NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Tacrolimus is a calcineurin inhibitor and potent immunosuppressive agent used largely as a means of prophylaxis against cellular rejection after transplantation. Tacrolimus therapy can be associated with mild serum enzyme elevations, and it has been linked to rare instances of clinically apparent cholestatic liver injury.
Background
Tacrolimus (ta kroe' li mas), also previously known as FK506, is a macrolide antibiotic which also has profound immunosuppressive properties, particularly affecting T cells and the cellular immune response. Tacrolimus acts as a calcineurin inhibitor which is responsible for activating an important signal transduction molecule in the pathway of T cell activation. Inhibition of this pathway results in a decrease in maturation of T lymphocytes and reduction in lymphokine production, including IL-2. Tacrolimus was approved for use in the United States in 1994 and rapidly became an important part of the primary regimen of immunosuppression after allogenic transplantation. Current indications are for prevention of organ rejection after transplantation. It is also used off label as therapy of active and recalcitrant forms of autoimmune diseases. Tacrolimus is available as capsules of 0.5, 1 and 5 mg in several generic forms and under the brand name of Prograf. It is also available in extended release forms and as a solution for intravenous administration (5 mg/mL). Because of variability in individual pharmacokinetics of tacrolimus, the maintenance dose varies greatly and proper dosing requires monitoring for drug levels, which is also important because of its many dose dependent side effects and drug-drug interactions. Tacrolimus should be used only by physicians with experience in immunosuppressive therapy and its complications. Tacrolimus is also available in topical forms for ophthalmologic and dermatologic conditions, as eye drops, creams ointments and lotions. Common side effects of oral and parenteral tacrolimus include headache, dizziness, paresthesias, neuropathy, hypertension, nephropathy, diabetes, acne, hirsutism and opportunistic infections. Less common but potentially severe adverse events include diabetes, renal failure, hyperkalemia, hypertension, severe neuropathy, blood dyscrasias, microangiopathy and anaphylactic reactions.
Hepatotoxicity
Tacrolimus therapy is associated with mild to moderate elevations in serum aminotransferase levels in 5% to 10% of patients. These elevations are usually mild, asymptomatic and self-limited, but are occasionally persistent and may require dose modification. Tacrolimus has also been implicated in instances of cholestatic hepatitis, but clinically apparent liver injury is rare. Because tacrolimus is used in the context of organ transplantation and often in liver transplantation, the causes of liver test abnormalities arising during therapy are many, and drug induced liver injury due to tacrolimus is sufficiently rare that its clinical features and typical course have not been defined.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
Tacrolimus undergoes extensive hepatic metabolism largely via the cytochrome P450 system (CYP 3A4) and is susceptible to many drug-drug interactions. Liver test abnormalities during therapy may be due to direct hepatotoxicity, its effects on levels of other medications, or its effects on the immune system.
Outcome and Management
The liver injury due to tacrolimus is usually mild and self-limiting and responses rapidly to dose adjustment or drug discontinuation. Other calcineurin inhibitors and immunosuppressants are generally tolerated, but rare instances of cross sensitivity to hepatic injury by cyclosporine and tacrolimus have been reported.
Agents used specifically for the prophylaxis against allograft rejection include cyclosporine, mycophenolate mofetil, sirolimus and tacrolimus, as well as azathioprine and corticosteroids.
Drug Class: Transplant Agents; Antirheumatic Agents, Major Immunosuppressive Agents
Other Drugs in the Class, Transplant Agents: Cyclosporine, Mycophenolate, Sirolimus
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Tacrolimus – Generic, Prograf®
DRUG CLASS
Transplant Agents
Product labeling at DailyMed, National Library of Medicine, NIH
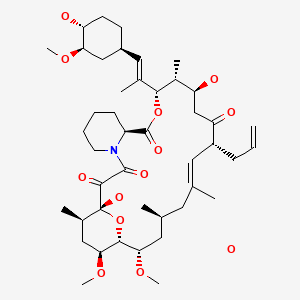
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Tacrolimus | 109581-93-3 | C44-H69-N-O12.H2-O |
|
ANNOTATED BIBLIOGRAPHY
References updated: 17 February 2020
- Zimmerman HJ. Cyclosporine. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 697-8.(Expert review of hepatotoxicity published in 1999; cyclosporine therapy was associated with a high rate of cholestatic liver enzyme elevations ranging from 4-86% and occasional instances of cholestatic hepatitis, some features of which were reproducible in animal models; tacrolimus, sirolimus, and mycophenolate are not discussed).
- Reuben A. Hepatotoxicity of immunosuppressive drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 569-92.(Review of hepatotoxicity of immunosuppressive agents mentions that reports of hepatotoxicity of cyclosporine have decreased since the 1980s, perhaps because of monitoring of serum levels and lower doses used; liver injury from tacrolimus, sirolimus and mycophenolate is rare and usually rapidly reversible).
- Krensky AM, Azzi JR, Hafler DA. Immnosuppressants and tolerogens. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 637-54.(Textbook of pharmacology and therapeutics).
- Mor E, Sheiner PA, Schwartz ME, Emre S, Guy S, Miller CM. Reversal of severe FK506 side effects by conversion to cyclosporine-based immunosuppression. Transplantation. 1994;58:380–2. [PubMed: 7519800](Severe side effects of tacrolimus therapy requiring dose modification or switching to cyclosporine occurred in 15 of 90 patients [17%] including nephropathy, diabetes, and neuropathy, but not hepatotoxicity).
- Fisher A, Mor E, Hytiroglou P, Emre S, Boccagni P, Chodoff L, Sheiner P, et al. FK506 hepatotoxicity in liver allograft recipients. Transplantation. 1995;59:1631–2. [PubMed: 7539961](Among 50 liver transplant recipients treated with tacrolimus, 6 had liver test abnormalities not explained by rejection or hepatitis, which resolved or improved on lowering dose or switching rejection therapy [bilirubin levels not given, ALT 121-744 U/L, Alk P 68-1293 U/L], biopsies showed centrolobular dropout).
- Kowdley KV, Keeffe EB. Hepatotoxicity of transplant immunosuppressive agents. Gastroenterol Clin North Am. 1995;24:991–1001. [PubMed: 8749908](Review of reports of hepatotoxicity from cyclosporine in form of mild hyperbilirubinemia, mild-to-moderate serum enzyme elevations and biliary sludge and stones).
- Tsamandas AC, Jain AB, Felekouras ES, Fung JJ, Demetris AJ, Lee RG. Central venulitis in the allograft liver: a clinicopathologic study. Transplantation. 1997;64:252–7. [PubMed: 9256183](Among 27 patients with central venulitis receiving cyclosporine or tacrolimus, there was no association with drug levels and the histological lesion was attributed to rejection rather than drug induced liver injury).
- Yuan QS, Zheng FL, Sun Y, Yu Y, Li Y. Rescue therapy with tacrolimus in renal graft patients with cyclosporine A-induced hepatotoxicity: a preliminary study. Transplant Proc. 2000;32:1694–5. [PubMed: 11119896](7 renal transplant recipients with suspected cyclosporine hepatotoxicity were switched to tacrolimus, ALT levels decreased from 28-119 to 24-43 U/L; no mention of bilirubin, Alk P or symptoms).
- Emre S, Genyk Y, Schluger LK, Fishbein TM, Guy SR, Sheiner PA, Schwartz ME, et al. Treatment of tacrolimus-related adverse effects by conversion to cyclosporine in liver transplant recipients. Transpl Int. 2000;13:73–8. [PubMed: 10743694](Among 388 liver transplant recipients treated with tacrolimus, 70 required conversion to cyclosporine because of side effects, including 6 for late hepatotoxicity, often for steatohepatitis or recurrent autoimmune hepatitis, with improvement in 4).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 270 [0.5%] were done for drug induced acute liver failure, 124 for acetaminophen and 137 for other drugs or toxins, but none for agents used to prevent transplant rejection).
- Shah S, Budev M, Blazey H, Fairbanks K, Mehta A. Hepatic veno-occlusive disease due to tacrolimus in a single-lung transplant patient. Eur Respir J. 2006;27:1066–8. [PubMed: 16707401](60 year old woman developed sinusoidal obstruction syndrome 18 months after lung transplant, which the authors attributed to tacrolimus therapy).
- Taniai N, Akimaru K, Ishikawa Y, Kanada T, Kakinuma D, Mizuguchi Y, Mamada Y, et al. Hepatotoxicity caused by both tacrolimus and cyclosporine after living donor liver transplantation. J Nippon Med Sch. 2008;75:187–91. [PubMed: 18648179](56 year old liver transplant recipient developed fluctuating ALT elevations [averaging 200-300 U/L] and bilirubin elevations [peak 11 mg/dL] that were not explained by rejection or infection and appeared to respond transiently to changing from tacrolimus to cyclosporine and then recurring, ultimately treated with lower doses of tacrolimus and mycophenolate).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, none were attributed to cyclosporine, tacrolimus, sirolimus or mycophenolate).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none to an agent used to prevent transplant rejection).
- Czubkowski P, Pawłowska J, Jankowska I, Teisseyre M, Kamińska D, Markiewicz M, Ryżko J. Successful sirolimus rescue in tacrolimus-induced thrombotic microangiopathy after living-related liver transplantation. Pediatr Transplant. 2012;16:E261–4. [PubMed: 22066835](One year old girl with biliary atresia developed tacrolimus induced microangiopathy [hemoglobin 5.6 mg/dL, indirect bilirubin 8.8 mg/dL, ALT 419 U/L, GGT 118 U/L] 8 months after living donor liver transplantation, which resolved after switching to sirolimus).
- Mesar I, Kes P, Hudolin T, Basic-Jukic N. Rescue therapy with sirolimus in a renal transplant recipient with tacrolimus-induced hepatotoxicity. Ren Fail. 2013;35:1434–5. [PubMed: 24028307](54 year old man with end stage renal disease developed evidence liver injury 11 days after transplant and starting tacrolimus, mycophenolate, corticosteroids and basiliximab [bilirubin 0.8 mg/dL, ALT 601 rising to 1242 U/L, GGT 144 to 212], rapidly resolving when he was switched to sirolimus).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to an anti-rejection agent used in transplantation).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, one was attributed to mycophenolate but none to cyclosporine, sirolimus or tacrolimus).
- Ko MS, Choi YH, Jung SH, Lee JS, Kim HS, Lee CH, Kim SG. Tacrolimus therapy causes hepatotoxicity in patients with a history of liver disease. Int J Clin Pharmacol Ther. 2015;53:363–71. [PubMed: 25740263](Analysis of electronic medical records from 2462 Korean patients with a history of liver taking tacrolimus or cyclosporine from 2002-2008, found that peak ALT levels were higher [50 vs 41 U/L] and time to peak was shorter [101 vs 142 days] in the tacrolimus treated patients).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, one case was attributed to cyclosporine, but none to tacrolimus, sirolimus or mycophenolate mofetil).
- Pandey N, Gupta AK, Gupta S. Tacrolimus-associated jaundice. Am J Ther. 2018;25:e723–e725. [PubMed: 29672330](46 year old man with nonalcoholic hepatitis and cirrhosis developed jaundice 6 days after liver transplant).
- Fiore M, Leone S, Maraolo AE, Berti E, Damiani G. Liver Illness and Psoriatic Patients. Biomed Res Int. 2018;2018:3140983. [PMC free article: PMC5818942] [PubMed: 29546055](Review of liver disease in psoriasis including drug induced liver injury due to methotrexate, acitretin, TNF inhibitors, cyclosporine, and leflunomide).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Cyclosporine.[LiverTox: Clinical and Researc...]Review Cyclosporine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Sirolimus.[LiverTox: Clinical and Researc...]Review Sirolimus.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Everolimus.[LiverTox: Clinical and Researc...]Review Everolimus.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Transplant Agents.[LiverTox: Clinical and Researc...]Review Transplant Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Calcineurin inhibitor minimisation versus continuation of calcineurin inhibitor treatment for liver transplant recipients.[Cochrane Database Syst Rev. 2012]Review Calcineurin inhibitor minimisation versus continuation of calcineurin inhibitor treatment for liver transplant recipients.Penninga L, Wettergren A, Chan AW, Steinbrüchel DA, Gluud C. Cochrane Database Syst Rev. 2012 Mar 14; (3):CD008852. Epub 2012 Mar 14.
- Tacrolimus - LiverToxTacrolimus - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...