NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Treadwell J, Mitchell M, Eatmon K, et al. Imaging Tests for the Diagnosis and Staging of Pancreatic Adenocarcinoma [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Sep. (Comparative Effectiveness Review, No. 141.)

Imaging Tests for the Diagnosis and Staging of Pancreatic Adenocarcinoma [Internet].
Show detailsIntroduction
In this chapter, we describe the results of the literature searches, and then present the results for each KQ.
A list of acronyms and abbreviations is available following the list of references for this report, along with a glossary of selected terms. The Appendixes include Appendix A, Search Strategy; Appendix B, Full-length Review of Excluded Studies; Appendix C, Evidence Tables; Appendix D, Analyses and Risk of Bias Assessments, and Appendix E, Sensitivity Analyses for Meta-analyses Involving Multiple Readers per Study. In Appendix C:
- The tables of comparative accuracy studies appear next, involving KQ1b and KQ2b. The first two tables present general study characteristics and patient characteristics, and are sorted in reverse chronology and then by last name of the first author. The next five tables provide test details, and are also sorted in reverse chronology and then by last name of the first author. The final table provides all the comparative accuracy data, and is first sorted by the component being assessed (e.g., vessel involvement), then by test comparison (e.g., MDCT vs. EUS-FNA), and finally by reverse chronology.
- The tables of harms studies appear next, involving KQ3, and are sorted in reverse chronology and then by last name of the first author. The first four tables summarize the pancreas-specific studies, and the others summarize the non-pancreas-specific studies.
- The tables of screening studies appear last, involving KQ4. and are sorted in reverse chronology and then by last name of the first author. The tables summarize general study characteristics, patient information, test details, and data.
Our quantitative analyses of comparative accuracy are summarized in Appendix D, along with the risk-of-bias assessments.
Results of Literature Searches
We summarize the study selection process in Figure 2 below (as recommended by Moher et al.[2009]).29 The literature searches identified 9,776 citations, and after duplicate review, we excluded 9,036 of them. The most common reason for exclusion was that the article did not involve diagnosis, staging, screening, or harms. We retrieved the other 740 articles in full, and after duplicate review, we excluded 610 of those. The most common reason was that the study reported data on only a single imaging test of interest and did not meet inclusion criteria for other KQs. See Appendix B for a list of the publications excluded at the full article level. We included the remaining 130 publications, which described 123 unique studies/reviews (7 publications reported overlapping patients). Of the 123, 15 were systematic reviews and 108 were studies.
We sent scientific information packet (SIP) letters and emails to the 11 identified relevant industry stakeholders requesting submission of published and unpublished information on their product(s). Additionally, a U.S. Federal Register notice was posted on August 27, 2013, requesting scientific information submissions (https://federalregister.gov/a/2013-20849). Two responses were subsequently received, and both responses indicated that the sender did not know of any pertinent studies.
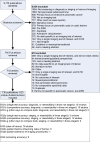
Figure 2
Literature flow diagram. Note: the numbers in the box above add to more than 123 because some studies/reviews addressed multiple Key Questions.
Test Performance of Imaging Modalities for Diagnosis
- KQ1.
Comparative Effectiveness of Imaging Techniques for Diagnosis
- KQ1a.
What is the accuracy of each imaging technique for diagnosis and assessment of resectability?
Key Points
- Evidence was insufficient to permit accuracy estimates for multidetector computed tomography (MDCT) angiography with or without three-dimensional (3D) reconstruction.
- For diagnosis using endoscopic ultrasound with fine-needle aspiration (EUS-FNA), four high-quality and recent systematic reviews yielded sensitivity estimates ranging from 85 percent to 93 percent and specificity estimates ranging from 94 percent to 100 percent. (Strength of evidence from published systematic reviews was not graded.)
- For diagnosis using magnetic resonance imaging (MRI), three systematic reviews yielded sensitivity estimates of 84 percent to 86 percent and specificity estimates of 82 percent to 91 percent. (Strength of evidence from published systematic reviews was not graded.)
- For diagnosis using positron emission tomography–computed tomography (PET/CT), three systematic reviews yielded sensitivity estimates of 87 percent to 90 percent and specificity estimates of 80 percent to 85 percent. (Strength of evidence from published systematic reviews was not graded.)
- For MDCT, in assessing the resectability of tumors in patients with unstaged disease, one systematic review yielded a sensitivity estimate of 81 percent (95% CI, 76% to 85%) and a specificity estimate of 82 percent (95% CI, 77% to 97%). (Strength of evidence from published systematic reviews was not graded.)
- For MRI, in assessing the resectability tumors in patients with unstaged disease, one systematic review yielded a sensitivity estimate of 82 percent (95% CI, 69% to 91%) and a specificity estimate of 78 percent (95% CI, 63% to 87%). (Strength of evidence from published systematic reviews was not graded.)
Detailed Synthesis
Thirteen systematic reviews44–56 met the inclusion criteria for this question, of which four were both recent (published 2009 or later) and of high quality (meeting all eight of the quality criteria deemed most important). All of the reviews are summarized in Appendix C, and their quality assessments are in Appendix D. The four recent high-quality reviews included only evidence on EUS-FNA and not on any of the other diagnostic modalities. The total number of included studies and patients for the four imaging technologies were:
- EUS-FNA: 58 studies, ~7,862 patients (precise counts not calculable because some reviews reported the study Ns differently)
- CT: At least 29 papers, at least 1,823 patients (precise counts not calculable due to unknown overlap between diagnostic and resectability studies)
- MRI: 25 papers, at least 1,169 patients (precise counts not calculable because one review did not provide sufficient information)
Diagnosis
For EUS-FNA in diagnosing pancreatic cancer, we included eight reviews,44–47,52,54,55,57 and of these, the four recent high-quality reviews44,46,47,57 reported summary sensitivity results ranging from 85 percent to 93 percent and summary specificity results ranging from 94 percent to 100 percent (see Appendix C).A threshold effect was apparent, as the reviews reporting the highest specificities were also the ones reporting the lowest sensitivities. A threshold effect was also seen within Madhoun’s review (2013),44 as FNA with a 25-gauge needle resulted in higher sensitivity and lower specificity than FNA with a 22-gauge needle. The difference in sensitivity was statistically significant; the difference in specificity was not.
CT was addressed in only one review,53 which was deemed not of high quality. It also is outdated, having been published in 2005.
MRI was addressed in three reviews,48,49,53 none of which were high quality. Two of the reviews48,49 were published by the same group of authors in different journals the same year. Study inclusion criteria in the two reviews were identical except for means of obtaining the reference diagnosis (histopathologic analysis only in 1 review,48 histopathologic analysis or clinical and imaging followup in the other49). All of the MRI studies included in the former review were also included in the latter. The reviews agreed on MRI sensitivity, with meta-analysis results ranging from 84 percent to 86 percent, but differed on specificity, with the two reviews from one group reporting 91 percent specificity and the other review reporting 82 percent. The difference may be because the most recent data in the third review53 is now 10 years old, and thus it does not reflect the current state of the art in MRI.
PET/CT was addressed in three reviews,48,50,56 none of which were high quality. The review by Wu et al. (2012)48 reported an erroneous confidence interval on sensitivity (82% to 81%), which is likely a typographical error. We attempted to contact the authors to obtain the correct confidence interval, but received no response.
Results across all modalities are summarized in Table 3. The limited quality of all the reviews on CT, MRI, and PET/CT preclude great confidence in the quantitative estimates of accuracy.
Table 3
KQ1a: Summary results of systematic reviews on diagnosis.
Comparative Test Performance of Imaging Modalities for Diagnosis
- KQ1b.
What is the comparative accuracy of the different imaging techniques for diagnosis and assessment of resectability?
Key Points
- For all other test comparisons involving diagnosis and the assessment of resectability in patients with unstaged disease, we deemed the evidence insufficient to permit conclusions, and the most common reason for insufficiency was imprecision.
Detailed Synthesis
Twenty-four studies met inclusion criteria for KQ1b or KQ2b on comparative accuracy for staging (or met criteria for both KQ1b and KQ2b). General study characteristics, patient characteristics, and test details appear in Appendix C. Ten of the 24 were conducted in Europe, 6 in the United States, 4 in Japan, and 4 in other countries. Nineteen of the 24 studies were conducted at universities. For the 19 studies reporting the dates of patient enrollment, the starting dates ranged from October 1995 to September 2008, and the median length of the patient enrollment period was 2 years (range 7 months to 5 years). Fifteen studies were prospective, and the other nine were retrospective. Eleven studies reported either the study funding source or whether there existed conflicts of interest (or both). Among these 11 studies, 6 specifically declared that authors had no conflicts of interest; 3 provided the funding source(s) but did not mention conflicts of interest; 2 reported that the authors had potential conflicts of interest. For these latter two—
- One study58 comparing EUS-FNA to MDCT was authored by individuals receiving grant money from the American Society for Gastrointestinal Endoscopy, however, the authors stated that “the funding sources had no role in the collection, analysis, or interpretation of the data or in the decision to submit the manuscript for publication.”
Regarding study design, the 24 studies performed multiple imaging tests on a single group of patients. One key advantage of this design is that patient factors and tumor characteristics are controlled; any observed difference in the accuracy of the tests could not be attributed to differences in the types of patients who received those tests (e.g., differences in tumor size profiles). The remainder of this section is divided into subsections based on the comparisons made by included studies (listed in Table 5 below).
Table 5
KQ1b: numbers of studies comparing different tests for diagnosis and resectability in patients with unstaged disease.
MDCT Angiography With 3D Reconstruction Versus Without 3D Reconstruction
One study21 addressed MDCT angiography with or without 3D reconstruction, and the study reported comparative accuracy in assessing resectability among patients with unstaged disease. The study was judged as low risk of bias. However, the study was performed by the developers of the 3D reconstruction software under consideration. We performed a statistical comparison of the sensitivity of MDCT without 3D reconstruction (89%; 95% CI, 68% to 97%) to the sensitivity of MDCT with 3D reconstruction (100%; 95% CI, 83% to 100%), and found no statistically significant difference, and we judged the evidence too imprecise to permit a conclusion. However, for detecting resectability, MDCT with 3D reconstruction (100%; 95% CI, 91% to 100%) was more accurate than MDCT without 3D reconstruction (79%; 95% CI, 64% to 89%); the rate differences were statistically significant. This means that, among patients whose disease was truly resectable, MDCT with 3D reconstruction identified a greater percentage as resectable than did MDCT without 3D reconstruction (the reference standard was the findings of an intraoperative exam). However, the potential for reporting bias (the authors may have published the article only because results favored their technology) and unknown consistency (i.e., there was only one study of this comparison) mean the evidence is insufficient to permit a general conclusion about comparative accuracy.
MDCT Versus EUS-FNA
Three studies58–60 compared MDCT versus EUS-FNA with respect to diagnostic accuracy. Two were judged as having moderate risk of bias, and one was judged as low risk of bias. We performed a meta-analysis of the three studies (see Figure 3) and found summary sensitivities for MDCT and EUS-FNA of 87 percent (95% CI, 82% to 91%) and 89 percent (95% CI, 85% to 93%), respectively, and we found summary specificities of 67 percent (95% CI, 53% to 78%) and 81 percent (95% CI, 68% to 90%). This evidence suggests a slight advantage of EUS-FNA, however statistical tests revealed no statistically significant differences, and we judged the evidence as too imprecise to permit a conclusion of similar accuracy (particularly notable was the uncertainty around specificities). Thus, we drew no conclusion.
One study58 compared MDCT and EUS-FNA for the assessment of resectability in those with disease not staged. The study was judged to have low risk of bias, and it found similar accuracy for the two technologies (truly unresectable patients were correctly deemed unresectable at rates of 64% and 68% for MDCT and EUS-FNA, respectively; and patients with truly resectable disease were correctly deemed resectable at rates of 92% and 88% for MDCT and EUS-FNA, respectively). We judged the study to be sufficiently precise to permit a conclusion of similar accuracy. However, consistency was unknown, which limits the confidence one can have in the conclusion.
Based on the study’s prevalence of unresectability of 53 percent, the results can be interpreted as follows: those whose disease is deemed unresectable by either MDCT or EUS-FNA have about an 88 percent chance of their disease actually being unresectable (positive predictive value), and those whose disease is deemed resectable by either test have about a 70 percent chance of their disease actually being resectable (negative predictive value). Translated to raw numbers, considerable a hypothetical example in which there are 1,000 patients being assessed for resectability, and 397 are deemed unresectable by the imaging test. About 88 percent of these patients, or 350 patients, would be truly be unresectable and would avoid unnecessary surgery (if the decision were based only on the test result). The other 47 patients would not undergo resection since the imaging test suggested unresectability (again simplistically assuming the resectability decision was based only on the test result). An additional 603 patients would be deemed resectable by the imaging tests, and about 70 percent of them would actually be resectable (423 patients) and therefore would have successful surgery, and the other 30 percent (180 patients) would undergo unsuccessful surgery. All of these hypothetical numbers are based on the findings of a single study, and they assume that the prevalence of unresectability is 53 percent.
MDCT Versus MRI
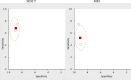
Seven studies61–67 compared MDCT and MRI with respect to diagnostic accuracy. Four62–65 were low risk of bias, and three61,66,67 were moderate risk of bias. Our meta-analysis found summary sensitivities of 89 percent for both technologies (95% CIs of 82% to 94% for MDCT and 81% to 91% for MRI), and summary specificities of 90 percent for MDCT (95% CI, 80% to 95%) and 89 percent for MRI (95% CI, 74% to 95%). These data we judged sufficiently precise to indicate similar accuracy. Plots in receiver operating characteristic (ROC) space appear in Figure 4 below. These plots show the similarity in accuracy between MDCT and MRI, with the filled squares in the same location of the plot, and the dashed area of 95 percent confidence slightly larger for MRI but with similar shapes and locations. The heterogeneity was lower for MDCT than EUS-FNA, and also was generally lower for sensitivity than specificity (tau=0.47 and 0.8 for MDCT sensitivity and MDCT specificity, respectively, as compared with tau=0.6 and 1.1 for EUS-FNA sensitivity and EUS-FNA specificity, respectively.)
To aid interpretation, we provide estimates for both positive predictive value (PPV) and negative predictive value (NPV). The median prevalence in the seven studies was 53 percent, and based on that prevalence, we estimate a PPV of 90 percent and an NPV of 88 percent. This means that a patient with a positive test result (on either MDCT or MRI) has approximately a 90 percent chance of having pancreatic adenocarcinoma, whereas a patient with a negative test result (on either MDCT or MRI) has only a 12 percent chance of having pancreatic adenocarcinoma.
Translated to raw numbers, considerable a hypothetical example in which there are 1,000 patients being diagnosed, and 521 test positive for pancreatic adenocarcinoma. About 90 percent of these patients, or 472 patients, would truly have the disease. The other 49 patients would be false alarms. An additional 479 patients would have tested negative, but only 421 of these would actually be negative for pancreatic adenocarcinoma. The other 58 of them (12 percent of 479) would be missed pancreatic adenocarcinomas. All of these hypothetical numbers are based on our meta-analystic summary sensitivity and specificity as well as an assumed prevalence of 53 percent.
We also performed 35 sensitivity analyses of this meta-analysis (Appendix E). Four of the seven studies reported data for multiple readers separately; the above analysis used only reader #1 from these four studies. The sensitivity analysis all found very similar results regardless of which permutation of readers we used (see all estimates in Appendix E).
For the above meta-analysis of seven studies, we also measured the correlation between the end date of patient recruitment (i.e., the month when the last patient was enrolled in the study) and the difference in logit sensitivities. To enable this, we needed the end month of patient recruitment for all seven studies, but only five reported this information, so we assumed that the end of enrollment had occurred 2.3 years before the study publication month (2.3 years was the average for all studies). This correlation of seven studies’ results did not reveal a convincing trend. The results for sensitivity showed an association (R2=0.78) suggesting that later studies favored MRI over MDCT for diagnosis, but examination of the graph suggested that the finding was being driven by a single study (the year-2000 study), and when it was removed, the R2 for the remaining six studies reduced to 0.26. For specificity, no correlation was apparent (R2=0.11).
Two studies64,68 compared MDCT and MRI for the assessment of resectability in patients with disease not staged; both were judged low risk of bias. Our meta-analysis of the two studies (see Figure 5) yielded summary sensitivities for MDCT and MRI of 68 percent (95% CI, 47% to 85%) and 52 percent (95% CI, 31% to 72%), respectively, and we found summary specificities of 89 percent (95% CI, 77% to 96%) and 91 percent (95% CI, 80% to 97%). These suggest neither an advantage of MDCT nor an advantage of MRI (statistical tests not significant), and we judged the data too imprecise to indicate equivalence, thus we drew no conclusion.
MDCT Versus PET/CT
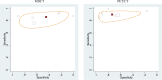
Six studies65,69–73 compared MDCT and PET/CT with respect to diagnostic accuracy. Two65,71 were low risk of bias, and four69,70,72,73 were moderate risk of bias. Our meta-analysis found summary sensitivities of 85 percent (95% CI, 80% to 90%) for MDCT and 91 percent (95% CI, 85% to 94%) for PET/CT, and summary specificities of 55 percent for MDCT (95% CI, 44% to 66%) and 72 percent for PET/CT (95% CI, 61% to 81%). Statistical tests showed no clear difference for sensitivity, but also no statistical difference for either sensitivity or specificity.
Plots in ROC space appear in Figure 6 below. These plots show large uncertainty around specificity estimates (horizontal ovals), and this uncertainty explains why the apparent specificity difference (55% for MDCT vs. 72% for PET/CT) was not statistically significant. The general uncertainty was mostly due to the heterogeneity among different studies (rather than small sample sizes), with tau values of logit specificity of 0.75 for MDCT and 1.09 for PET/CT. The corresponding heterogeneity values for sensitivity (0.38 for MDCT and 0.21 for PET/CT) indicate higher consistency among study results for detecting pancreatic adenocarcinoma. Overall, given the wide uncertainty (caused by inconsistency among study results, particularly in the ability of these tests to rule out pancreatic adenocarcinoma), we drew no conclusion about this comparison.
For the above meta-analysis of six studies, we also measured the correlation between the end date of patient recruitment and the difference in logit sensitivities. This correlation did not reveal any trend (R2=0.03 for both sensitivity and specificity).
EUS-FNA Versus PET/CT
One study74 compared EUS-FNA and PET/CT with respect to diagnostic accuracy; we judged its risk of bias as moderate. Results statistically favored neither technology for either sensitivity (EUS-FNA, 81%; 95% CI, 62% to 91%; vs. PET/CT, 89%; 95% CI, 72% to 96%) or specificity (EUS-FNA, 84%; 95% CI, 62% to 94%, vs. PET/CT, 74%; 95% CI, 51% to 88%). Furthermore, we judged the evidence too imprecise to conclude similar accuracy. Thus, no conclusion is warranted.
MRI Versus PET/CT
One study75 compared MRI and PET/CT with respect to diagnostic accuracy; we judged its risk of bias as low. Results statistically favored neither technology for either sensitivity (MRI, 85%; with 95% CI, 64% to 95%; vs. PET/CT, 85%; 95% CI, 64% to 95%) or specificity (MRI, 72%; 95% CI, 49% to 87%; vs. PET/CT, 94%; 95% CI, 74% to 99%). Furthermore, we judged the evidence too imprecise to conclude similar accuracy. Thus, no conclusion is warranted.
For other subquestions under KQ1(c through g), no included studies reported pertinent data.
Conclusions for KQ1
For single-test accuracy of diagnosis and resectability in patients with unstaged disease, we included nine systematic reviews, and drew the following conclusions:
- Evidence was insufficient to permit accuracy estimates for MDCT angiography with or without 3D reconstruction.
- For diagnosis using EUS-FNA, three high-quality and recent systematic reviews yielded sensitivity estimates ranging from 83 percent to 92 percent and specificity estimates ranging from 95 percent to 100 percent. (Strength of evidence from published systematic reviews was not graded.)
- For diagnosis using MRI, three systematic reviews yielded sensitivity estimates of 84 percent to 85 percent and specificity estimates of 82 percent to 91 percent. (Strength of evidence from published systematic reviews was not graded.)
- For diagnosis using PET/CT, two systematic reviews yielded sensitivity estimates of 87 percent and 90 percent and specificity estimates of 83 percent and 90 percent. (Strength of evidence from published systematic reviews was not graded.)
- For MDCT, in assessing the resectability tumors in patients with unstaged disease, one systematic review yielded a sensitivity estimate of 81 percent (95% CI, 76% to 85%) and a specificity estimate of 82 percent (95% CI, 77% to 97%). (Strength of evidence from published systematic reviews was not graded.)
- For MRI, in assessing the resectability of tumors in patients with unstaged disease, one systematic review yielded a sensitivity estimate of 82 percent (95% CI, 69% to 91%) and a specificity estimate of 78 percent (95% CI, 63% to 87%). (Strength of evidence from published systematic reviews was not graded.)
For comparative test accuracy of diagnosis and resectability in patients with unstaged disease, our assessments of the evidence are summarized in Table 6 below. Of the eight sets of evidence listed in the table, we deemed five insufficient to permit conclusions because of imprecision. A sixth was insufficient because of the existence of only a single study and the possibility of publication bias. The other two rows represent our conclusions for KQ1b:
Table 6
Summary of evidence on KQ1b.
Test Performance of Imaging Modalities for Staging
- KQ2.
Comparative Effectiveness of Imaging Techniques for Staging
- KQ2a.
What is the staging accuracy of each imaging technique (for tumor size, lymph node status, vessel involvement, metastases, stage I–IV, and resectability)?
Key Points
- Two low-quality systematic reviews reported on CT for assessing vascular invasion. Both concluded that sensitivity and specificity were worse for the subset of studies using older or single-slice CT scanners than for the studies using newer multi-slice CT. Summary sensitivity values for the newer scanners ranged from 80 percent to 85 percent while summary specificity ranged from 82 percent to 97 percent. The evidence base in both reviews was small: four or five studies each. (Strength of evidence from published systematic reviews was not graded.)
- One low-quality systematic review reported on MR for assessing vascular invasion, concluding it had sensitivity of 63 percent and specificity of 93 percent. The evidence base was only four studies. (Strength of evidence from published systematic reviews was not graded.)
- One review of PET/CT included only a single study, which had reported 82 percent sensitivity and 97 percent specificity for detecting liver metastasis. (Strength of evidence from published systematic reviews was not graded.)
Detailed Synthesis
We included three reviews for this subquestion; none were high quality.51,56,76 No reviews reported on the ability of these imaging modalities to assess overall stage (I-IV) or TNM (tumor, lymph node, metastasis) components of staging.
Two reviews addressed the diagnosis of vascular invasion (Table 7): both included CT results and one also reviewed MRI data. Zhao (2009) analyzed CT (5 studies, 452 patients excluding single-slice CT studies) and provided separate analyses of the full set of CT studies and a subset of studies that used multi-slice scanners.51 Sensitivity was considerably higher for the later studies than for the rest, with no corresponding loss of specificity. The review of MRI76 found only four studies (total 143 patients) and thus had a large uncertainty in its results.
A review of PET for pancreatic cancer staging56 also tabulated a subset of studies using integrated PET/CT scanners. It found only one such study, which reported on only 50 patients (Table 8 and Table 9)
Table 7
KQ2a: Summary results of systematic reviews on vascular invasion.
Table 8
KQ2a: Summary results of systematic reviews on nodal metastasis.
Table 9
KQ2a: Summary results of systematic reviews on liver metastasis.
Comparative Test Performance of Imaging Modalities for Staging
- KQ2b.
What is the comparative staging accuracy of the different imaging techniques?
Key Points
- For all other test comparisons involving staging and the assessment of resectability in patients with staged disease, we deemed the evidence insufficient to permit conclusions, and the most common reason for insufficiency was imprecision.
Detailed Synthesis
For an overview of the studies included for comparative accuracy, including study locations and patient characteristics, see the section Key Question1b above entitled “Comparative Test Performance of Imaging Modalities for Diagnosis.” This section is divided into subsections based on the comparisons made by included studies (listed in Table 10 below).
Table 10
KQ2b: Numbers of studies comparing different tests for staging and resectability in staged patients.
MDCT Versus EUS-FNA
One study58 compared MDCT and EUS-FNA with respect to T staging. Authors reported the data as the percentages of patients whose disease was accurately staged (41% by MDCT, vs. 67% by EUS-FNA), the percentage whose disease was overstaged (14% by MDCT, 18% by EUS-FNA), and the percentage whose disease was understaged (44% by MDCT, and 14% by EUS-FNA). (The reference standard was based on intraoperative findings.) The difference in accuracy was statistically significant by an exact McNemar’s test for paired binary data (our odds ratio for paired binary data also indicated statistical significance). However, consistency was unknown, which limits the confidence one can have in the conclusion.
One study77 compared MDCT with EUS-FNA for the assessment of vessel involvement. Results statistically favored neither technology for either sensitivity (MDCT 56%; 95% CI, 34% to 75%; vs. EUS-FNA, 61%; 95% CI, 39% to 80%) or specificity (MDCT, 94%; 95% CI, 80% to 98%; vs, EUS-FNA, 91%; 95% CI, 76% to 97%). Furthermore, we judged the evidence too imprecise to conclude similar accuracy. Thus, no conclusion is warranted.
MDCT Versus MRI
One study78 compared MDCT and MRI with respect to T staging. MDCT yielded an accurate T stage in 73 percent, whereas MRI yielded an accurate stage in 62 percent. This result was not statistically significant by the odds ratio for paired binary data, and also was not indicative of equivalence (95% confidence interval 0.93 to 2.9), so we drew no conclusion.
One study78 compared these technologies with respect to N staging. Results statistically favored neither technology for either sensitivity (MDCT, 38%; 95% CI, 21% to 57%; vs. MRI, 15%; 95% CI, 5% to 36%) or specificity (MDCT, 79%; 95% CI, 63% to 90%; vs. MRI, 93%; 95% CI, 78% to 98%). Furthermore, we judged the evidence too imprecise to conclude similar accuracy. Thus, no conclusion is warranted.
Five studies63,65,78–80 compared these technologies with respect to the assessment of metastases. Our meta-analysis yielded sensitivity estimates of 48 percent (95% CI, 31% to 66%) and 50 percent (95% CI, 19% to 81%) for MDCT and MRI, respectively. For specificity, the meta-analytic estimates were 90 percent (95% CI, 81% to 95%) and 95 percent (95% CI 91% to 98%) for MDCT and MRI, respectively. The comparisons were not statistically significant and we judged them as too imprecise to indicate equivalence, thus we drew no conclusion.
Plots in ROC space appear in Figure 7 below representing the five studies for assessment of metastases. The wide variability in sensitivity is shown graphically by the vertically shaped ovals, and the generally high specificity is shown by the fact that the ovals are on the left side of the ROC plot. Heterogeneity was low for MDCT (tau 0.50 for sensitivity and 0.53 for specificity) and MRI specificity (tau 0.14), but large for MRI sensitivity (tau 1.03). This latter value was caused by the five studies’ sensitivity estimates encompassing most of the 0 percent to 100 percent scale (sensitivities of MRI of 87%, 73%, 57%, 30%, and 0% in the five studies). The study80 that had found 0 percent sensitivity for both tests had investigated paraaortic lymph node metastases, which may be relatively hard to detect, thereby yielding such low values.
We also performed two sensitivity analyses of this meta-analysis (see Appendix E). One of the five studies had reported data for three readers separately; the above analysis used only reader #1 from that study. The sensitivity analyses all found very similar results regardless of which reader we used (see all estimates in Appendix E).
For the above meta-analysis of five studies, we also measured the correlation between the end date of patient recruitment and the difference in logit sensitivities. To enable this, we needed the end month of patient recruitment for all five studies, but only four reported this information, so we assumed that the end of enrollment had occurred 2.3 years before the study publication month (2.3 was the average for all studies). This correlation of five studies’ results did not reveal a convincing trend. The results for sensitivity showed an association (R2=0.58) suggesting that later studies favored MRI over MDCT for assessing metastases, but examination of the graph suggested that the finding was being driven by a single study (the year-2004 study),78 and when it was removed, the R2 for the remaining six studies reduced to 0.17. For specificity, no correlation was apparent (R2=0.16).
One study78 compared MDCT and MRI for the assessment of precise stage. MDCT was accurate in 46 percent and MRI was accurate in 36 percent; this difference was not statistically significant by the odds ratio for paired binary data, and furthermore the data were too imprecise to indicate equivalence (95 percent confidence interval 0.88 to 2.6).
Three studies64,68,78 compared these technologies for the assessment of vessel involvement (two were low risk of bias, and one was moderate risk of bias). The data are shown in Figure 8. The meta-analysis found similar accuracy (sensitivity 68% for MDCT with 95% CI, 55% to 79%; and 62% for MRI with 95% CI, 48% to 74%; specificity 97% for MDCT with 95% CI, 94% to 98%; and 96% for MRI with 95% CI 93% to 98%). Given the relatively wide confidence intervals, as well as the fact that there were only three studies, we deemed the evidence imprecise. Overall, we judged this as moderate evidence for concluding equivalent accuracy. Given the median prevalence of 13 percent, we computed a positive predictive value of 73%, and a negative predictive value of 5%.
Translated to raw numbers, consider a hypothetical example in which there are 1,000 patients being assessed for vessel involvement, and 114 test positive. About 73 percent of these patients, or 85 patients, would truly have vessel involvement. The other 29 patients would be false alarms. An additional 886 patients would have tested negative for vessel involvement, and almost all of these (95% or 840 patients) would actually be negative. The other 46 of them (5 percent of 886) would have undetected vessel involvement. All of these hypothetical numbers are based on our meta-analystic summary sensitivity and specificity as well as an assumed prevalence of 13 percent.
One study78 compared these technologies for assessing resectability in those with staged disease. Results statistically favored neither technology for sensitivity (MDCT, 67% with 95% CI, 48% to 81%; vs. MRI, 57% with 95% CI, 37% to 74%) or specificity (MDCT, 97% with 95% CI, 84% to 99%; vs. MRI, 90% with 95% CI, 74% to 96%). Furthermore, we judged the evidence too imprecise to conclude similar accuracy. Thus, no conclusion is warranted.
MDCT Versus PET/CT
One study73 compared MDCT and PET/CT with respect to N staging. Study results suggested similar accuracy:
- MDCT sensitivity, 26 percent; 95 percent CI, 14 percent to 43 percent
- PET/CT sensitivity, 32 percent; 95 percent CI, 19 percent to 50 percent
- MDCT specificity, 75 percent; 95 percent CI, 50 percent to 90 percent
- PET/CT specificity, 75 percent; 95 percent CI, 50 percent to 90 percent
However, because it was only a single study, and it was at moderate risk of bias, we drew no conclusions.
Two studies65,81 compared these technologies with respect to the assessment of metastases. Neither study used dynamic IV contrast for PET-CT. The data are shown in Figure 9. Our meta-analysis yielded summary sensitivities of 57 percent (95% CI, 36% to 75%) and 67 percent (95% CI, 47% to 83%) for MDCT and PET/CT, respectively, and the summary specificities were 91 percent (95% CI 81% to 97%) and 100 percent (95% CI 95% to 100%) for MDCT and PET/CT, respectively. The difference in specificity was statistically significant in favor of PET/CT. The sensitivities were not statistically different, however, both studies slightly favored PET-CT. One65 of the two studies was moderate risk of bias, and the other81 was low risk of bias, and we judged their results as consistent. Taken together, the evidence permits a conclusion that PET/CT is more accurate than MDCT in the assessment of metastases. PET/CT had 100% specificity in both studies, and neither study commented on possible reason(s) for these findings.
To help interpret the data, we note that the two studies had an average prevalence of metastases of 39 percent, and at this prevalence, a positive MDCT scan indicates an 80 percent chance of actually having metastases, whereas a positive PET/CT scan indicates a 100 percent chance. A negative MDCT scan indicates a 23 percent of having metastases, whereas a negative PET/CT scan indicates a 17 percent chance of having metastases.
Translated to raw numbers, considerable a hypothetical example in which there are 1,000 patients being assessed for metastases. 277 test positive on MDCT, 80 percent truly do have metastases (222 patients). By contrast, 261 test positive on PET-CT, and all of them truly do have metastases. Turning to negative tests, on MDCT 723 test negative, and 76 percent of them are truly negative (555 patients), with the other 168 patients (23 percent) having missed metastases. By contrast, 739 test negative on PET-CT, and 610 of them (83 percent) are truly negative, and the other 129 patients (17 percent) had missed metastases. All of these hypothetical numbers are based on our meta-analystic summary sensitivity and specificity as well as an assumed prevalence of 39 percent.
EUS-FNA Versus MRI
One study82 compared EUS-FNA and MRI for the assessment of precise TNM stage. EUS-FNA provided an accurate stage in 71 percent of patients (34/48), whereas MRI did so in 75 percent (36/48). The matched pairs odds ratio was not statistically significant, and data were too imprecise to suggest equivalence (95 percent confidence 0.43 to 1.5). Thus, we drew no conclusion.
MRI Versus PET/CT
One study65 compared MRI and PET/CT with respect to the assessment of metastases. One study compared these technologies with respect to N staging. Results statistically favored neither technology for either sensitivity (MDCT, 57%; 95% CI, 25% to 84%; vs. PET/CT, 86%; 95% CI, 48% to 97%) or specificity (MDCT, 86%; 95% CI, 48% to 97%; vs. PET/CT, 94%; 95% CI, 64% to 100%). Furthermore, we judged the evidence too imprecise to conclude similar accuracy. Thus, no conclusion is warranted.
For other subquestions under KQ2 (c through g), no included studies reported pertinent data.
Conclusions for KQ2
For single-test accuracy of staging and resectability in patients with staged disease, we included one low-quality systematic review published in 2009 that addressed this question, and assessed the accuracy of CT in assessing vascular involvement. When the review considered only studies published since 2004, the review estimated the sensitivity of CT to be 85 percent (95% CI, 78% to 91%) and specificity to be 82 percent (95% CI, 74% to 88%). We did not grade the strength of evidence from published systematic reviews.
For comparative test accuracy of staging and resectability in patients with staged disease, our assessments of the evidence are summarized in Table 11 below. Of the 12 sets of evidence listed in the table, we deemed 7 insufficient to permit conclusions due to imprecision. Two others were insufficient because of the existence of only a single study and a moderate risk of bias. The other three rows represent our conclusions for KQ2b:
Table 11
Summary of evidence on KQ2b.
Harms of Imaging Modalities
- KQ3.
Harms of Imaging Techniques for Diagnosis and/or Staging
Key Points
- Procedural harms were reported in many EUS-FNA imaging studies, but not in many studies of the other imaging technologies.
- The most commonly reported procedural harms of EUS-FNA were pancreatitis (with rates ranging from 0% to 3.7%), postprocedural pain (0.1% to 2.0%), and bleeding/puncture/perforation (0% to 4.3%).
Detailed Synthesis
We included 78 studies for this key question. Fifty described harms due to imaging tests for the diagnosis/staging of pancreatic cancer and were published in the year 2000 or later, and the other 28 were not specific to pancreatic cancer and were published in the year 2009 or later. This later date was chosen given the anticipated large amount of studies addressing harms of imaging in patients with any indication for the procedures.
Pancreas-specific studies were published from 1991 to 2013, and studies evaluated as few as 50 patients83 or as many as 1,034 patients.84 One study by Eloubeidi et al. (2004)85 contacted 27 EUS training centers in the United States to request information regarding total number of EUS-FNAs of solid pancreatic masses performed, the duration of time over which these procedures were performed, and cases of pancreatitis. Authors reported that patient-specific information was not provided, but self-reported episodes of acute pancreatitis were provided.85 Settings included university hospitals, tertiary care medical centers, and cancer centers. Most studies enrolled patients suspected of pancreatic adenocarcinoma or patients with solid pancreatic lesions. The percentage of female patients ranged from 29 percent to 83 percent. Nonpancreas-specific studies were published from 2009 to 2013; one integrated retrospective analysis included trials conducted as early as 1993.86 Studies evaluated as few as 1 patient or as many as 106,000 patients.87 Settings included outpatient radiology centers, university hospitals, tertiary care medical centers, and cancer centers.
Below, the procedural harms data are discussed in separate sections for each imaging technology. Each technology’s section is further divided into studies in which the imaging test was used specifically in the diagnosis and staging of pancreatic adenocarcinoma (“Harms studies specific to pancreatic cancer”), and other studies in which the imaging test was used for other purposes (“Recent harms studies not specific to pancreatic cancer”).
MDCT
Harms Studies Specific to Pancreatic Cancer
Two MDCT studies reported adverse events.60,88 Sakamoto et al. (2008)88 enrolled 119 patients suspected of having a pancreatic solid tumor because of abdominal screening findings on EUS-FNA or CT. The percentage of female patients was 39 percent and the mean age was 68.7 years. The study was conducted in Japan at Kink University from March 2002 to August 2006. Three patients experienced an allergic eruption to the contrast agent (100 mL Optiray 320).
Agarwal et al. (2004)60 enrolled patients with primary pancreatic neoplasm undergoing distal pancreatectomy and had no previous pancreatic resection or metastatic neoplasm. A total of 179 patients were included, and 114 were female. The mean age was 61 years, with a range of 19–86 years. The study was conducted in the United States at a cancer center between November 2000 and November 2001. Eighty-one of the enrolled patients underwent MDCT and two patients experienced postprocedural abdominal pain that completely subsided within 24 hours. The reported harms rate was 2.5 percent (2/81).
Recent Harms Studies Not Specific to Pancreatic Cancer
CT-related adverse events (range 0.13% to 61.5% [all moderate]) were evaluated in more than 180,497 patients in 12 studies.87,89–99 Most studies evaluated CT, however three studies evaluated CT and CT angiography91,92,97 and two studies evaluated CT coronary angiography.96,98 Eight studies included at-risk patients.89,90,92–96,98
Non-ionic contrast agents, introduced in the 1970s, have a lower osmolarity than blood and are therefore less likely to cause adverse reactions.99 Non-ionic contrast agents evaluated included iopromide,87,89,92,99 iomeprol,89,90,99 iohexol,89,90,97 iopamidol,90,91,96,98,99 iodixanol,89,98 and ioversol.90,94,99
One study retrospectively reviewed extravasation (an inadvertent leakage of fluid from an intravenous site into the surrounding soft tissue) and allergic-like reactions from 24,826 injections (12,142 previously warmed) of intravenous (IV) iopamidol in CT and CT angiography examinations.91 The authors indicated that extrinsic warming (to 37 °C) appeared to affect adverse event rates for iopamidol 370 (8 events [warming] vs. 26 events [no warming]) but did not affect rates for iopamidol 300 (74 events [warming] vs. 69 events [no warming]).
Another study reported more delayed adverse reactions from iohexol (37/258) than controls (7/281).97 Delayed adverse reactions are typically defined as occurring 1 hour or more after administration of a contrast medium.97 In this report, Loh et al. reported statistically significantly more delayed adverse reactions (e.g., skin rashes, itching, headache) occurred with contrast-enhanced CT compared with controls. Kingston et al. (2012)92 focused on rates of extravasation in 26,854 patients. Results indicated that the “presence of cancer, hypertension, smoking and recent surgery was associated with higher extravasation rates.” Extravasations most commonly occurred at the elbow (71.4%).
Cadwallader et al. (2011)95 reported results from 198 scans of at-risk patients to determine the risk of fatal cancer induction. Forty-one (20.7%) scans did not alter case management of the patient and were thus deemed as unnecessarily exposing patients to CT radiation. According to the National Cancer Institute, the extra risk of one person to develop a fatal cancer from a CT procedure is about 1 in 2,000.100
Two studies reported only mild-to-moderate harms;89,98 two studies included at-risk patients.89,98 Six studies, however, reported serious/severe adverse events;87,90,93,94,96,99 five (42%) studies enrolled at-risk patients.90,93,94,96,99 Two studies reported 15 deaths within 45 days93 after CT. Mitchell et al. (2012)93 enrolled 633 patients; 174 undergoing computed tomography pulmonary angiography (CTPA) to exclude pulmonary embolism and 459 patients who did not undergo CTPA (non-CTPA). Study groups were similar for presumptive risk factors for contrast-induced nephropathy (CIN), such as anemia, diabetes mellitus, and history of hypertension and baseline renal insufficiency; however, significantly more CTPA patients had vascular disease (15% vs. 8%) and congestive heart failure (12% vs. 5%). Seventy patients (11%) developed CIN; slightly more were patients undergoing CTPA than patients without (14% vs. 10%). All-cause 45-day mortality rate was slightly higher in CTPA patients (3% vs. 2%) with 15 deaths during this time. Three patients undergoing CTPA went into severe renal failure, with two ultimately dying. The authors indicated that the “development of CIN was associated with an increased risk of death from any cause (relative risk = 12, 95 percent CI 3 to 53).”
Kobayashi et al. (2013)90 reported 23 (0.06%) severe reactions including shock, hypotension, desaturation, and airway obstruction in a retrospective cohort study of 36,472 patients. Patients received various nonionic low-osmolar contrast agents; approximately half of the study population was diabetic (19.5%) or hypertensive (28.6%). Vogl et al. (2012)94 reported anaphylactoid adverse reactions requiring hospitalization in 4 (0.03%) patients receiving ioversol. Of the 10,836 patients enrolled at 72 centers in Germany, more than 5,000 had 1–7 concomitant diseases, including diabetes mellitus and renal insufficiency. Jung et al (2102)99 focused on cutaneous adverse reactions in 47,388 patients receiving various nonionic monomers such as iomeprol. Severe reactions such as severe generalized urticaria and facial edema occurred in 16 patients. The three remaining studies reported shortness of breath (5 patients)87 and one case of atrial fibrillation (patient on peritoneal dialysis),96 See Appendix C for details on CT-related adverse events in these studies.
EUS-FNA
Harms Studies Specific to Pancreatic Cancer
The four most commonly reported harms of EUS-FNA were pancreatitis; pain; perforation, puncture, or bleeding; and tumor seeding. Sixteen studies reported that there were no harms or complications resulting from EUS-FNA procedures.83,101–115
Twenty-four studies reported pancreatitis as an adverse event.84,85,104,113,116–136 The number of patients at risk of pancreatitis ranged from 24 to 4,909, and the mean age range was 57–68.2 years. The range of rates reported for pancreatitis was 0 percent to 3.7 percent. Fifteen studies indicated patients experienced mild or acute pancreatitis,85,116,117,119–122,124,127–130,132,133,135 three studies reported moderate pancreatitis,84,116,125 and one study reported severe pancreatitis.84 Eight did not specify whether pancreatitis was mild/acute, moderate, or severe.113,118,122,123,126,131,134,136 Table 12 below provides further details.
Table 12
Rates of pancreatitis after EUS-FNA.
Ten studies reported pain as an adverse event.88,116,120,123,124,132,142–145 The number of patients at risk of pain ranged from 28 to 677, and the mean age range was 62–66.5 years. The range of rates reported for pain was 0.1 percent to 2.0 percent. Six studies specifically mentioned the development of abdominal pain.88,120,124,132,143,145 Table 13 provides further details.
Table 13
Rates of pain after EUS-FNA.
Twenty studies reported perforation, puncture, or bleeding as an adverse event.84,88,113,116,118,120,123–127,131–134,142,143,146–149 We combined these three concepts because all three can be caused by endoscopic ultrasound and/or fine-needle aspiration, and studies may use different words to describe the event (e.g., a perforation that results in bleeding could be described by one study as perforation but by another study as bleeding). The number of patients at risk of perforation, puncture, or bleeding ranged from 52 to 1,034, and the mean age range was 47–68.2 years. The range of rates reported for this outcome was 0 percent to 4.3 percent. Table 14 provides further details.
Table 14
Rates of bleeding, perforation, or puncture after EUS-FNA.
Four studies88,103,104,113 specifically reported that tumor seeding after EUS-FNA had not occurred; these studies had enrolled a total of 418 patients. Additionally, other complications, such as infection, brief hypoxia, and reversal of medication, were reported. Five studies reported infection, with rates ranging from 0 percent to 6.7 percent.113,118,121,125,126 Two studies reported brief hypoxia,121,124 with rates ranging from 0.3 percent to 0.6 percent, and two studies reported reversal of medication usage,124,125 with rates ranging from 0.2 percent to 0.6 percent.
Recent Harms Studies Not Specific to Pancreatic Cancer
Katanuma et al. (2013)116 reported 11 harms (1 moderate) in 316 patients. In multivariate analysis, tumors measuring 20 mm or less in diameter (odds ratio [OR], 18.48; 95% CI, 3.55 to 96.17; p<0.001) and pancreatic neuroendocrine tumors (OR, 36.50; 95% CI, 1.73 to 771.83; p=0.021) were significant independent risk factors for post-procedural events.
EUS-related adverse events (range 0.06% to 14.4% [mostly minor] were reported in more than 21,088 patients in five studies.150–154 Two studies enrolled at-risk patients and focused on sedation-related complications.150,151 One study enrolled 799 patients (more than 60% classified as ASA Class III)150 (see Appendix C for further details). In multivariate analysis, male sex (OR, 1.75; 95% CI, 1.08 to 2.85; p=0.02), ASA class 3 or more (OR, 1.90; 95% CI, 1.11 to 3.25; p=0.02), and body mass index (OR, 1.05; 95% CI, 1.01 to 1.09; p=0.009) were independent predictors of airway modifications. More than 65 percent of patients randomly assigned to midazolam/meperidine or propofol in another study were ASA Class III or higher (18% ASA Class IV).151 Of the 151 patients enrolled, 34 patients underwent EUS. No significant differences were reported in overall cardiopulmonary complication rates.
Forty-two (0.4%) serious adverse events were reported by Niv et al. (2011)153 in a 7-year retrospective review of physician reporting. Harms from EUS-FNA and endoscopic retrograde cholangiopancreatography (ERCP) included perforation (69%), bleeding (4.8%), cardiovascular and respiratory (4.8%), teeth trauma (2.4%) and other (19%). “Critical outcomes” for the 42 patients involved included 15 deaths (35.7%) and 18 (42.9%) patients with residual damage. The incidence of mortality for EUS-related procedures (diagnostic and interventional) has reportedly varied between 0 percent and 0.06 percent.152 Eloubeidi et al. (2009)154 reported cervical esophageal perforations in three (0.06%) patients at the time of endoscopic ultrasound intubation. One patient reported chest pains and two patients reported excessive salivation and sore throat prompting a physical exam. All patients underwent surgical repair and resumed swallowing without complications. Lastly, Kalaitzakis et al. (2011)152 reported 9 (0.2%) EUS-FNA related harms including desaturation, supraventricular tachycardia, and gallbladder and duodenal perforations. Jenssen et al. (2012) indicated that gastrointestinal perforations from EUS-FNA typically occurred either at areas of angulation, in the presence of unexpected anatomical alterations, or in luminal obstruction.155
See Appendix C for details on EUS-FNA–related adverse events in these studies.
MRI
Harms Studies Specific to Pancreatic Cancer
No included studies reported procedural harms of MRI.
Recent Harms Studies Not Specific to Pancreatic Cancer
MRI-related adverse events (range 0% to 64.6%) were evaluated in more than 156,962 patients in 11 studies.86,87,156–164 Adverse events from contrast-enhanced MRIs were the focus of 10 (91%) studies.86,87,156–163 The following contrast agents were administered in nine studies:
- Gadobenate dimeglumine (Gd-BOPTA)156
- Gadodiamide (Gd-DTPA-BMA)86
- Gadoversetamide (Gd-DTPA-BMEA)86
- Gadoxetic acid disodium salt (Gd-EOB-DTPA)160
- Gadoteridol (Gd-HP-DO3A)86
- Manganese chloride tetrahydrate (CMC-001)157
- Oral manganese (McCl2)162
See Appendix C for a list of currently marketed gadolinium [GD] agents for MRI. Contrast-enhanced MRIs, widely used for more than 20 years, provide increased sensitivity and specificity of lesion detection.165 Although relatively safe in most patients, contrast agents may be quite harmful to others.
The American College of Radiology Manual on Contrast Media (2013)166 indicates that patients with a history of prior allergy-like reaction to contrast media, history of asthma, renal insufficiency, significant cardiac disease, and elevated anxiety are at an increased risk of experiencing adverse IV contrast-material reactions.166 Some reactions, in fact, may be life threatening. In 2006, some gadolinium-based contrast agents (GBCAs) were linked with nephrogenic systemic fibrosis (NSF), a scleroderma-like, fibrosing condition, that could be potentially fatal in patients with renal failure.167
The American College of Radiology manual166 estimates that “patients with end-stage chronic kidney disease (CKD) (CKD5, eGFR [estimated glomerular filtration rate] <15 ml/min/1.73 m2) and severe CKD (CKD4, eGFR 15 to 29 ml/min/1.73 m2) have a 1 percent to 7 percent chance of developing NSF after one or more exposures to at least some GBCAs.” In 2010, the U.S. Food and Drug Administration (FDA) issued a warning for use of GBCAs in patients with kidney dysfunction. Agents such as Magnevist, Omiscan, and Optimark, the agency states, place certain patients with kidney dysfunction at higher risk for NSF than other GBCAs.168 FDA had previously issued a Public Health Advisory (2006) about the possible link between exposure to GBCAs for magnetic resonance angiography and NSF in patients with kidney failure.169 The FDA later (2007) required a box warning on product labeling of all GBCAs used in MRIs regarding the risk of NSF in patients with severe kidney insufficiency, patients just before/just after liver transplantation, or individuals with chronic liver disease.170
Six MRI-related studies enrolled at-risk patients;86,158,160,161,163,164 five studies evaluated GBCAs in patients at-risk for kidney or liver disease.86,158,160,161,163 The largest study (N=84,621) surveyed 19,354 (22.9%) patients at-risk with renal and liver dysfunctions, history of allergies, hypertension, chronic heart disease, and central nervous system disorders who received manual (74.5%) or automated (25.5%) injections of Gd-DOTA.158 In the study, 421 adverse events (65 different) occurred in 285 (0.34%) patients. Eight serious adverse events (less than 0.01%) were reported; life-threatening events in 3 patients. Ishiguchi and Takahashi (2010)161 also evaluated the safety of Gd-DOTA and reported a less than 1 percent overall incidence of adverse events. The authors indicated that general condition, liver disorder, kidney disorder, complication, concomitant treatments, and Gd-DOTA dose were statistically significant risk factors for adverse reactions.
Ichikawa et al. (2010) reported mostly mild adverse events in 178 patients with suspected focal hepatic lesions160 after undergoing MRI with a single injection of Gd-EOB-DTPA. Voth et al. (2011)86 retrospectively reviewed 34 clinical studies that had enrolled 4,549 patients receiving Gd-BT-DO3A and 1,844 patients receiving comparator agents (e.g., Gd-DTPA, Gd-HP-DO3A, Gd-DTPA-BMEA, or Gd-DTPA-A-BMA). Results indicated similar overall adverse event rates for both groups (4.0%) although slightly more serious adverse events occurred in the Gd-BT-DO3A group (0.4% vs. 0.2%). Lastly, Hammerstingl et al. (2009)163 reported no serious or severe adverse events after randomly assigning patients with known focal liver lesions or suspected liver lesions to gadobutrol (N=292) or gadopentetate-enhanced MRI (N=280).
Five studies, also evaluating GBCA-enhanced MRIs, reported no harms,156 mild gastrointestinal harms,162 mild burns from an MR coil,87 and two severe adverse drug reactions (ADRs).157,159 One integrated retrospective analysis of six clinical studies159 (N=14,299) indicated that the “occurrence of ADRs…following…gadobutrol is comparable with the published data of other Gd-based contrast agents.” Lastly, one study focusing on general harms from MRI164 enrolled 365 patients at-risk for developing breast cancer and reported significant MRI discomfort was mainly due to noise of the machine (64.6%). See Appendix C for details on MRI-related adverse events in these studies.
PET/CT
Harms Studies Specific to Pancreatic Cancer
There were no pancreas-specific studies on harms of PET/CT.
Recent Harms Studies Not Specific to Pancreatic Cancer
PET/CT-related harms were reported in 3,359 patients in one study.87 A retrospective review of 3,359 PET/CT scans (106,800 scans overall)87 reported four severe adverse events including chest pain (2) and shortness of breath (2). See Appendix C for details on PET/CT–related adverse events in these studies.
- KQ3a.
How are patient factors related to the harms of different imaging techniques?
No included studies addressed this subquestion.
- KQ3b.
What are patient perspectives on the tolerance of different imaging techniques and the balance of benefits and harms of different imaging techniques?
Key Points
- No pertinent evidence exists on other screening tests, or any imaging tests for diagnosis/staging.
Detailed Synthesis
One study addressed this question.171 Authors in the Netherlands enrolled 69 patients at high risk of having (or developing) pancreatic adenocarcinoma in a screening program. In the study “high risk” was defined as anyone with a first-degree relative with pancreatic cancer or anyone with a gene mutation prone to pancreatic cancer. The screening examinations involved both EUS and MRI, and all patients enrolled had received both imaging tests (testing interval between tests was a maximum of 2 weeks).
Patients were asked a question about their comfort level during EUS and MRI with this wording: “How did you experience undergoing an MRI? Was this experience: not uncomfortable, slightly uncomfortable, very uncomfortable or extremely uncomfortable.” For EUS, 10 percent of patients found it very uncomfortable, and for MRI this percentage was 11 percent. For EUS, the stated reason for lack of comfort involved either inadequate sedation or oversedation, whereas for MRI the stated reason involved claustrophobia. The authors also reported “there was no statistically significant difference in the frequency that respondents were dreading the procedure,” but they did not report the percentages of patients feeling dread beforehand.
Conclusions for KQ3
In the diagnosis and staging of pancreatic adenocarcinoma, different imaging tests are associated with different types of harms. MDCT and PET/CT use radiation and therefore can theoretically contribute to future development of malignancy, but the size of the risk is not possible to estimate specifically when used for diagnosis/staging of pancreatic adenocarcinoma. EUS-FNA risks are due to the physical invasiveness of the procedure and primarily involve pancreatitis, postprocedural pain, and puncture, perforation, or bleeding. Regarding patient tolerance, one study of screening found that about 10 percent of patients state that EUS-FNA and MRI are very uncomfortable.
Test Performance of Imaging Modalities for Screening Asymptomatic Adults at High-Risk
- KQ4.
Imaging Techniques for Screening Asymptomatic People
Key Points
- No accuracy estimates are possible for any single imaging modality because the six included screening studies provided accuracy data only for combinations of imaging tests.
- Of 46 patients with a pathological specimen from either biopsy or surgery in the six screening studies, 17 total (1.1% to 9.0% of HRIs screened) had true-positive findings (i.e., pathology-confirmed precursor lesions or pancreatic adenocarcinoma); 19 total (0% to 9.8% of HRIs screened) had major false-positive findings (i.e., patient had surgical resection based on imaging and pathology that showed a benign lesion, e.g., branch duct intraductal papillary mucinous neoplasia [BD-IPMN] with low-grade dysplasia); seven total (0% to 9.2% of HRIs screened) had a minor false-positive finding (i.e., patient had a FNA biopsy based on imaging but pathology was normal, so no surgery was performed). An additional three patients (0% to 1.5% of HRIs screened) had false-negative findings (i.e., patient’s cancer was missed on image screening but found on later screening with pathology confirmation).
Detailed Synthesis
Six primary studies met inclusion criteria for this question, of which five were recent (published in 2009 or later). The group of studies was heterogeneous in the populations studied, imaging tests examined, the study design, and reporting of results, which limits generation of conclusions.
Differences in populations studied resulted from different definitions of high-risk. Most studies used a combination of personal and family history of pancreatic cancer and/or a familial cancer syndrome (i.e., familial pancreatic cancer) and/or a hereditary predisposition to tumors (i.e., Peutz-Jeghers syndrome). One study172 screened only individuals with a known p16 gene mutation, which is associated with various cancers and found most prominently in pancreatic cancer.
Studies varied in the choice of imaging studies and screening protocols. One study172 examined the use of MRI only for screening HRIs, whereas the others looked at a combination of MRI/magnetic resonance cholangiopancreatography (MRCP) with EUS-FNA, some with the addition of MDCT and also ERCP. Two studies173,174 looked at one-time-only initial screening of HRIs, whereas four studies had followup screening annually or more frequently for individuals from whom it was indicated. Followup times ranged from 5 to 50.4 months through the studies.
All six studies were prospective, and none were randomized controlled trials; however, there were other differences in study designs. One study175 had a control arm of 149 non-HRIs. Two studies173,175 reported some level of blinding to test interpretation; radiologists blinded to results of other imaging reports and endoscopists blinded to imaging results, and in one study, pathologists were unaware of clinical or radiologic findings.175 In two studies,175,176 only one endoscopist performed all EUS-FNA procedures, and in two studies,175,177 only one radiologist performed radiologic interpretations of interest in the study (MRI and MDCT). However in other studies it was unclear and there was no stated accounting for variability in performance of EUS-FNA or interpretation of radiologic and EUS images. Studies’ reference standard was not confirmed surgical pathology for all cases because most were not treated. Reference standard was “followup,” which in some cases was a pathologic specimen (biopsy or surgical) only for those who warranted such interventions clinically, but in most cases was a clinical visit or followup imaging. The clinical judgment for surgery was determined in most cases by a multidisciplinary team according to institution-specific standards, in some cases with parameters around appropriate indications. In one multi-site study,173 standard of care for surveillance and treatment was determined by each individual site.
Most of these studies were not designed to assess accuracy of individual imaging modalities for screening of HRIs, but rather the accuracy of screening HRIs with a combination of imaging modalities as deemed clinically appropriate. Similarly, they were not designed to assess comparative accuracy of imaging modalities. Therefore, studies did not uniformly nor comprehensively report results for each imaging modality performed, which prevents conclusions about accuracy of any particular imaging tests or comparative accuracy.
Data tables in Appendix D present the findings from the screening studies. From three of the six screening studies that reported such data, 52 percent to 63 percent of HRIs had completely normal imaging studies (for any imaging modality—MDCT, EUS-FNA, MRI) throughout the study periods. An additional 18 percent to 45 percent of HRIs from the same three studies had some abnormal imaging findings, but not sufficient to warrant biopsy or surgery during the study periods. Some of these abnormal findings, such as changes suggestive of pancreatitis, although noteworthy, were not deemed precursor lesions to pancreatic adenocarcinoma for which biopsy or surgical intervention was necessary. So within the three studies, two studies stated that 97 percent and one study stated that 81 percent of HRIs screened had no concerning imaging findings (by any imaging modality) that resulted in additional intervention.
Fourteen individuals (2.1%) from all six studies (total N=665) were found to have in situ or frank adenocarcinoma. While these rates are still relatively low, they are significantly higher among the HRI population than among the general population with an annual incidence of 0.014 percent.1 However the desirable approach to screening is to uncover precursor lesions before they become adenocarcinoma. Among all HRIs enrolled from all 6 studies, a total of 46 individuals (7% of all enrolled HRIs from 6 studies, with individual studies ranging from 2% to 18% of HRIs screened) had abnormal findings on imaging findings (on any imaging modality) that resulted in either a biopsy or surgery.
Of the total of 46 HRIs with a pathological specimen from either biopsy or surgery from all 6 screening studies, 17 total had true-positive findings (i.e., pathology confirmed precursor lesions or pancreatic adenocarcinoma). The true-positive findings for individual studies ranged from 1.1 percent to 9.0 percent of HRIs screened. Canto et al. (2012)173 acknowledged a more conservative approach and had a lower rate of true positives along with Al-Sukhni (2012).177 However Al-Sukhni also had two cases of false negatives in which biopsy-confirmed cancer lesions and precursor lesions were found on subsequent imaging screenings (4th and 5th rounds) that were not seen on initial imaging. Although we categorized this as a “false-negative” with respect to the imaging modalities’ ability to detect a precursor lesion, it is arguable whether the biology of the tumor is such that the rapid cancer development in HRIs is the primary attributable factor. The Vasen et al. (2011) study reported one false negative study in a patient who was diagnosed with pancreatic adenocarcinoma when symptoms developed 4 months after the negative screening MRI.172 The Canto et al. (2012)173 study did not report any false negatives, but the authors also focused on a one-time initial screening and had an average followup period of 28 months (range, 14–47.2 months), perhaps not allowing for additional cases of undetected pancreatic cancers to be detected. Higher rates of true positives were observed in the studies by Verna (2010),174 Vasen (2011),172 and Canto (2006)175 (4.9%, 9.0%, 5.1%, respectively); also, these studies had higher rates of major false positives (5.1% to 9.8%) (i.e., patients went to surgery for benign lesions that were not deemed precancerous lesions). The challenge remains that certain lesions such as the various grades of IPMN and PanIN 1–3 cannot be reliably distinguished by imaging modalities. Even with FNA biopsy, Langer et al. (2009)176 also reported high rates of false positives. In that same study, there were 7 cases of “minor false positives” in which the HRI had an FNA biopsy based on imaging and pathology that was normal, but surgery was avoided.
Individual study observations suggest that CT alone as an imaging modality for screening HRI may be insufficient.173,175 In Canto et al. (2012),173 the authors noted fewer pancreatic lesions were detected by MDCT than by MRI and EUS-FNA. Individual study observations also suggest that EUS-FNA alone may “overcall suspicious lesions,”177 but in combination with additional imaging such as MRI, it may be useful to prevent unnecessary surgery.
Conclusions for KQ4
The six included studies were not designed to assess accuracy of individual imaging modalities for screening of HRIs, but rather the accuracy of screening HRIs with a combination of imaging modalities as deemed clinically appropriate. Similarly, they were not designed to assess comparative accuracy of imaging modalities. Studies did not uniformly or comprehensively report results for each imaging modality performed, which prevents conclusions about accuracy for any particular imaging test or comparative accuracy. However, we describe some observations from the studies on various imaging modalities below.
MDCT has been the most used imaging modality for screening. Its advantages are that it is widely available, noninvasive, well-tolerated, and less dependent on test operators and interpreters. In certain populations at high risk for pancreatic cancer (e.g., Peutz-Jeghers syndrome, BRCA2 mutation) who are also at high risk of developing other cancers (ovarian cancer, melanoma), there may be additional utility to screening with imaging modalities such as MDCT (and MRI) because of the possibility of detecting other cancers outside of the pancreas and outside of the range of EUS. In a few studies174,175,177 extrapancreatic neoplasms were detected among HRIs (located in ovaries, kidneys, lung). In some studies, MDCT missed lesions that were detected through EUS.175
Some studies report that MRI/MRCP has similar abilities to detect precursor lesions.176 Moreover, MRI/MRCP has the advantage of not exposing patients to radiation, which is important, given the repeated nature of some screening regimens proposed. As mentioned above, MRI also has the ability to detect extrapancreatic lesions.
Advantages of EUS-FNA appear to include detection of pancreatic masses smaller than 1 cm, and FNA also allows for tissue sampling to aid in diagnosis. Disadvantages to EUS-FNA include that it is less readily available, more operator dependent, and more invasive than other imaging modalities. Authors of one study believe that EUS-FNA “overcalled” or overdiagnosed suspicious lesions, leading to unnecessary surgical resection.175 Studies reviewed have suggested the use of EUS-FNA as an adjunct to another screening modality such as CT or MRI.175,177 Taken as a whole, the studies examined provide no evidence for conclusions about which imaging modalities are best for screening asymptomatic HRIs for pancreatic cancer screening.
- Introduction
- Results of Literature Searches
- Test Performance of Imaging Modalities for Diagnosis
- Comparative Test Performance of Imaging Modalities for Diagnosis
- Test Performance of Imaging Modalities for Staging
- Comparative Test Performance of Imaging Modalities for Staging
- Harms of Imaging Modalities
- Test Performance of Imaging Modalities for Screening Asymptomatic Adults at High-Risk
- Results - Imaging Tests for the Diagnosis and Staging of Pancreatic Adenocarcino...Results - Imaging Tests for the Diagnosis and Staging of Pancreatic Adenocarcinoma
Your browsing activity is empty.
Activity recording is turned off.
See more...