NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Voclosporin is an orally available calcineurin inhibitor and potent immunosuppressive agent which is used in combination with mycophenolate mofetil and corticosteroids to treat acute lupus nephritis. Voclosporin may be associated with low rate of transient serum enzyme elevations during treatment but has not been linked to instances of clinically apparent acute liver injury with jaundice.
Background
Voclosporin (voe” kloe spor’ in) is a potent immunosuppressive agent that is used as therapy for patients with systemic lupus erythematosus and acute lupus nephritis. Voclosporin is a derivative of cyclosporin A, a cyclic polypeptide of 11 amino acids, initially isolated from a fungus species (Beauveria nivea). Cyclosporine and voclosporin have potent immunosuppressive activity, acting as calcineurin inhibitors which block T cell activation decreasing cytokine secretion and immunologically mediated cell injury. Voclosporin differs from cyclosporine by a single amino acid, but appears to have a higher degree of selectivity and somewhat more potency. Voclosporin has been developed as an immunomodulatory agent for therapy of autoimmune conditions rather than as an antirejection agent for organ transplantation. In several clinical trials, voclosporin in combination with mycophenolate mofetil and low doses of corticosteroids was shown to alleviate the renal injury in acute lupus nephritis, decreasing proteinuria and preventing major worsening of estimated glomerular filtration rate (eGFR). Voclosporin was approved for use in adults with acute lupus nephritis in the United States in 2021. It is available in capsules of 7.9 mg under the brand name Lupkynis. The typical dose is 27.7 mg (3 capsules) twice daily used in combination with mycophenolate mofetil (2 grams daily) and high dose corticosteroids followed by a rapid taper to maintenance dosing. Therapy requires careful monitoring for renal function and should be administered only by physicians with expertise in management of lupus nephritis and complications of calcineurin inhibitor therapy. Common side effects include nausea, diarrhea, abdominal discomfort, dizziness and paresthesias. Voclosporin can also cause worsening of renal function and hypertension, and dose adjustments are often required (in up to 50% of cases). Less common but potentially severe adverse events include increased risk of severe infections and malignancies, drug-drug interactions, prolongation of the QT interval, hyperkalemia and neurotoxicity.
Hepatotoxicity
In large randomized controlled trials of voclosporin, serum aminotransferase levels were transiently elevated in 3% of patients, but rates were similar in controls receiving conventional therapy (2%). Similar to cyclosporine, voclosporin therapy is associated with minor increases in alkaline phosphatase and bilirubin levels, although values usually remain within the normal range. In the prelicensure studies of voclosporin, there were no instances of clinically apparent liver injury attributable to therapy. Clinical experience with this agent, however, has been limited and other calcineurin inhibitors have been found to cause rare instances of hepatic injury, which are generally cholestatic, mild-to-moderate in severity and self-limited in course once treatment is stopped.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which voclosporin might cause liver injury is unknown. Other calcineurin inhibitors such as cyclosporine and tacrolimus are associated with serum enzyme elevations and rare instances of cholestatic liver injury. Voclosporin is metabolized in the liver, largely by CYP 3A4 and is susceptible to drug-drug interactions, particularly with strong inducers (such as rifampin and St. John's wort) or strong inhibitors of CYP 3A4 (such as ketoconazole) which should be avoided.
Outcome and Management
Serum enzyme elevations during therapy with voclosporin are generally mild and without accompanying symptoms or jaundice. Appearance of liver injury during therapy should lead to a search for other possible causes. But if none are found, dosing should be interrupted if values rise above 5 times the upper limit of normal (ULN), with caution in restarting if they subsequently fall into the normal range.
Drug Class: Immunosuppressive Agents
Other Drugs in the Subclass, Calcineurin Inhibitors: Cyclosporine, Tacrolimus
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Voclosporin – Lupkynis®
DRUG CLASS
Immunosuppressive Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Voclosporin | 515814-01-4 | C63-H111-N11-O12 |
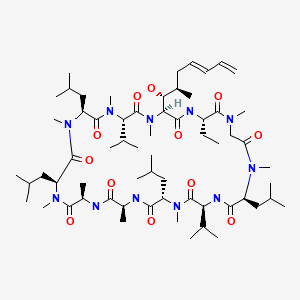
|
| Cyclosporine | 59865-13-3 | C62-H111-N11-O12 |
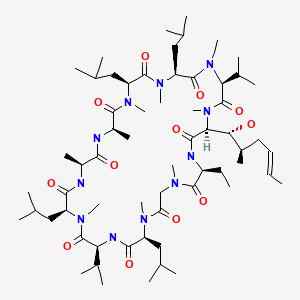
|
ANNOTATED BIBLIOGRAPHY
References updated: 30 August 2021
Abbreviations used: eGFR, estimated glomerular filtration rate.
- Zimmerman HJ. Hepatotoxic effects of oncotherapeutic and immunosuppressive agents. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 673-708.(Expert review of hepatotoxicity published in 1999; voclosporin is not mentioned but cyclosporine is discussed; cyclosporine therapy is associated with a high rate of cholestatic liver enzyme elevations ranging from 4-86% and has been linked to occasional instances of cholestatic hepatitis, some features of which are reproducible in animal models).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2021/213716Orig1s000MultidisciplineR.pdf. (FDA Drug Approvals website that has product labels [package inserts], letters of approval and full FDA integrated review of the voclosporin application for safety and efficacy which mentions [pages 202-3] that mean values of ALT, Alk P, GGT and bilirubin remained in the normal range although mean levels of Alk P and bilirubin increased slightly during therapy; the rate of ALT or AST elevations above 3 times ULN was similar in incidence to that in placebo recipients [3% vs 2%] and were transient and asymptomatic, except for one case of liver injury with jaundice in a patient with pulmonary tuberculosis who was receiving antimycobacterial therapy). - Bissonnette R, Papp K, Poulin Y, Lauzon G, Aspeslet L, Huizinga R, Mayo P, et al. ISA247 Psoriasis Study Group. A randomized, multicenter, double-blind, placebo-controlled phase 2 trial of ISA247 in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2006;54:472–8. [PubMed: 16488299](Among 201 adults with plaque psoriasis treated with voclosporin [0.5 mg/kg or 1.5 mg/kg] or placebo daily for 12 weeks, improvements in psoriasis occurred in 15% and 45% of voclosporin vs 0% of placebo recipients, and adverse events more frequent with voclosporin included nausea, diarrhea, abdominal pain, vomiting, dizziness, and paresthesias, while there were “no significant changes” in hepatic laboratory features).
- Busque S, Cantarovich M, Mulgaonkar S, Gaston R, Gaber AO, Mayo PR, Ling S, et al. PROMISE Investigators. The PROMISE study: a phase 2b multicenter study of voclosporin (ISA247) versus tacrolimus in de novo kidney transplantation. Am J Transplant. 2011;11:2675–84. [PubMed: 21943027](Among 334 patients undergoing renal transplantation treated with either voclosporin [3 doses] or tacrolimus for 24 weeks, rates of biopsy proven acute cellular rejection were comparable in the 4 groups as were adverse event rates).
- Rovin BH, Solomons N, Pendergraft WF 3rd, Dooley MA, Tumlin J, Romero-Diaz J, Lysenko L, et al. AURA-LV Study Group. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 2019;951:219–231. [PubMed: 30420324](Among 265 patients with active lupus nephritis treated with voclosporin [23.7 mg or 39.5 mg] vs placebo twice daily for 48 weeks, complete renal responses occurred in 49% and 40% vs 24%, while serious adverse events were more frequent with voclosporin [28% and 25% vs 16%] as were infections [12% and 14% vs 8%] and all-cause mortality [11% and 2.3% vs 1%]).
- Heo YA. Voclosporin: first approval. Drugs. 2021;81:605–610. [PMC free article: PMC8010786] [PubMed: 33788181](Review of the mechanism of action, history of development, clinical efficacy and safety of voclosporin shortly after its approval for use in lupus nephritis mentions common adverse events as being transient decline in GFR, hypertension, headache, anemia and cough).
- Rovin BH, Teng YKO, Ginzler EM, Arriens C, Caster DJ, Romero-Diaz J, Gibson K, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021;397(10289):2070–2080. [PubMed: 33971155](Among 457 patients with active lupus nephritis treated with voclosporin [23.7 mg] or placebo twice daily for 52 weeks, “complete renal response” rates were higher with voclosporin [41% vs 23%], while serious adverse events were similar [21% vs 21%] and deaths were fewer in number [1 vs 5]).
- Voclosporin (Lupkynis) for lupus nephritis. Med Lett Drugs Ther. 2021;63(1631):134–6. [PubMed: 34544103](Concise summary of the mechanism of action, clinical efficacy, safety and costs of voclosporin, a calcineurin inhibitor approved for lupus nephritis mentions that therapy can increase the risk of bacterial, viral and fungal infections, can prolong the QT interval, and can cause hyperkalemia and neurotoxicity; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Voclosporin: A Novel Calcineurin Inhibitor for the Treatment of Lupus Nephritis.[Ann Pharmacother. 2022]Voclosporin: A Novel Calcineurin Inhibitor for the Treatment of Lupus Nephritis.McArn AC, Nixon AR, Jarrell KL. Ann Pharmacother. 2022 Feb 15; :10600280221075331. Epub 2022 Feb 15.
- Review Voclosporin: a novel calcineurin inhibitor for the treatment of lupus nephritis.[Expert Rev Clin Pharmacol. 2022]Review Voclosporin: a novel calcineurin inhibitor for the treatment of lupus nephritis.van Gelder T, Lerma E, Engelke K, Huizinga RB. Expert Rev Clin Pharmacol. 2022 May; 15(5):515-529. Epub 2022 Jul 11.
- A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis.[Kidney Int. 2019]A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis.Rovin BH, Solomons N, Pendergraft WF 3rd, Dooley MA, Tumlin J, Romero-Diaz J, Lysenko L, Navarra SV, Huizinga RB, AURA-LV Study Group. Kidney Int. 2019 Jan; 95(1):219-231. Epub 2018 Nov 9.
- Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial.[Lancet. 2021]Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial.Rovin BH, Teng YKO, Ginzler EM, Arriens C, Caster DJ, Romero-Diaz J, Gibson K, Kaplan J, Lisk L, Navarra S, et al. Lancet. 2021 May 29; 397(10289):2070-2080. Epub 2021 May 7.
- Review Voclosporin: a novel calcineurin inhibitor for the management of lupus nephritis.[Expert Rev Clin Immunol. 2021]Review Voclosporin: a novel calcineurin inhibitor for the management of lupus nephritis.Mejía-Vilet JM, Romero-Díaz J. Expert Rev Clin Immunol. 2021 Sep; 17(9):937-945. Epub 2021 Aug 25.
- Voclosporin - LiverToxVoclosporin - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...