rarely been implicated in causing clinically apparent acute liver injury.
Background
The vinca alkaloids are antineoplastic agents that act by binding to intracellular tubulin, the basic protein subunit of microtubules which are important in many intracellular processes including mitosis and cell division. The vinca alkaloids inhibit cell division by blocking mitosis; they also inhibit purine and RNA synthesis causing death of rapidly dividing cells. Vincristine and vinblastine were initially isolated from periwinkle (vinca rosea), extracts of which were found to have antitumor activity. Subsequently, they have been synthesized, although their structure is quite complex. Vinorelbine is a semisynthetic derivative of extracts of periwinkle. Vincristine (vin kris' teen) was approved for use in cancer chemotherapy in 1963, vinblastine (vin blas' teen) in 1965 and vinrelbine (vin or' el been) in 1994. Vincristine and vinblastine have become major components of many combination anticancer regimens, used particularly in treatment of acute leukemia, Hodgkin disease and other lymphomas, various sarcomas, Wilms tumor, neuroblastoma, and breast and lung cancer. Vinorelbine has had a more restricted use and is approved only for advance non-small cell lung cancer. The vinca alkaloids are given intravenously, typically at one or two week intervals in cycles with other agents. Importantly, the vinca alkaloids should only be given intravenously; if given intrathecally, they cause a progressive neurological syndrome, ascending paralysis and death. The vinca alkaloids are available in generic forms and under the trade names Oncovin (vincristine), Velban (vinblastine) and Navelbine (vinorelbine). Side effects are common and include nausea, vomiting, fatigue, headache, dizziness, peripheral neuropathy, hoarseness, ataxia, dysphagia, urinary retention, constipation, diarrhea, bone marrow suppression, alopecia and phlebitis at the infusion site. Uncommon but potential severe adverse events include severe neutropenia, bleeding, peripheral neuropathy, pulmonary toxicity, hypersensitivity reactions and embryo-fetal toxicity.
Hepatotoxicity
Despite being cytotoxic for cancer cells and metabolized actively by the liver, the vinca alkaloids have only rarely been associated with significant hepatic toxicity. Because they are usually given with other anticancer agents and/or radiotherapy, which may also be hepatotoxic, the role that they play in causing liver injury is not always clear. When given on their own, the vinca alkaloids are associated with transient and asymptomatic elevations in serum aminotransferase levels in 5% to 10% of patients. However, clinically apparent liver injury attributed to the vinca alkaloids has been rare and not well defined. Both vincristine and vinblastine may increase the risk of sinusoidal obstruction syndrome, also known as venoocclusive disease of the liver, caused by radiation, dactinomycin or the alkylating agents, but not when given on their own. In these situations, the risk of sinusoidal obstruction syndrome is greater with higher doses of radiation, dactinomycin or cyclophosphamide and younger age (in children).
Likelihood score, vincristine: C (probable rare cause of clinically apparent liver injury).
Likelihood score, vinblastine: E* (unproven but suspected rare cause of clinically apparent liver injury)
Likelihood score, vinorelbine: E* (unproven but suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The reason why the vinca alkaloids are not particularly toxic to liver cells is not known, but cell division and mitosis (the major targets of vincristine and vinblastine) are rare in the resting liver. Both agents are extensively metabolized in the liver and excreted in bile.
Outcome and Management
Serum enzyme elevations are not uncommon during cancer chemotherapy and may occasionally be dose limiting; however, the vinca alkaloids are rarely the sole or major reason for significant or persistent enzyme elevations or clinically apparent liver injury. Patients who develop sinusoidal obstruction syndrome, but recover, can be safely treated with further courses of vincristine alone or combined with reduced doses of the alkylating agent or dactinomycin.
Drug Class: Antineoplastic Agents
CASE REPORT
Case 1. Fatal sinusoidal obstruction syndrome after abdominal irradiation and vincristine therapy.(1)
A 30 year old woman with poorly differentiated lymphocytic nodular lymphoma was treated with prednisone and weekly infusions of vincristine and once weekly oral streptonigrin (an antibiotic and bioreductive antineoplastic agent). Vincristine was withdrawn after 4 cycles because of neuropathy. After 6 cycles of chemotherapy, she underwent mediastinal (2000 rads) and abdominal (2225 rads) irradiation over a two month period with another cycle of vincristine (day 88). On day 111, which was 10 days after finishing radiation therapy and 33 days after the fifth and last infusion of vincristine, she developed pancytopenia, fever, abdominal pain, ascites and jaundice. Serum aminotransferase levels were markedly elevated with only modest increases in alkaline phosphatase. The fever and pancytopenia resolved with antibiotic therapy, but she developed progressive jaundice and hepatic failure and died 4 weeks later. Autopsy showed an enlarged, congested liver with sinusoidal obstruction and severe centrilobular necrosis. The autopsy showed no residual evidence of lymphoma.
Key Points
| Medication: | Vincristine, hepatic irradiation |
|---|---|
| Pattern: | Hepatocellular (R=~100) |
| Severity: | 5+ (hepatic failure and death) |
| Latency: | 111 days after starting vincristine, 10 days after irradiation |
| Recovery: | None |
| Other medications: | Prednisone, streptonigrin |
Laboratory Values
| Days After Starting | Chemo Therapy | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| 1 | Started oral prednisone and weekly doses of vincristine and streptonigrin | ||||
| 11 | V & S | 9 | 105 | 0.8 | |
| 17 | V & S | 10 | 100 | ||
| 24 | V & S | 11 | 105 | ||
| 31 | S | 13 | 90 | ||
| 38 | S | 11 | 90 | ||
| 53 | Rad | 15 | 135 | ||
| 88 | Rad & V | 25 | 140 | ||
| 111 | Admission | 4000 | 255 | 5.8 | Jaundice, ascites, fever |
| 113 | Day 2 | 1550 | 230 | 6.0 | |
| 117 | Day 6 | 185 | 265 | 10.0 | |
| 122 | Day 11 | 88 | 405 | 20.0 | |
| 131 | Day 20 | 62 | 340 | 20.5 | |
| 141 | Day 30 | Died of hepatic failure | |||
| Normal Values | <30 | <275 | <1.2 | ||
- *
Abbreviations: Rad=radiation therapy, S=oral streptonigrin, V=vincristine infusion.
Comment
The clinical presentation with weight gain, abdominal pain, ascites and jaundice is typical of sinusoidal obstruction syndrome. Hepatic irradiation, dactinomycin, and the alkylating agents (dacarbazine, cyclophosphamide, myleran and busulfan) are most frequently associated with this form of liver injury. The injury is probably due to direct toxicity to sinusoidal lining cells with their subsequent necrosis and extrusion into the sinusoidal spaces, which causes obstruction, hemorrhage and local ischemia to hepatocytes. Signs of portal hypertension appear early, followed (in severe cases) by jaundice and hepatic failure. The dose of radiation used in this patient (<3000 rads) was not considered adequate to cause sinusoidal obstruction syndrome on its own, which suggested that vincristine might have increased the risk of this complication.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Vinblastine – Generic, Velban®
Vincristine – Generic, Oncovin®
Vinorelbine – Generic, Navelbine®
DRUG CLASS
Antineoplastic Agents
COMPLETE LABELING (Vinblastine)
COMPLETE LABELING (Vincristine)
COMPLETE LABELING (Vinorelbine)
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Vinblastine | 865-21-4 | C46-H58-N4-O9 |
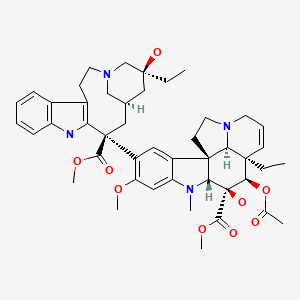
|
| Vincristine | 57-22-7 | C46-H56-N4-O10 |
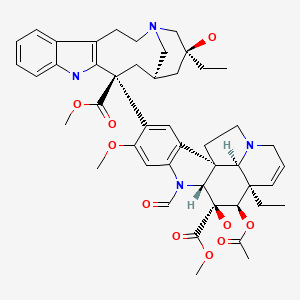
|
| Vinorelbine | 71486-22-1 | C45-H54-N4-O8 |
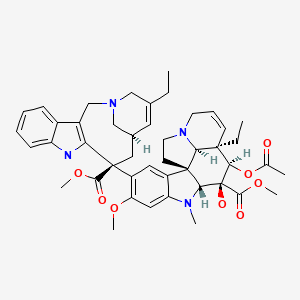
|
CITED REFERENCE
- 1.
- Hansen MM, Ranek L, Walbom S, Nissen NI. Fatal hepatitis following irradiation and vincristine. Acta Med Scand. 1982;212:171–4. [PubMed: 7148508]
ANNOTATED BIBLIOGRAPHY
References updated: 12 September 2020
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine and dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone; CHOP, chemotherapy regimen of cyclophosphamide, doxorubicin, vincristine and prednisone; G-CHOP, obinutuzumab with CHOP; R-CHOP, rituximab with CHOP; SOS, sinusoidal obstruction syndrome.
- Zimmerman HJ. The vinca alkaloids. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 692-4.(Expert review of hepatotoxicity published in 1999 mentions that vincristine and vinblastine appear to cause little hepatic injury in humans or experimental animals).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 549-68.(Review of hepatotoxicity of published in 2007; mentions that vincristine may increase the risk of sinusoidal obstruction syndrome caused by dactinomycin).
- Chabner BA, Bertino J, Clearly J, Ortiz T, Lane A, Supko JG, Ryan DP. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1677-730.(Textbook of pharmacology and therapeutics).
- Costa G, Hreshchyshyn MM, Holland JF. Initial clinical studies with vincristine. Cancer Chemother Rep. 1962;24:39–44. [PubMed: 14023264](Preliminary studies of vincristine against various cancers; dose limiting side effects included peripheral neuropathy and bone marrow suppression; 5 of 12 patients had patchy hepatic necrosis on autopsy despite having no abnormalities of liver tests before death).
- Bohannon RA, Miller DG, Diamond HD. Vincristine in the treatment of lymphomas and leukemias. Cancer Res. 1963;23:613–21. [PubMed: 13968454](Review of mechanism of action, clinical efficacy and toxicity of vincristine; AST elevations occurred in a small proportion of patients, but were not dose-limiting).
- Raine J, Bowman A, Wallendszus K, Pritchard J. Hepatopathy-thrombocytopenia syndrome–a complication of dactinomycin therapy for Wilms' tumor: a report from the United Kingdom Childrens Cancer Study Group. J Clin Oncol. 1991;9:268–73. [PubMed: 1846405](Among 355 children with Wilms tumor who received the combination of dactinomycin and vincristine, 5 developed “hepatopathy-thrombocytopenia syndrome” [bilirubin 1.3-6.1 mg/dL, ALT 335-5723 U/L], but none of 146 given vincristine alone developed this syndrome).
- Hansen MM, Ranek L, Walbom S, Nissen NI. Fatal hepatitis following irradiation and vincristine. Acta Med Scand. 1982;212:171–4. [PubMed: 7148508](30 year old woman with lymphoma developed SOS after liver irradiation and chemotherapy with vincristine [bilirubin 6.0 rising to 21.0 mg/dL, AST 4000 U/L, Alk P 250 U/L], with progressive multiorgan failure and death 4 weeks later: Case 1).
- el Saghir NS, Hawkins KA. Hepatotoxicity following vincristine therapy. Cancer. 1984;54:2006–8. [PubMed: 6090004](49 year old woman with lung cancer treated with VP-16, cyclophosphamide and vincristine developed ALT elevations [2 to 6 times ULN] a week after chemotherapy with normal bilirubin levels and liver biopsy; ALT elevations recurred when vincristine was given alone).
- Green DM, Finklestein JZ, Norkool P, D'Angio GJ. Severe hepatic toxicity after treatment with single-dose dactinomycin and vincristine. A report of the National Wilms' Tumor Study. Cancer. 1988;62:270–3. [PubMed: 2838151](5 children, ages 1 to 8 years, developed severe hepatic injury [SOS] 2-9 days after the 2nd or 3rd course of dactinomycin and vincristine, and 1 died; no recurrence when vincristine was restarted alone or with reduced and split doses of dactinomycin).
- Green DM, Norkool P, Breslow NE, Finklestein JZ, D'Angio GJ. Severe hepatic toxicity after treatment with vincristine and dactinomycin using single-dose or divided-dose schedules: a report from the National Wilms' Tumor Study. J Clin Oncol. 1990;8:1525–30. [PubMed: 2167951](Retrospective analysis of 154 children with Wilms tumor treated with dactinomycin and vincristine found higher rate of hepatotoxicity with higher single dose dactinomycin [14%] than with lower [4%] or split doses [3%]; clinical presentation with ascites, elevated AST [529 to 8208 U/L] and 50% with jaundice).
- Zhou XJ, Rahmani R. Preclinical and clinical pharmacology of vinca alkaloids. Drugs. 1992;44 Suppl 4:1–16. [PubMed: 1283846](Review of structure, mechanism of action, pharmacology, clinical efficacy and toxicities of vinca alkaloids; hepatotoxicity not discussed).
- Bisogno G, de Kraker J, Weirich A, Masiero L, Ludwig R, Tournade MF, Carli M. Veno-occlusive disease of the liver in children treated for Wilms tumor. Med Pediatr Oncol. 1997;29:245–51. [PubMed: 9251728](Among 511 children with Wilms tumor treated with dactinomycin and vincristine with or without other agents or irradiation, 64 [12%] had hepatotoxicity including 41 [8%] with SOS [all with hepatomegaly, 83% ascites, 28% jaundice, 6% fatal]; rates higher in children <1 years of age and those who received irradiation [12% vs 7%]).
- Ortega JA, Donaldson SS, Ivy SP, Pappo A, Maurer HM. Venoocclusive disease of the liver after chemotherapy with vincristine, actinomycin D, and cyclophosphamide for the treatment of rhabdomyosarcoma. A report of the Intergroup Rhabdomyosarcoma Study Group. Childrens Cancer Group, the Pediatric Oncology Group, and the Pediatric Intergroup Statistical Center. Cancer. 1997;79:2435–9. [PubMed: 9191535](Among 821 children [ages 2 to 15 years] with rhabdomyosarcoma treated with vincristine, dactinomycin and cyclophosphamide, 10 [1.2%] developed SOS and 1 died; single major risk factor was higher doses of cyclophosphamide).
- Nishihori Y, Yamauchi N, Kuribayashi K, Sato Y, Morii K, Hirayama Y, Sakamaki S, et al. Rinsho Ketsueki. 2000;41:1231–7. [Severe hemolysis and SIADH-like symptoms induced by vincristine in an ALL patient with liver cirrhosis] Japanese. [PubMed: 11193445](Abstract only: 11 year old boy with leukemia and chronic hepatitis C developed severe hemolysis and died of hepatic failure while being treated with vincristine).
- van der Hul RL, Seynaeve C, van Geel BN, Verweij J. Low dose methotrexate and vinblastine, given weekly to patients with desmoid tumours, is associated with major toxicity. Sarcoma. 2003;7:153–7. [PMC free article: PMC2395524] [PubMed: 18521380](Among 10 patients with desmoid tumors given methotrexate [50 mg/week] and vinblastine [10 mg/week], severe side effects were common and 2 developed transient ALT elevations [3 to 10 times ULN] without jaundice).
- Arndt C, Hawkins D, Anderson JR, Breitfeld P, Womer R, Meyer W. Age is a risk factor for chemotherapy-induced hepatopathy with vincristine, dactinomycin, and cyclophosphamide. J Clin Oncol. 2004;22:1894–901. Erratum in: J Clin Oncol 2004; 22: 3434. [PubMed: 15143082](Among 339 children with rhabdomyosarcoma treated with vincristine, dactinomycin and cyclophosphamide, 18 [6%] developed hepatotoxicity and 3 died [1%]; risk of liver injury was greater in children <3 years of age [15% vs 4%]).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury and 2 to antineoplastic agents [melphalan and gemtuzumab], but none to vincristine or vinblastine).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contains 9036 hepatic adverse drug reactions in children; vincristine accounted for 46 instances, ranking 26th).
- Langholz B, Skolnik JM, Barrett JS, Renbarger J, Seibel NL, Zajicek A. Arndt CAs. Dactinomycin and vincristine toxicity in the treatment of childhood cancer: a retrospective study from the Children's Oncology Group. Pediatr Blood Cancer. 2011;57:252–7. [PMC free article: PMC3467305] [PubMed: 21671362](Retrospective review of six pediatric cancer clinical trials, comprising 4567 patients; the review found an overall risk for hepatotoxicity due to dactinomycin to be between 10-15%, depending upon age and type of tumor being treated; patients <1 year of age were at greater hepatotoxicity risk; vincristine was not associated with liver injury in this review).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to a vinca alkaloid).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to a vinca alkaloid).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 363 [36%] were attributed to antibiotics, only one of which was attributed to a vinca alkaloid, vincristine in a 42 year old woman with HIV infection and lymphoma treated with combination chemotherapy and developing jaundice within a few weeks, bilirubin rising to 17.9, ALT 82 U/L, Alk P 265 U/L; resolving and later tolerating the other drugs in the combination).
- Wang J, Zheng R, Wang Z, Yang Y, Wang M, Zou W. Efficacy and safety of vinorelbine plus cisplatin vs. gemcitabine plus cisplatin for treatment of metastatic triple-negative breast cancer after failure with anthracyclines and taxanes. Med Sci Monit. 2017;23:4657–64. [PMC free article: PMC5629993] [PubMed: 28957036](Among 48 patients with refractory metastatic breast cancer treated with cisplatin combined with either vinorelbine or gemcitabine, objective response rates were similar [46%], as was time to progression and adverse events; mild [grade 1 or 2] “hepatic dysfunction” arose in 27% of both groups and did not require dose modification or progress to clinically apparent liver injury).
- Eslami A, Mathur AD, Jha KK, Wang H. Acute liver failure secondary to ABVD use. BMJ Case Rep. 2018;2018:bcr2018225474. [PMC free article: PMC6067149] [PubMed: 30061135](79 year old woman with Hodgkin disease was found unconscious 3 hours after completing a first cycle of ABVD [doxorubicin, bleomycin, vinblastine and dacarbazine], with liver injury arising over the next 24 hours [bilirubin 2.6 mg/dL on day 2, ALT 980 U/L, Alk P 126, lactate 5.3 mmol/L], with rapid recovery).
- Tanoshima R, Khan A, Biala AK, Trueman JN, Drögemöller BI, Wright GEB, Hasbullah JS, et al. Canadian Pharmacogenomics Network for Drug Safety Consortium. Analyses of adverse drug reactions-nationwide active surveillance network: Canadian Pharmacogenomics Network for Drug Safety Database. J Clin Pharmacol. 2019;59:356–63. [PubMed: 30452777](Among 10,475 adverse drug reaction reports made to a Canadian pharmacovigilance registry between 2005 and 2017 from 26 medical centers, 73% were pediatric and the most commonly implicated drugs were antineoplastic agents, including methotrexate, vincristine, doxorubicin, cisplatin, L-asparaginase, cyclophosphamide, dexamethasone, cytarabine and etoposide, hepatotoxicity being the 10th most common type of reaction [194 cases]).
- Peng S, Yang K, Xu Z, Chen S, Ji Y. Vincristine and sirolimus in the treatment of kaposiform haemangioendothelioma. J Paediatr Child Health. 2019;55:1119–24. [PubMed: 30604513](Metaanalysis of trials of vincristine and sirolimus for Kaposiform hemangioendothelioma identified 5 studies in 75 patients of vincristine, with adverse event reports from 16 subjects, the most common events being neuropathy [38%], abdominal pain [19%], anorexia [6%] and transient ALT elevations [12%]; no mention of clinically apparent liver injury).
- Younes A, Sehn LH, Johnson P, Zinzani PL, Hong X, Zhu J, Patti C, et al. PHOENIX investigators. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37:1285–95. [PMC free article: PMC6553835] [PubMed: 30901302](Among 838 patients with large B cell lymphoma treated with R-CHOP with or without ibrutinib, overall survival was not improved by ibrutinib, but serious side effects were greater [53% vs 34%]; no mention of ALT elevations or hepatotoxicity).
- Poeschel V, Held G, Ziepert M, Witzens-Harig M, Holte H, Thurner L, Borchmann P, et al. FLYER Trial Investigators. German Lymphoma Alliance. Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): a randomised, phase 3, non-inferiority trial. Lancet. 2019;394(10216):2271–81. [PubMed: 31868632](Among 592 patients with B cell non-Hodgkin lymphoma treated with 6 cycles of R-CHOP vs 4 cycles with 2 further cycles of rituximab alone, progression free survival was similar in the two groups while adverse events were more with the 6 cycles; no mention of ALT elevations or hepatotoxicity).
- Brühwiler LD, Schwappach DL. Safe vincristine use in Switzerland: still a long way to go? J Oncol Pharm Pract. 2020;26:51–9. [PubMed: 30866715](The medical error of administration of vincristine intrathecally instead of intravenously leads to progressive decerebration and death within a few days, and international recommendations to prevent this error have not been rigorously adopted as shown by surveys of pharmaceutical services in Switzerland).
- Sehn LH, Martelli M, Trněný M, Liu W, Bolen CR, Knapp A, Sahin D, et al. A randomized, open-label, Phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-Cell lymphoma: final analysis of GOYA. J Hematol Oncol. 2020;13:71. [PMC free article: PMC7276080] [PubMed: 32505213](Among 1414 patients with large B cell lymphoma treated with CHOP and either rituximab [R-CHOP] or obinutuzumab [G-CHOP] [both monoclonal antibodies to CD20], progression-free survival rates were similar [64% and 63%] as were total adverse events, but serious events were more frequent with G-CHOP [44% vs 38%]; no mention of ALT elevations or hepatotoxicity).
- Dalal M, Gupta J, Price K, Zomas A, Miao H, Ashaye A. Efficacy and safety of front-line treatments for advanced Hodgkin lymphoma: a systematic literature review. Expert Rev Hematol. 2020 Aug 4;:1–16. Epub ahead of print. [PubMed: 32749937](Systematic review of first line therapies of advance Hodgkin disease focused on ABVD [doxorubicin, bleomycin, vinblastine and dacarbazine] and BEACOPP [bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone], discussing side effects of pulmonary toxicity, secondary malignancies, neutropenia and peripheral neuropathy but not hepatotoxicity or ALT elevations).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Interaction of vinca alkaloids with tubulin: a comparison of vinblastine, vincristine, and vinorelbine.[Biochemistry. 1996]Interaction of vinca alkaloids with tubulin: a comparison of vinblastine, vincristine, and vinorelbine.Lobert S, Vulevic B, Correia JJ. Biochemistry. 1996 May 28; 35(21):6806-14.
- The Biological Activity of the Novel Vinca Alkaloids 4-chlorochablastine and 4-chlorochacristine.[Curr Cancer Drug Targets. 2019]The Biological Activity of the Novel Vinca Alkaloids 4-chlorochablastine and 4-chlorochacristine.Montag G, Stopper H, Ngo QA, Hintzsche H. Curr Cancer Drug Targets. 2019; 19(3):222-230.
- Kinetic analysis in living cells of the inhibition of the P-glycoprotein-mediated efflux of anthracyclines by vinca alkaloids.[Chem Biol Interact. 1998]Kinetic analysis in living cells of the inhibition of the P-glycoprotein-mediated efflux of anthracyclines by vinca alkaloids.Pereira E, Tarasiuk J, Garnier-Suillerot A. Chem Biol Interact. 1998 Jul 3; 114(1-2):61-76.
- Review Vinca alkaloids as a potential cancer therapeutics: recent update and future challenges.[3 Biotech. 2023]Review Vinca alkaloids as a potential cancer therapeutics: recent update and future challenges.Banyal A, Tiwari S, Sharma A, Chanana I, Patel SKS, Kulshrestha S, Kumar P. 3 Biotech. 2023 Jun; 13(6):211. Epub 2023 May 24.
- Review Vinca alkaloids.[Int J Prev Med. 2013]Review Vinca alkaloids.Moudi M, Go R, Yien CY, Nazre M. Int J Prev Med. 2013 Nov; 4(11):1231-5.
- Vinca Alkaloids - LiverToxVinca Alkaloids - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...