NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Forman-Hoffman V, Middleton JC, Feltner C, et al. Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder: A Systematic Review Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 May. (Comparative Effectiveness Review, No. 207.)

Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder: A Systematic Review Update [Internet].
Show detailsIntroduction
This systematic review uses current methods to update a report published in 2013 that evaluated psychological and pharmacological treatments of adults with posttraumatic stress disorder (PTSD). This review focuses on updating the earlier work, expanding the range of treatments examined, addressing earlier uncertainties, identifying ways to improve care for PTSD patients, and reducing variation in existing treatment guidelines. Treatments examined are shown in Table A. The analytic framework that guides our review is shown in Figure A.
Table A
Psychological and pharmacological interventions used for treatment of patients with PTSD.
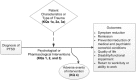
Figure A
Analytic framework for the comparative effectiveness of psychological treatments and pharmacological treatments for adults with PTSD. KQ = Key Question; PTSD = posttraumatic stress disorder.
Results/Key Findings
- We used information from 207 published articles reporting on 193 studies to answer our Key Questions (KQs).
- KQ 1 (Psychological Treatment) Findings (Table B)
- –
Two types of cognitive behavioral therapy (CBT) treatments had high strength of evidence (SOE) of benefit in reducing PTSD-related outcomes. These treatments included CBT-exposure and CBT-mixed treatments (CBT-mixed was a term we used to combine CBT treatments that had different types of CBT characteristics).
- –
Other psychological treatments with moderate SOE of benefit included cognitive processing therapy (CPT), cognitive therapy (CT), eye movement desensitization and reprocessing (EMDR), and narrative exposure therapy (NET).
- –
Moderate strength of evidence favored CBT-exposure over relaxation for reducing PTSD-related outcomes.
- KQ 3 (Psychological Versus Pharmacological Treatment) Findings
- –
Insufficient evidence from a single study examined the comparative effectiveness of a psychological and pharmacological treatment.
- KQ 4 (Adverse Events of Treatments)
- –
Most studies did not describe methods used to systematically assess adverse event information.
- –
Insufficient evidence was found for all serious adverse event comparisons between and across psychological and pharmacological treatments.
- –
When looking at the treatments with at least moderate SOE of benefit, the only adverse event found to have at least moderate SOE was nausea, with venlafaxine.
- Contextual Question (CQ) 1a (Components of Efficacious Interventions)
- –
One study determined that the most frequently identified components of efficacious PTSD psychological interventions include psychoeducation, coping skills and emotion regulation, cognitive processing and restructuring (i.e., “meaning making”), imaginal exposure, emotions, and memory processing.
- CQ 1b (Fidelity of Efficacious Treatments When Implemented in Clinical Practice Settings)
- –
No identified studies tested the degree of fidelity of psychological interventions found to be effective in study settings when implemented in clinical practice settings.
Table B
Summary of efficacy and strength of evidence of PTSD psychological treatments.
Table C
Summary of efficacy and strength of evidence of PTSD pharmacological treatments.
Discussion/Findings in Context: What Does the Review Add to What Is Already Known?
Our review found high SOE of efficacy for CBT-exposure and CBT-mixed treatments and moderate SOE of efficacy for CPT, CT, EMDR, and NET. Among pharmacotherapies, we found moderate SOE of efficacy for fluoxetine, paroxetine, and venlafaxine. Few studies compared treatments with each other, including psychological versus pharmacological treatments, although moderate SOE favors CBT-exposure over relaxation for reduction in PTSD-related outcomes. We did not find sufficient information to comment on whether patients with different types of trauma exposure or other characteristics benefited from a particular type of treatment. For the most part, we found insufficient information about adverse events; insufficient evidence for serious adverse events was found for all of the treatments examined.
Our findings are similar to existing guidelines and systematic reviews that have shown that some psychological therapies and some pharmacological treatments are effective treatments for adults with PTSD. The recently published American Psychological Association (APA) review found evidence to strongly recommend CPT, CT, CBT, prolonged exposure (PE), and to, a slightly lesser degree, recommend EMDR, NET, and brief eclectic psychotherapy (BEP).87 Each of these psychological treatments had at least moderate or high strength of evidence of efficacy to reduce PTSD symptoms in this updated review, with the single exception of BEP having insufficient strength of evidence for reduction in PTSD symptoms and low strength of evidence for both loss of PTSD diagnosis and reduction in depression symptoms. The APA group also recommended fluoxetine, paroxetine, venlafaxine, and sertraline, the same four medications recommended in the Department of Defense/Veterans Administration guidelines;88 this updated review found moderate strength of evidence in support for fluoxetine, paroxetine, venlafaxine as well, with the exception of limited evidence for sertraline (low SOE), driven by heterogeneity in individual study findings.
For the most part, the conclusions made in this update remain unchanged from our prior review published in 2013 on this topic.89 Additional evidence prompted the increase of a few of the SOE grades for psychological treatments (e.g., CBT-mixed from moderate to high for reduction in PTSD symptoms, loss of PTSD diagnosis, and reduction in depression symptoms; CBT-exposure from moderate to high for loss of PTSD diagnosis; and EMDR from low to moderate for reduction in PTSD symptoms). Conversely, some of the SOE grades decreased from the last review for some of the pharmacological treatments after reassessing the SOE (fluoxetine from moderate to low for no difference for reduction in depression symptoms, sertraline from moderate to low for reduction in PTSD symptoms and from low [for benefit] to low for no difference for reduction in depression symptoms, and topiramate from moderate to low for reduction in PTSD symptoms), although the SOE changed from insufficient to moderate for loss of PTSD diagnosis and low to moderate for reduction in depression symptoms for venlafaxine (reduction in PTSD symptoms remained at moderate). The SOE moved from insufficient to low for reduction in PTSD symptoms for four treatments—trauma affect regulation (TAR), imagery rehearsal therapy (IRT), prazosin, and olanzapine. Consistent with the prior review, the evidence included in this update yielded mostly insufficient evidence regarding comparative effectiveness and harms associated with treatments of interest. Finally, our searches yielded no evidence of studies that met our inclusion/exclusion criteria that tested any of the newly added treatment types (energy psychology/emotional freedom techniques, and the three atypical antipsychotics, ziprasidone, aripiprazole, and quetiapine).
Despite evidence of benefit of several types of psychological and pharmacological treatments for PTSD, however, clinicians still are uncertain about which treatment to select for individual patients. Our findings suggest that clinicians might need to consider other factors in selecting a treatment for PTSD: patient preference of treatment, whether the patient has care available to them, whether they can afford the treatment, whether they have tried any treatments already, or whether the patient has other co-occurring problems like substance use or depression.
Key Limitations and Research Gaps
Key limitations include the following.
- We did not find studies that met our inclusion/exclusion criteria and studied the efficacy or effectiveness of several types of PTSD treatments such as energy psychology, escitalopram, fluvoxamine, desvenlafaxine, duloxetine, tricyclic antidepressants, other second generation antidepressants, newer antipsychotics (e.g., ziprasidone, aripiprazole and quetiapine), benzodiazepines, and other medications such as naltrexone, cycloserine, and inositol. Of note, none of these interventions are currently approved by the Food and Drug Administration to treat PTSD.
- We did not find many studies of comparative effectiveness that directly compared the benefits of two types of treatments.
- Few studies examined whether particular treatments are better or worse for particular kinds of patients.
- Few studies provided information about adverse events associated with PTSD treatments.
Research gaps include the following.
- Comparing psychological and pharmacological treatments with known benefits in reducing PTSD-related outcomes with each other.
- Examining benefits associated with new PTSD treatments and also the currently used treatments (e.g., energy psychology, escitalopram, fluvoxamine, desvenlafaxine, duloxetine, tricyclic antidepressants, other second generation antidepressants).
- Determining whether certain treatments work better or worse for particular types of patients.
- Designing studies to search and record adverse events for patients enrolled in research studies.
A summary of the review is presented in Table D.
Table D
Summary of review characteristics.
Important Studies Underway
One trial of mirtazapine (https://clinicaltrials.gov/show/NCT00302107) and one trial of mindfulness based stress reduction (https://clinicaltrials.gov/show/NCT01532999) described as completed in clinicaltrials.gov but findings not yet published.
Footnotes
NOTE: The references for the Evidence Summary are included in the reference list that follows the appendixes.
- Evidence Summary - Psychological and Pharmacological Treatments for Adults With ...Evidence Summary - Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder: A Systematic Review Update
Your browsing activity is empty.
Activity recording is turned off.
See more...