NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Balk E, Adam GP, Kimmel H, et al. Nonsurgical Treatments for Urinary Incontinence in Women: A Systematic Review Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Aug. (Comparative Effectiveness Review, No. 212.)

Nonsurgical Treatments for Urinary Incontinence in Women: A Systematic Review Update [Internet].
Show detailsThe structure of the Result section is as follows:
We first describe and summarize the full network meta-analyses for urinary incontinence (UI) outcomes (cure, improvement, and satisfaction). The full network meta-analyses included and compared, as possible, all interventions (or intervention categories) across Key Questions (KQ) 1 to 4. The network meta-analyses comparing intervention categories (e.g., alpha agonists, neuromodulation) are described in detail. The network meta-analyses of specific interventions are summarized briefly but are otherwise presented only in the appendixes, as described below. All presented odds ratios (ORs) for UI outcomes are based on network meta-analytic combinations of direct and indirect evidence; none are based on pairwise (standard) meta-analysis of direct evidence only.
Following the description of the full network meta-analyses, are four sections with results specific to each of the four KQs. These include results from the network meta-analyses on UI outcomes (cure, improvement, satisfaction) that are specific to each KQ, quality of life, and adverse events. The analyses and results for the UI outcomes for each KQ are identical to those presented for the full network meta-analyses, but they focus on the relevant KQ.
Overview of the Evidence Base Addressing All Key Questions
The update searches returned 7840 new citations across all databases searched, of which we excluded 7117 during abstract screening (Figure 4). Of the 723 articles screened in and reviewed in full text, 613 were found to be irrelevant, primarily because they did not include the population of interest (more than 90% women with urinary incontinence; 298 studies). Other reasons for exclusion were mostly study-type factors, including a lack of peer review (119 studies), a small sample size in nonrandomized or noncomparative studies (62 studies), a lack of primary results (61 studies), new reports from studies included in the 2012 Agency for Healthcare Research and Quality (AHRQ) review that did not give new outcomes of interest (57 studies), no intervention or comparison of interest (12 studies), and languages we could not translate (4 studies). A full list of excluded studies is in Appendix B. The 109 new studies identified were combined with the 134 studies from the original report that were deemed to meet eligibility criteria. Thus, we included a total of 233 studies in 244 articles, of which 140 reported UI outcomes, 96 reported quality of life outcomes, and 127 reported adverse events.
The eligible studies evaluated the following intervention categories (and combinations thereof):
- Nonpharmacological
- Behavioral therapy
- Intravesical pressure release
- Neuromodulation
- Pharmacological
- Anticholinergics
- Alpha agonists
- Hormones
- Onabotulinum toxin A (BTX)
- Antiepileptic
- Periurethral bulking
Following Figure 4, we first describe the total body of evidence across all KQs and intervention types (nonpharmacological and pharmacological).
Risk of Bias
Risk of bias across all studies is presented in Figure 5. Full risk of bias evaluations by study are given in Appendix D.
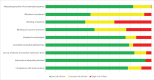
Figure 5
Risk of bias for all studies with urinary incontinence or quality of life outcomes.
Study Characteristics of Studies With Urinary Incontinence Outcomes
The characteristics of the 140 included studies (in 151 articles) that reported UI outcomes are summarized in Appendix C, Table C-3. They are listed in alphabetical order. We identified 51 studies in the update search and included 89 from the 2012 AHRQ report.
Across all trials, the mean or median age of enrollees ranged between 33 and 85 (median 55; interquartile range [IQR] 50 to 59) years. Analyzed sample sizes ranged between 18 and 2393; median 85 (IQR 50 to 218).
Study Characteristics of Studies With Quality of Life Outcomes
Quality of life outcomes were evaluated in 96 studies. Appendix E (Table E-1) gives the baseline data for these studies. Ninety-five studies were randomized controlled trials (RCTs), and one was an nonrandomized comparative study (NRCS); 60 were newly identified in the update. The mean or median ages ranged from 32 to 85 years. Analyzed sample sizes ranged between 14 and 2393 for the RCTs, with a median of 57 (interquartile range [IQR] 33 to 128); the non-randomized study had 6844 participants. Appendix C contains details of study design and baseline (Table C-1) and interventions (Table C-2) for the new studies; information for the studies in the 2012 AHRQ report are given in the appendixes of that report.
The studies evaluated several quality of life domains: bother, daily activities, distress, general health, mental health, pain, sexual health, and sleep/energy. Results are given by KQ below. Appendix E contains summary (Table E-2) and detailed (Table E-3) quality of life results. Quality of life was not evaluated by network meta-analysis, but instead by within-study statistical significance and directionality.
Study Characteristics of Studies Reporting Adverse Events
Adverse events outcomes were evaluated in 127 studies. Most included studies are RCTs or NRCS, but 16 are single group (noncomparative) studies. Results for adverse events are given for each KQ below. Details of design and intervention for the new studies are given in Appendix C; information for the studies in the 2012 AHRQ review are given in the appendixes of that report. Full adverse events data are given in Appendix F.
Key Questions 1 to 4. Network Meta-Analyses for Urinary Incontinence Outcomes Across All Interventions
We first describe the findings from the network meta-analyses for cure, improvement, and satisfaction with control of UI symptoms, together with subgroup analyses. In the following sections we address each Key Question separately, with a focused summary of the UI outcomes and descriptions of the quality of life and adverse event findings.
The evidence graphs in Figures 6 and 7 show that across studies there are 80 comparisons that have been conducted among 51 interventions (Figure 6), which fall into 14 intervention categories (Figure 7). Studies of antiepileptics (pregabalin) and beta-adrenergic agonists (mirabegron) did not report on UI outcomes. The 80 comparisons of specific interventions represent a small percentage of the 1275 possible combinations among the 51 interventions. Most interventions have been compared only with placebo (or sham or no treatment, node P).
Groups of interventions that have not been compared with other groups are readily identified in the figure. For example, four periurethral bulking agents, coded U1, U2, U4, and U7 in Figure 6, have been compared with each other, but have not been compared with any other treatment in the graph. Some treatments, such as intravesical pressure release (intervention “V” in the figures) have been compared with only sham/placebo/no treatment (node P).
![This is the topology map of the network meta-analysis of all the individual compared treatments. Treatments are displayed by circles representing individual treatments or combinations of treatments, grouped together by treatment type. The interventions categories and individual interventions are: alpha agonist (duloxetine, midodrine), onabotulinum toxin A, anticholinergic (oxybutynin, solifenacin, tolterodine, tropsium, fesoterodine, flavoxate, phenylpropanolamine, propantheline, propiverine), hormones (vaginal estrogen, oral estrogen, subcutaneous estrogen, transdermal estrogen, raloxifene), neuromodulation (electroacupuncture, InterStim, magnetic stimulation, transcutaneous electrical nerve stimulation [TENS]), behavioral therapy (bladder training, education, heat therapy, pelvic floor muscle training [PFMT], bladder support, biofeedback, mindfulness-based stress reduction [MBSR], yoga, vaginal weights), periurethral bulking (polyacrylamide, collagen, autologous fat, carbonated beads, polydimethylsiloxane, porcine collagen, dextranomer hyaluronate), intravesical pressure release, and sham/no treatment/placebo. Combinations of aforementioned individual treatments are displayed, and are generally combinations between two or three treatments. Many of the combination treatments are within the behavioral therapy and neuromodulation categories. Lines between treatment circles indicate a direct study comparison of the treatments, and several of the treatment circles connect to the central sham/no treatment/placebo circle. Not all of the treatment circles are connected to each other within the treatment categories or across categories.](/books/NBK534614/bin/ch5f3.gif)
Figure 6
Evidence graph depicting all compared individual treatments in randomized controlled trials. Abbreviations: MBSR = mindfulness-based stress reduction, PFMT = pelvic floor muscle training, TENS = transcutaneous electrical nerve stimulation.
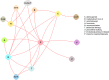
Figure 7
Evidence graph depicting all compared categories of treatments in randomized controlled trials.
Network Meta-Analysis of Cure (Across All Interventions)
The evidence graph for cure with respect to individual treatments is relatively sparse compared to all possible comparisons among treatments (or treatment categories) (Figures 8 and 9). Of note in Figure 8, there are three subgraphs. Three periurethral bulking agents (nodes U1, U2, and U4) have only been compared with each other and not with any of the other interventions in the graph. Also, a combination of vaginal estrogen and bladder support (H1+T5) has been compared with vaginal estrogen only (H1) and not with any other treatment in the graph. All other treatments in the graph have been compared with each other directly or indirectly.
![This is the topology map of the network meta-analysis of all the treatments that evaluated cure. Treatments are displayed by circles representing individual treatments or combinations of treatments, grouped together by treatment type. The interventions categories and individual interventions are: alpha agonist (duloxetine, midodrine), onabotulinum toxin A, anticholinergic (oxybutynin, solifenacin, tolterodine, tropsium, fesoterodine, flavoxate, phenylpropanolamine, propantheline, propiverine), hormones (vaginal estrogen, oral estrogen, subcutaneous estrogen, transdermal estrogen, raloxifene), neuromodulation (electroacupuncture, InterStim, magnetic stimulation, transcutaneous electrical nerve stimulation [TENS]), behavioral therapy (bladder training, education, heat therapy, pelvic floor muscle training [PFMT], bladder support, biofeedback, mindfulness-based stress reduction [MBSR], yoga, vaginal weights), periurethral bulking (polyacrylamide, collagen, autologous fat, carbonated beads, polydimethylsiloxane, porcine collagen, dextranomer hyaluronate), intravesical pressure release, and sham/no treatment/placebo/placebo. Combinations of aforementioned individual treatments are displayed, and are generally combinations between two or three treatments. Many of the combination treatments are within the behavioral therapy and neuromodulation categories. Lines between treatment circles indicate a direct study comparison of the treatments, and several of the treatment circles connect to the central sham/no treatment/placebo circle. Not all of the treatment circles are connected to each other within the treatment categories or across categories. There are also three connected subgraphs: one that connects polyacrylamide, collagen, carbonated beads, and dextranomer hyluronate to each other; one of vaginal estrogen to vaginal estrogen and bladder support; and all remaining nodes.](/books/NBK534614/bin/ch5f5.gif)
Figure 8
Evidence graph of all randomized controlled trials evaluating cure across individual interventions. Abbreviations: MBSR = mindfulness-based stress reduction, PFMT = pelvic floor muscle training, TENS = transcutaneous electrical nerve stimulation.
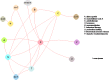
Figure 9
Evidence graph of all randomized controlled trials evaluating cure across types of interventions.
Comparisons Across Intervention Categories
In total, 51 RCTs (7049 women) were included in this analysis of cure; studies ranged in size from 29 to 2487 people. 3,27–74 Table 5 describes the intervention categories compared, the number of women who received each intervention, and the numbers of studies (and women) analyzing each comparison between intervention categories. Forty-five RCTs (83%) were deemed to be at low or moderate risk of bias.
Table 5
Summary description of all studies reporting on cure.
Table 6 shows the ORs for cure comparing all 14 intervention categories that have been evaluated. Further details about the network meta-analyses, including the analysis of individual interventions in each intervention category, are in Appendix G. Only 20 of 91 possible comparisons are informed by direct (head-to-head) comparisons, of which 9 are comparisons with no treatment. In Table 6, the direct comparisons are in the unshaded cells. Shaded cells correspond to comparisons that were inferred from the network meta-analysis model but had not been examined in the included RCTs. For example, alpha agonists have been compared only with neuromodulation and placebo; periurethral bulking and intravesical pressure release devices have each only been compared with no or sham treatment. Comparisons with other active intervention categories are indirect through comparisons other interventions. Indirect comparisons are more uncertain than those for which head-to-head data exist. The added uncertainty of indirect comparisons is partly reflected in the width of their respective confidence intervals, which are broader (often much broader) than for interventions with direct comparisons. For all comparisons that are empirically observed with direct comparisons (all nonshaded cells in the table), results using only head-to-head data (i.e., standard pairwise meta-analysis) agree well with the results from the network meta-analysis (data not shown).
Full Network Summary
First, we describe results from the full network regardless of their primary use for urgency or stress UI or whether they are first, second, or third line therapies. In the subgroup analyses section below, we restrict summaries to those interventions used primarily for stress UI separately from those interventions used primarily for urgency UI.
All active treatments appear to result in higher rates of cure than sham, placebo, or no treatment. The differences versus no treatment are statistically significant for the following active interventions: BTX, anticholinergics, combined hormones and behavioral therapy, neuromodulation, combined neuromodulation and behavioral therapy, and behavioral therapy (alone). There was also a near-significant effect compared with placebo (based on statistically nonsignificant ORs of >2.0 for which the lower bound of the confidence interval is ≥0.80) for combined anticholinergics and behavioral therapy and for intravesical pressure release.
Regarding comparisons of active interventions, based on only statistically significant differences, bladder BTX results in higher cure rates than alpha agonists, anticholinergics, and periurethral bulking. Both neuromodulation (alone) and behavioral therapy (alone) result in higher cure rates than either alpha agonists or anticholinergics. Combined neuromodulation and behavioral therapy also results in higher cure rates than alpha agonists.
Other evidence of possible comparative benefits suggest that intravesical pressure release devices and combined anticholinergics and behavioral therapy each result in higher rates of cure than no treatment; combined hormones and behavioral therapy is favored over alpha agonists; combined neuromodulation and behavioral therapy is favored over anticholinergics; BTX is favored over behavioral therapy; and periurethral bulking agents result in lower rates of cure than neuromodulation, behavioral therapy, and the combination of the two.
Of note, the evidence regarding hormones, combined hormones and anticholinergics, combined anticholinergics and behavioral therapy, and combined hormones, neuromodulation, and behavioral therapy was generally too sparse to allow confident comparisons with other interventions (and in most instances no treatment); 95 percent confidence intervals for all comparisons with these interventions were very wide.
Table 6
Odds ratios for cure between all intervention categories.
The league table (Table 7) offers complementary information from the same analysis. For each intervention category, it shows the mean and forecasted (from the network meta-analysis model) cure rates across the included RCTs. Bladder BTX (B), neuromodulation (N), behavioral therapy (T), and their evaluated combinations (N+T, H+T, H+N+T) had mean cure rates in the 30 to 45 percent range. Anticholinergics with or without behavioral therapy (C, C+T) hormones (H), alpha-agonists (A), periurethral bulking (U) or intravesical pressure release devices (V) had mean cure rates in the 14 to 28 percent range. Sham, placebo, or no treatment had a mean cure rate of 12 percent.
It should be noted that these summary results do not take into account characteristics of the women included in the studies that may be associated with resistance to treatment; thus, the summary findings may be confounded by study. In other words, the network meta-analyses assume that the women across all studies (and all other study characteristics) are generally similar. For example, they do not account for possible differences among women being considered for (and treated with) oral medications, injected or invasive interventions, or nonpharmacological interventions. Subgroup meta-analysis results are presented in the next section.
Descriptions of the comparisons across all individual interventions can be found in Appendix G. Briefly, the results of the analyses of intervention categories are congruent with the corresponding results of the analyses of individual interventions. However, many more of the specific comparisons have very broad confidence intervals because the comparisons across individual interventions are even more sparse than for comparisons of intervention categories.
Table 7
Mean and forecasted cure rates by intervention category (all).
Subgroup Analyses
Key Question Subgroups
For most of the subgroups of particular interest to the stakeholders (women athletes and those engaging in high-impact physical activity, military women or veterans, and racial and ethnic minorities) data within or between studies were sparse or not available. Therefore, no descriptions of these subgroups are possible.
Older Women
Analyses limited to studies with mean age greater than 60 years were congruent with the overall analyses presented here; although different specific comparisons reached statistical significance. Evidence graphs, odds ratio tables, and league tables for these studies can be found in Appendix H, Figure H-4, Tables H-3A and H-3B, and Table H-11. Only 7 studies provided data specifically for women at least 60 years of age. In brief, anticholinergics were found to be (statistically significantly) less likely to result in cure than combined hormones and behavioral therapy (OR 0.09, 95% confidence interval [CI] 0.02 to 0.47), combined neuromodulation and behavioral therapy (OR 0.09, 95% CI 0.02, 0.48), and behavioral therapy alone (OR 0.36, 95% CI 0.14 to 0.92). Combinations of behavioral therapy with either hormones or with neuromodulation were both significantly more likely to achieve cure than no treatment.
Other evidence of possible comparative benefits, based on statistically nonsignificant ORs of >2.0 (for which the lower bound of the confidence interval is ≥0.80) suggest that combinations of behavioral therapy with either hormones or with neuromodulation are favored over behavioral therapy alone and achieve cure in about 70 percent of older women (see Appendix H Table H-11). Cure was achieved in about 36 percent of older women using behavioral therapy alone. Lower rates of cure were found for other interventions.
Stress, Urgency, and Mixed UI Subgroups
Stress UI
Twenty-nine of the 140 studies reported on cure among women who have only stress UI. Evidence graphs, odds ratio tables, and league tables for these studies can be found in Appendix H, Figure H-1A, Tables H-1A, H-1B, and Table H-10 (left side). The smaller number of studies focusing on stress UI translated into relatively fewer possible comparisons of interventions, which included alpha agonists, hormones, behavioral therapy (alone), combination hormones and behavioral therapy, combination hormones and anticholinergics, neuromodulation, combination neuromodulation and behavioral therapy, periurethral bulking, intravesical pressure release, and placebo/sham/no therapy. Of note, hormones and combination hormones and anticholinergics have been compared only with each other and form a separate subgraph than the other interventions.
Only combination hormones and behavioral therapy, behavioral therapy (alone), and neuromodulation have been found to be statistically significantly more effective than placebo/no treatment, with ORs of 11.4, 5.6, and 3.5, respectively.
Alpha agonists were found to be less effective than behavioral therapy (OR 0.22, 95% CI 0.06 to 0.74) or combination hormones and behavioral therapy (OR 0.11, 95% CI 0.01 to 0.84). Periurethral bulking was also less effective than behavioral therapy (OR 0.23, 95% CI 0.06 to 0.98). Statistically nonsignificant comparisons suggest that alpha agonists are also less effective than neuromodulation (OR 0.35, 95% CI 0.12 to 1.00), and periurethral bulking is less effective than combination hormones and behavioral therapy (OR 0.12, 95% CI 0.01, 1.03).
Overall, the studies found that 64 percent of women with stress incontinence achieved cure with combination hormones and behavioral therapy and 46 percent with behavioral therapy alone. About 30 to 35 percent of women achieved cure with neuromodulation, intravesical pressure release, and combination neuromodulation and behavioral therapy. Lower rates of cure were reported with other interventions.
Urgency UI
Only 10 studies reported on cure among women who have only urgency UI. The small number of studies focusing on urgency UI translated into relatively few possible comparisons of interventions, which included BTX, anticholinergics, behavioral therapy, combination anticholinergics and behavioral therapy, neuromodulation, and placebo/sham/no therapy. Evidence graphs, odds ratio tables, and league tables for these studies can be found in Appendix H, Figure H-1B, Table H-2, and Table H-10 (right side).
All five active interventions were statistically significantly more likely result in cure than placebo/sham/no treatment, with ORs ranging from 1.80 (for anticholinergics) to 4.94 (for BTX, see Appendix H, Table H-2 for details). Among active interventions, BTX was statistically significantly more effective than anticholinergics (OR 2.7) or combination of anticholinergics and behavioral therapy (OR 2.2). No other statistically significant differences were found and none of the statistically nonsignificant findings met our criteria for possible effect (OR ≥2 with a lower bound of the confidence interval ≥0.80).
Overall, the studies found that 43 percent of women with urgency incontinence achieved cure with BTX, 25 to 30 percent achieved cure with neuromodulation, behavioral therapy (alone), and combination anticholinergic and behavioral therapy; 21 percent achieved cure with anticholinergics (alone) and 13 percent with no treatment.
Mixed UI
No study that reported cure explicitly included (or reported on) only women with mixed UI (with symptoms of both urgency and stress UI). Therefore, no conclusions are possible beyond a qualitative combination of findings for urgency and stress UI treatments.
Stress and Urgency UI Subgroups Based on Categorization of Interventions
Because only a subset of studies specifically evaluated women with either stress or urgency UI, as noted, the networks are relatively sparse, and findings are less robust than for the overall network meta-analysis. Therefore, we resummarize the overall network in two subgroups, focusing on those intervention categories typically used primarily either for urgency or for stress UI. Interventions commonly used for both are included in both subanalyses.
Stress UI Subanalysis
Among first- and second-line therapies used for stress UI (behavioral therapy, alpha agonists, and hormones, and combinations of these), behavioral therapy was found to be more than twice as effective in achieving cure as alpha agonists alone (OR 2.50, 95% CI 1.19 to 5.26). This finding is supported only by indirect evidence across studies. All other comparisons of first- and second-line therapies (with both direct and indirect comparisons) were statistically nonsignificant with wide confidence intervals.
An indirect comparison found only an imprecise estimate of the comparative effectiveness of the two third-line therapies, periurethral bulking agents and intravesical pressure release, with wide confidence intervals.
Urgency UI Subanalysis
Among first-and second-line therapies used for urgency UI (behavioral therapy, anticholinergics, hormones, and combinations of these), behavioral therapy was found to be significantly more likely to achieve cure than anticholinergics (OR 1.56, 95% CI 1.02 to 2.43). This finding is supported by both direct and indirect evidence. All other comparisons of first- and second-line therapies (with both direct and indirect comparisons) were statistically nonsignificant with wide confidence intervals.
Among third-line therapies used for urgency UI (BTX, neuromodulation, and combinations of neuromodulation with first- or second-line therapies), no therapy was found to be statistically significantly more effective than others.
Of note, several studies directly compared second- with third-line therapies in the same samples of women. Among these, studies found that BTX and neuromodulation are each more likely to achieve cure than anticholinergics (BTX: OR 2.91, 95% CI 1.43 to 5.93; neuromodulation: OR 1.72, 95% CI 1.03 to 2.86; each estimate combining direct and indirect evidence).
Network Meta-Analysis of Improvement (Across All Interventions)
The evidence graph for improvement with respect to individual treatments is sparse (Figure 10) and comprises 2 subgraphs (U1/U2/U4/U7 and all others including U5). Figure 11 shows the evidence graph with respect to types of interventions. All intervention categories are connected in one subgraph.
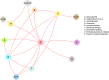
Figure 11
Evidence graph of all randomized controlled trials evaluating improvement across types of interventions.
Comparisons Across Intervention Categories
In total, 64 RCTs (13375 women) were included in this analysis; studies ranged in size from 29 to 5584 women. 17,27–29,32,33,36–38,41–43,48,52,54,55,57,58,60,63,65,67–70,73,75–111 Table 8 describes the intervention categories compared, the number of women who received each intervention, and the numbers of studies (and women) analyzing each comparison between intervention categories. Studies reported improvement outcomes for evaluations of 13 intervention categories. In contrast with the cure outcome, no studies reported improvement for the combination of hormones and behavioral therapy. Fifty-five RCTs (89%) were deemed to be at low or moderate risk of bias.
Table 8
Summary description of all studies reporting on improvement.
Table 9 shows the ORs for improvement comparing all 13 intervention categories that have been evaluated. Further details about the network meta-analyses, including the analysis of individual interventions in each intervention category, are in Appendix G. Only 19 of 78 possible comparisons are informed by direct (head-to-head) comparisons, of which 9 are comparisons with no treatment. In Table 9, the direct comparisons are in the unshaded cells. Shaded cells correspond to comparisons that were inferred from the network meta-analysis model but had not been examined in the included RCTs. For example, alpha agonists have been compared only with neuromodulation and placebo; periurethral bulking and intravesical pressure release devices have each only been compared with no or sham treatment. Comparisons with other active intervention categories are indirect through comparisons with other interventions. Indirect comparisons are more uncertain than those for which head-to-head data exist. The added uncertainty of indirect comparisons is partly reflected in the width of their respective confidence intervals, which are broader (often much broader) than for interventions with direct comparisons. For all comparisons that are empirically observed with direct comparisons (all nonshaded cells in the table), results using only head-to-head data (i.e., standard pairwise meta-analysis) agree well with the results from the network meta-analysis (data not shown).
Full Network Summary
First, we describe results from the full network regardless of their primary use for urgency or stress UI or whether they are first, second, or third line therapies. In the subgroup analyses section below, we restrict summaries to those interventions used primarily for stress UI separately from those interventions used primarily for urgency UI.
All active treatments appear to result in higher rates of improvement than sham, placebo, or no treatment. Hormones and combined hormones and anticholinergics had estimates with wide confidence intervals not suggesting a difference compared with placebo. The differences versus no treatment are statistically significant for the following active interventions: alpha agonists, BTX, anticholinergics, combined anticholinergics and behavioral therapy, neuromodulation, combined neuromodulation and behavioral therapy, the triple combination of hormones and neuromodulation and behavioral therapy, behavioral therapy (alone), and intravesical pressure release. More interventions were found to be more effective to achieve improvement, compared with placebo, than to achieve cure. Alpha agonists and the triple combination of hormones, neuromodulation, and behavioral therapy were not significantly different than no treatment to achieve cure. Intravesical pressure release and combined anticholinergics and behavioral therapy were only nonsignificantly more likely to achieve cure.
Regarding comparisons of active interventions, based on only statistically significant differences, hormones are less effective than all other interventions (excepting combined hormones and anticholinergics and periurethral bulking, for which there are nonsignificant differences). Alpha agonists were found to be statistically less effective to achieve improvement than neuromodulation, behavioral therapy, and combined neuromodulation and behavioral therapy. Anticholinergics were found to be significantly less effective than behavioral therapy.
Other evidence of possible comparative benefits, based on statistically nonsignificant ORs of >2.0 (for which the lower bound of the confidence interval is ≥0.80) suggest that alpha agonists are also less effective to achieve satisfaction than the triple combination of hormones, neuromodulation and behavioral therapy. Anticholinergics are also less effective than the same triple therapy, combined neuromodulation and behavioral therapy, and behavioral therapy alone.
Of note, the evidence regarding periurethral bulking and intravesical pressure release was generally too sparse to allow confident comparisons with other interventions; 95 percent confidence intervals for almost all comparisons with these interventions were very wide.
Table 9
Odds ratios for improvement between all intervention categories.
The league table (Table 10) offers complementary information from the same analysis. For each intervention category, it shows the mean and forecasted (from the network meta-analysis model) improvement rates across the included RCTs. With use of most interventions between about 60 and 70 percent of women had improvement in their symptoms (variously defined across the studies). These more successful interventions included behavioral therapy, neuromodulation, both combinations of the two and with addition of hormones, BTX, combination anticholinergics and behavioral therapy, and intravesical pressure release. With use of anticholinergics (alone), periurethral bulking, and alpha agonists, about 40 to 50 percent of women had improvement. About 25 percent of women on no treatment or placebo had improvement.
It should be noted that these summary results do not take into account characteristics of the women included in the studies that may be associated with resistance to treatment; thus, the summary findings may be confounded by study. In other words, the network meta-analyses assume that the women across all studies (and all other study characteristics) are generally similar. For example, they do not account for possible differences among women being considered for (and treated with) oral medications, injected or invasive interventions, or nonpharmacological interventions. Subgroup meta-analysis results are presented in the next section.
Descriptions of the comparisons across all individual interventions can be found in Appendix G. Briefly, the results of the analyses of intervention categories are congruent with the corresponding results of the analyses of individual interventions. However, many more of the specific comparisons have very broad confidence intervals because the comparisons across individual interventions are even more sparse than for comparisons of intervention categories.
Table 10
Mean and forecasted improvement rates by intervention category (all).
Subgroup Analyses
Key Question Subgroups
For most of the subgroups of particular interest to the stakeholders (women athletes and those engaging in high-impact physical activity, military women or veterans, and racial and ethnic minorities) data within or between studies were sparse or not available. Therefore, no descriptions of these subgroups are possible.
Older Women
Analyses limited to studies with mean age greater than 60 years were congruent with the overall analyses presented here; although different specific comparisons reached statistical significance. Evidence graphs, odds ratio tables, and league tables for these studies can be found in Appendix H, Figure H-5, Table H-6, and Table H-13. Only 18 studies provided data specifically for women at least 60 years of age. In brief, alpha agonists were significantly less likely to achieve improvement than the triple combination of hormones and neuromodulation and behavioral therapy (OR 0.12, 95% CI 0.01 to 0.92) and than behavioral therapy alone (OR 0.17, 95% CI 0.03 to 0.97). Hormone therapy was also less effective than either triple therapy (OR 0.10, 95% CI 0.02 to 0.50) or behavioral therapy alone (OR 0.15, 95% CI 0.03 to 0.67). In addition, hormone therapy was found to be less effective than anticholinergics (OR 0.18, 95% CI 0.03 to 0.97).
Improvement was achieved in about 50 to 70 percent of older women using combined neuromodulation and behavioral therapy, the triple therapy (with hormones), behavioral therapy alone, and anticholinergics. About one-third of older women using neuromodulation alone or combination hormones and anticholinergics were reported to have improvement. With other interventions, including no treatment, 17 or 18 percent of older women had improvement.
Stress, Urgency, and Mixed UI Subgroups
Stress UI
Fifty-two of the studies reported on improvement among women who have only stress UI. Evidence graphs, odds ratio tables, and league tables for these studies can be found in Appendix H, Figure H-2A, Table H-4, and Table H-12 (left side). The small number of studies focusing on stress UI translated into relatively few possible comparisons of interventions, which included alpha agonists, anticholinergics, behavioral therapy, hormones, neuromodulation, combination neuromodulation and behavioral therapy, the triple combination of hormones and neuromodulation and behavioral therapy, intravesical pressure release, and periurethral bulking.
Most evaluated interventions were statistically more effective than placebo/no treatment, including triple therapy (OR 11.6), behavioral therapy (OR 7.0), intravesical pressure release (OR 4.4), neuromodulation (OR 4.0), and alpha agonists (OR 2.3). Other interventions had mostly imprecise estimates of effectiveness.
Across active intervention, in studies of women with stress UI alpha agonists were found to be more effective to achieve improvement than hormones (OR 4.5) but less effective than triple therapy, neuromodulation and behavioral therapy (ORs ranging from 0.2 to 0.6, see Appendix H, Table H-4 for details). Combined hormones and anticholinergics (which are typically used for urgency UI) were less effective than triple therapy and behavioral therapy (OR 0.08 and 0.13, respectively). Hormone therapy (alone) was also less effective than triple therapy, neuromodulation, behavioral therapy, and intravesical pressure release (OR ranging from 0.04 to 0.13). Combined neuromodulation and behavioral therapy was found to be less effective than triple therapy (OR 0.09) and both neuromodulation and behavioral therapy alone (OR 0.25 and 0.14, respectively); however, the latter two comparison results are driven largely by studies that found the combination therapy to be no more effective than no treatment, together with studies that found neuromodulation alone to be highly effective compared with no treatment. Therefore, these indirect comparisons of combination neuromodulation and behavioral therapy with either neuromodulation or behavioral therapy alone are unlikely to be valid.
Statistically nonsignificant findings meeting our criteria for possible effect (OR ≥2 with a lower bound of the confidence interval ≥0.80) suggested that intravesical pressure release was possibly more effective than combination neuromodulation and behavioral therapy (OR 4.4), triple therapy was possibly more effective than periurethral bulking agents (OR 5.9), and neuromodulation was possibly more effective than combination hormones and anticholinergics (OR 4.5, see Appendix H, Table H-4 for details).
Improvement was achieved in about 50 to 80 percent of women with stress UI using triple therapy (hormones, neuromodulation, and behavioral therapy), neuromodulation alone, behavioral therapy alone, and intravesical pressure release. About 40 to 50 percent of women had improvement with alpha agonists and periurethral bulking agents.
With other interventions, including no treatment, 16 to 27 percent of women with stress UI had improvement.
Urgency UI
Only 18 of the of the 140 studies reported on improvement among women who have only urgency UI. The small number of studies focusing on urgency UI translated into relatively few possible comparisons of interventions, which included BTX, anticholinergics, behavioral therapy, combination anticholinergics and behavioral therapy, and neuromodulation. Evidence graphs, odds ratio tables, and league tables for these studies can be found in Appendix H, Figure H-2B, Table H-5, and Table H-12 (right side).
All five active interventions were statistically significantly more likely result in improvement than placebo/sham/no treatment, with ORs ranging from 1.80 (for anticholinergics) to 7.50 (for behavioral therapy, see Appendix H, Table H-5 for details). Among active interventions, anticholinergics were significantly less effective than all other evaluated therapies, with ORs ranging from 0.24 to 0.49. No other significant differences were found.
Overall, women with urgency UI using all interventions had high rates of improvement. Among women using anticholinergics alone, 60 percent had improvement. With all other active interventions between 75 and 86 percent of women reported improvement. With no treatment, 46 percent had improvement.
Mixed UI
Four studies reported on improvement in women with mixed UI.75,76,81,112 In one study each, compared to placebo, both the alpha agonist duloxetine76 (OR 2.10, 95% CI 1.45 to 3.05) and the anticholinergic tolterodine112 (OR 1.85, 95% CI 1.37 to 2.51) were effective to achieve improvement. In two small studies,75,81 neuromodulation was nonsignificantly more likely to achieve improvement than no treatment (summary OR 2.44, 95% CI 0.83, 7.19).
Stress and Urgency UI Subgroups Based on Categorization of Interventions
Because only a subset of studies specifically evaluated women with either stress or urgency UI, as noted, the networks are relatively sparse, and findings are less robust than for the overall network meta-analysis. Therefore, we resummarize the overall network in two subgroups, focusing on those intervention categories typically used primarily either for urgency or for stress UI. Interventions commonly used for both are included in both subanalyses.
Stress UI Subanalysis
Among first- and second-line therapies used for stress UI (behavioral therapy, alpha agonists, and hormones), behavioral therapy was found to be statistically significantly more than twice as effective in achieving improvement as alpha agonists (OR 2.50, 95% CI 1.39 to 4.55) and hormones (OR 10.0, 95% CI 3.57 to 33.3). In addition, behavioral therapy was found to possibly be more effective than combination hormones and anticholinergics (OR 5.28, 95% CI 0.86 to 32.5) across studies, with a similar estimate across studies of women with stress UI. Alpha agonists were also found to be significantly more effective than hormones (OR 4.10, 95% CI 1.40 to 11.9). The comparison with alpha agonists versus behavioral therapy is supported by both direct and indirect comparisons, but the other two comparisons are based on only indirect evidence across studies.
An indirect comparison found only an imprecise estimate of the comparative effectiveness of the two third-line therapies periurethral bulking agents and intravesical pressure release, with wide confidence intervals.
Urgency UI Subanalysis
Among first-and second-line therapies used for urgency UI (behavioral therapy, anticholinergics, hormones, and combinations of these), behavioral therapy was found to be significantly more likely to achieve improvement than anticholinergics (OR 1.82, 95% CI 1.03 to 3.23) and hormones (OR 10, 95% CI 3.57 to 33.3). Hormones were found to be significantly less effective than combination anticholinergics and behavioral therapy (OR 0.10, 95% CI 0.02 to 0.45) or anticholinergics alone (OR 0.18, 95% CI 0.06 to 0.54). However, all these findings were supported only by indirect comparisons.
Among third-line therapies used for urgency UI (BTX, neuromodulation, and combinations of neuromodulation with first- or second-line therapies), no therapy was found to be statistically significantly more effective than others. No study directly compared BTX with the various neuromodulation therapies.
Network Meta-Analysis of Satisfaction (Across All Interventions)
The evidence graph for improvement with respect to individual treatments is sparse (Figure 12), and comprises two subgraphs (B/N2 and all others). Figure 13 shows the evidence graph with respect to types of interventions. All intervention types are connected.
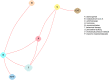
Figure 13
Evidence graph of all randomized controlled trials evaluating satisfaction across intervention categories.
Comparisons Across Intervention Categories
In total, 12 RCTs (3008 people) were included in this analysis; studies ranged in size from 24 to 975 women. 29,31,32,35,52,88,92,103,113,114 Table 11 describes the intervention categories compared, the number of women who received each intervention, and the numbers of studies (and women) analyzing each comparison between intervention categories. Five RCTs (63%) were deemed to be at low or moderate risk of bias.
Table 11
Summary description of all studies reporting on satisfaction.
Table 12 shows the ORs for achieving satisfaction with control of UI symptoms. The table includes all seven intervention categories evaluated. Further details about the network meta-analyses, including the analysis of individual interventions in each intervention category, are in Appendix G. Only 8 of 21 possible comparisons are informed by direct (head-to-head) comparisons, of which 3 are comparisons with no treatment. In Table 12, the direct comparisons are in the unshaded cells. Shaded cells correspond to comparisons that were inferred from the network meta-analysis model but had not been examined in the included RCTs. For example, BTX has been compared only with neuromodulation. Comparisons with other active intervention categories are indirect through comparisons with other interventions. Indirect comparisons are more uncertain than those for which head-to-head data exist. The added uncertainty in indirect comparisons is partly reflected in the width of their respective 95 percent confidence intervals, which are broader (often much broader) for comparisons without direct comparisons than for those with direct comparisons. For all comparisons that are empirically observed with direct comparisons (all nonshaded cells in the table), results using only head-to-head data (i.e., standard pairwise meta-analysis) agree well with the results from the network meta-analysis (data not shown).
Full Network Summary
First, we describe results from the full network regardless of their primary use for urgency or stress UI or whether they are first, second, or third line therapies. In the subgroup analyses section below, we restrict summaries to those interventions used primarily for stress UI separately from those interventions used primarily for urgency UI.
All active treatments appear to result in higher rates of satisfaction with control of UI symptoms than sham, placebo, or no treatment.
Regarding comparisons of active interventions, based on only statistically significant differences, treatment with either BTX or neuromodulation resulted in more women being satisfied than anticholinergics or combination anticholinergics and behavioral therapy. Behavioral therapy alone was found to be significantly more effective than anticholinergics alone.
Other evidence of possible comparative benefits, based on statistically nonsignificant ORs of >2.0 (for which the lower bound of the confidence interval is ≥0.80) suggests that anticholinergics are also less effective than combination neuromodulation and behavioral therapy.
Table 12
Odds ratios for satisfaction between all intervention categories.
The league table (Table 13) offers complementary information from the same analysis. For each intervention category, it shows the mean and forecasted (from the network meta-analysis model) satisfaction rates across the included RCTs. Most women were satisfied with any of the active treatments. BTX, neuromodulation, behavioral therapy, and combination neuromodulation and behavioral therapy achieved satisfaction rates of about 80 to 85 percent; 66 percent of women using combination anticholinergics and behavioral therapy, as did 55 percent of women using anticholinergics alone. Only 32 percent of women in no treatment study arms achieved satisfactory control of their UI symptoms.
It should be noted that these summary results do not take into account characteristics of the women included in the studies that may be associated with resistance to treatment; thus, the summary findings may be confounded by study. In other words, the network meta-analyses assume that the women across all studies (and all other study characteristics) are generally similar. For example, they do not account for possible differences among women being considered for (and treated with) oral medications, injected or invasive interventions, or nonpharmacological interventions. Subgroup meta-analysis results are presented in the next section.
Descriptions of the comparisons across all individual interventions can be found in Appendix G. Briefly, the results of the analyses of intervention categories are congruent with the corresponding results of the analyses of individual interventions. However, many more of the specific comparisons have very broad confidence intervals because the comparisons across individual interventions are even more sparse than for comparisons of intervention categories.
Table 13
Mean and forecasted satisfaction rates by intervention category (all).
Subgroup Analyses
Key Question Subgroups
For most of the subgroups of particular interest to the stakeholders (women athletes and those engaging in high-impact physical activity, military women or veterans, and racial and ethnic minorities) data within or between studies were sparse or not available. Therefore, no descriptions of these subgroups are possible.
Older Women
Analyses limited to studies with mean age greater than 60 years were congruent with the overall analyses presented here; although different specific comparisons reached statistical significance. Evidence graphs, odds ratio tables, and league tables for these studies can be found in Appendix H, Figure H-6, Table H-9, and Table H-15. Only 2 studies provided data specifically for women at least 60 years of age. Only anticholinergics, behavioral therapy, and no treatment were compared among these studies. In brief, both active interventions were more effective than no treatment (anticholinergics: OR 2.30, 95% CI 1.11 to 4.75; behavioral therapy: OR 8.01, 95% CI 4.01 to 16.0). Behavioral therapy was significantly more likely to achieve satisfactory control of UI symptoms.
Satisfaction was achieved in about three-quarters of older women using behavioral therapy, about half of women using anticholinergics, and about one-quarter of women in no treatment study arms.
Stress, Urgency, and Mixed UI Subgroups
Stress UI
Six of the studies reported on satisfaction among women who have only stress UI. Evidence graphs, odds ratio tables, and league tables for these studies can be found in Appendix H, Figure H-3A, Table H-7, and Table H-12 (left side). The small number of studies focusing on stress UI translated into relatively few possible comparisons of interventions, which included only neuromodulation, behavioral therapy, and no treatment.
Both active interventions were statistically more effective than placebo/no treatment (neuromodulation OR 8.4; behavioral therapy OR 5.5). The estimate of comparative effectiveness between the two active interventions was imprecise (with a very wide confidence interval).
Satisfaction was achieved in 82 percent of women with stress incontinence using neuromodulation, 75 percent using behavioral therapy, and 35 percent in no treatment study arms.
Urgency UI
Twelve of the studies reported on satisfaction among women who have only urgency UI. The small number of studies focusing on urgency UI translated into relatively few possible comparisons of interventions, which included anticholinergics, behavioral therapy, combination anticholinergics and behavioral therapy, BTX, and neuromodulation. The comparisons fell into two nonoverlapping subgraphs: one comparing BTX with neuromodulation; one comparing the other interventions. Evidence graphs, odds ratio tables, and league tables for these studies can be found in Appendix H, Figure H-3B, Tables H-8A and H-8B, and Table H-12 (right side).
Only behavioral therapy was found to be significantly more likely to result in satisfaction with control of UI symptoms than no treatment (OR 8.2, 95% CI 1.70 to 39.4). However, note that BTX and neuromodulation could not be directly or indirectly compared with no treatment. Among active interventions that could be compared, BTX was nonsignificantly favored over neuromodulation (OR 1.40, 95% CI 0.93 to 2.12). Note, though, that neuromodulation is typically used for stress UI.
Among women using behavioral therapy 75 percent had satisfaction. For all other active interventions between about 50 and 60 percent had satisfaction. With no treatment, 27 percent had satisfaction.
Mixed UI
One study reported on satisfaction specifically in women with mixed UI.112 Women with mixed UI who used the anticholinergic tolterodine had greater satisfaction than with placebo (OR 2.60, 95% CI 1.89 to 3.57).
Stress and Urgency UI Subgroups Based on Categorization of Interventions
Because only a subset of studies specifically evaluated women with either stress or urgency UI, as noted, the networks are relatively sparse, and findings are less robust than for the overall network meta-analysis. Therefore, we resummarize the overall network in two subgroups, focusing on those intervention categories typically used primarily either for urgency or for stress UI. Interventions commonly used for both are included in both subanalyses.
Stress UI Subanalysis
The only intervention commonly used for stress UI that has been evaluated for satisfaction is behavioral therapy. Therefore, no comparison with other stress UI interventions can be made.
Urgency UI Subanalysis
Among first-and second-line therapies used for urgency UI (behavioral therapy, anticholinergics, and combination anticholinergic and behavioral therapy), behavioral therapy was found to be significantly more likely to achieve satisfaction than anticholinergics (OR 3.13, 95% CI 1.85 to 5.26).
Among third-line therapies used for urgency UI (BTX, neuromodulation, and combinations of neuromodulation with first- or second-line therapies), no therapy was found to be statistically significantly more effective than others.
Key Question 1. What are the benefits and harms of nonpharmacological treatments of UI in women, and how do they compare with each other?
In the sections for KQ 1 to 4, we summarize the UI outcome data (cure, improvement, satisfaction) pertinent to each KQ. In these summaries we focus on the comparisons of interventions specific to stress UI and urgency UI separately. These summaries recapitulate the findings (pertinent to each KQ) summarized in the Subgroup Analyses subsections of the Key Questions 1 to 4: Network Meta-Analyses section above. Here we also summarize the quality of life and adverse event data.
Key Points
- Trials evaluated 5 intervention categories (including sham or no intervention) and 38 specific interventions. Trials directly compared 7 of 10 possible comparisons of intervention categories, of which 3 were comparisons between active interventions (not sham or no treatment). Trials directly compared 50 of 703 possible comparisons of specific interventions, of which 30 were comparisons of active interventions.
- For women with stress UI,
- Behavioral therapy was the only first- (or second-) line nonpharmacological treatment evaluated. Behavioral therapy was significantly more effective than no treatment to achieve cure (OR 3.1 across all studies; OR 5.6 in stress UI studies), improvement (OR 5.4 across all studies; OR 7.0 in stress UI studies), and satisfaction (OR 8.0 across all studies; OR 5.5 in stress UI studies).
- In studies of women with stress UI, intravesical pressure release and neuromodulation were evaluated as third-line nonpharmacological treatments. Based on data from these studies, comparisons of neuromodulation or combination neuromodulation and behavioral therapy with intravesical pressure release were mostly very imprecise (based on indirect comparisons). However, intravesical pressure release may result in higher rates of improvement than combination neuromodulation and behavioral therapy (OR 4.3, 95% CI 0.9, 20.0).
- In studies of women with stress UI, neuromodulation alone was significantly more likely than no treatment to achieve cure (OR 3.5), improvement (OR 4.0), and satisfaction (OR 8.4).
- Intravesical pressure release may be more effect than no treatment to achieve cure based on nonsignificant summary effect (OR 2.7, 95% CI 0.8, 9.0) across all studies, but no significant difference was found in stress UI studies. However, intravesical pressure release was significantly more likely to achieve improvement than no treatment (OR 4.6 across all studies; OR 4.4 in stress UI studies). The studies of intravesical pressure release did not report satisfaction.
- Studies of women with stress UI directly compared third-line neuromodulation and combined neuromodulation and behavioral therapy with first-line behavioral therapy. Studies found no significant differences in rates of cure, improvement, or satisfaction.
- For women with urgency UI,
- Behavioral therapy was the only first- (or second-) line nonpharmacological treatment evaluated. Behavioral therapy was significantly more effective than no treatment to achieve cure (OR 3.1 across all studies; OR 2.8 in urgency UI studies), improvement (OR 5.4 across all studies; OR 7.5 in urgency UI studies), and satisfaction (OR 8.0 across all studies; OR 8.2 in urgency UI studies).
- Neuromodulation and BTX were the third-line nonpharmacological treatments evaluated in women with urgency UI; although neuromodulation is typically used only for women with stress UI. Neuromodulation was significantly more effective than no treatment to achieve cure (OR 3.3 across all studies; OR 2.8 in urgency UI studies), improvement (OR 5.4 across all studies; OR 7.5 in urgency UI studies), and satisfaction (OR 8.0 across all studies; no comparison possible from urgency UI studies. BTX was nonsignificantly favored over neuromodulation to achieve satisfaction in studies of women with urgency UI (OR 1.40).
- Studies of women with urgency UI directly compared third-line neuromodulation with first-line behavioral therapy. Studies found no significant differences in rates of cure or improvement. Satisfaction was not reported in these studies.
- There were 78 studies that reported on quality of life outcomes.
- Studies reported on 15 nonpharmacological interventions (including combinations of interventions) that were compared with sham or no interventions. All interventions evaluated by more than one study were found to have a statistically significant improvement in at least one aspect of quality of life by at least one study, except for PFMT.
- Studies reported on 19 nonpharmacological interventions that were compared with each other. Home, supervised, or group PFMT was found to result in better quality of life than a more basic PFMT regimen. Combined PFMT and biofeedback has been found to improve the daily activities quality of life, but not other quality of life domains, compared with PFMT alone.
- There were 58 studies that reported on adverse events of nonpharmacological treatments.
- Among interventions for which at least two studies reported any specific adverse event, no (undefined or nonmajor) adverse events were reported with bladder training (2 studies, 106 women), education (4 studies, 277 women), PFMT (21 studies, 1560 women), combined PFMT and biofeedback (3 studies, 83 women), and combined PFMT, TENS, and biofeedback (2 studies, 107 women) (all low SoE).
- Among 10 studies of TENS, 8 reported no adverse events in 396 women, but two reported any or any moderate adverse event in a total of 13 of 67 women (19%). Overall, adverse events were reported in 2.8% of 463 women receiving TENS (moderate SoE).
- In 3 studies of TENS, 11% of women (n = 217) reported urinary tract infections; however, within studies, these rates were similar to or lower than urinary tract infection rates on an anticholinergic (6%) or with BTX (35%).
- In two studies of magnetic stimulation, 3 of 110 women total (2.7%) had undefined adverse events.
Findings
Table 14 repeats simplified data from Tables 7 (cure), 10 (improvement), and 13 (satisfaction) limited to nonpharmacological treatments (pertinent to this KQ). It summarizes the mean cure rates of the nonpharmacological treatments based on the network meta-analysis models of all studies and of the two subsets of studies of women with stress UI or with urgency UI.
Cure
All nonpharmacological treatments were associated with better cure rates than sham or no treatment (Table 14). Across all studies, the point estimates for the cure rates are between 27 and 35 percent for the active treatments versus 12 percent for sham or no treatment. With one exception, cure rates were similar in the studies restricted to stress or urgency UI (among relevant interventions). Behavioral therapy (1st line therapy) was somewhat higher in studies of women with stress UI (46%) than across all studies (30%). Except for intravesical pressure release, all nonpharmacological treatments resulted in statistically significantly higher cure rates than no or sham treatment, with ORs ranging from 3.1 to 4.0 across all studies (see Table 6). Intravesical pressure release (3rd line therapy) attained near-significance against no treatment (OR 2.7, 95% CI 0.8 to 9.0) across all studies, although the estimate was highly imprecise in studies of women with stress UI only.
With respect to cure, there were no statistically significant differences between categories of nonpharmacological interventions. From Table 6, the point estimates of the ORs between any two nonpharmacological treatments are smaller than 1.45 (or greater than its inverse). However, the confidence intervals are generally broad and cannot exclude relatively large differences. Estimates of differences were similar, but less precise, from studies restricted to women with either stress UI or urgency UI (Appendix H, Tables H-1 to H-2).
Table 14
Summary league table displaying percent of women with UI outcomes for each nonpharmacological treatment.
Improvement
All nonpharmacological treatments were associated with better improvement rates than sham or no treatment (Table 14). Across all studies, the point estimates for the improvement rates are between about 60 and 70 percent for the active treatments versus 25 percent for sham or no treatment. Improvement rates were for all evaluated nonpharmacological treatments were higher in studies of women with urgency UI only than across all studies. In studies of women with stress UI combination neuromodulation and behavioral therapy resulted in a low average rate of improvement (27%). All nonpharmacological treatments resulted in statistically significantly higher improvement rates than no or sham treatment, with ORs ranging from 4.2 to 6.7 across all studies (see Table 9). Estimates of effect compared with no or sham treatment were similar in studies restricted to women with stress UI or urgency UI, except that combination neuromodulation and behavioral therapy was not significantly different than control (see Appendix H, Tables H-4 and H-5).
With respect to improvement, there were no statistically significant differences between categories of nonpharmacological interventions. From Table 9, the point estimates of the ORs between any two nonpharmacological treatments are nonsignificant and imprecise, with confidence intervals that cannot exclude relatively large differences. Estimates of differences were mostly similar from studies restricted to women with either stress UI or urgency UI, except that in studies of stress UI, combination neuromodulation and behavioral therapy (3rd line therapy) was significantly less effective than either neuromodulation (3rd line therapy) or behavioral therapy (1st line therapy) alone (Appendix H, Tables H-4 and H-5).
Satisfaction
The three evaluated nonpharmacological treatments were associated with better satisfaction rates than sham or no treatment (Table 14). Across all studies, the point estimates for the satisfaction rates are about 80 percent for the active treatments versus 32 percent for sham or no treatment. Rates of satisfaction after behavioral therapy were similar in studies of women with only stress UI or urgency UI. Satisfaction rates after neuromodulation were also similar in studies of women with stress UI, but were lower (51%) in studies of women with urgency UI. All nonpharmacological treatments resulted in statistically significantly higher satisfaction rates than no or sham treatment, with ORs ranging from 8.0 to 10.7 across all studies (see Table 12). Estimates of effect compared with no or sham treatment were similar in studies restricted to women with stress UI or urgency UI (see Appendix H, Tables H-7 to H-8).
With respect to satisfaction, there were no statistically significant differences between categories of nonpharmacological interventions. From Table 12, the point estimates of the ORs between any two nonpharmacological treatments are nonsignificant and imprecise, with confidence intervals that cannot exclude relatively large differences. From studies restricted to women with either stress UI, the only available comparison found a nonsignificant difference between neuromodulation (3rd line therapy) and behavioral therapy (1st line therapy) among women with stress UI, based in part on direct head-to-head comparisons (Appendix H, Tables H-7 to H-8). Among studies of women with urgency UI, BTX (3rd line therapy) was nonsignificantly favored to achieve satisfaction over neuromodulation (OR 1.40, 95% CI 0.93 to 2.12).
Quality of Life
Seventy-eight studies reported on quality of life outcomes for the comparison of nonpharmacological interventions versus placebo or other nonpharmacological interventions. 28,31,35,43,46,49,51–53,56,63,66,69,74,93,98,103,108,109,111,115–170 The summary results are presented in Tables 15 and 16. The table cells show the number of studies and the number of people (in parentheses), followed by the number of studies with the number of studies that found statistically significant differences and which intervention was favored, the number of studies that found discordant results (that is, within the same study, significant differences favoring one intervention were found on one scale or subscale, but nonsignificant differences were found on others), and the number of studies with nonsignificant differences. Study-level details, including citations, are presented in Appendix E.
Nonpharmacological Interventions Versus Sham or No Treatment
Fifteen nonpharmacological interventions (including combinations of interventions) were compared with sham or no treatments in 36 studies (Table 15). Twenty-three of these studies evaluated behavioral interventions, which included bladder training, education, PFMT, biofeedback, electroacupuncture, weight loss, and yoga, and combinations of these interventions, 35,49,52,53,56,69,93,98,103,109,111,117,119–121,125,127,134,137,142,147,148,150,155,156,162–165,167–169 three combinations of behavioral and neuromodulation,28,43,162 and one an intravesical pressure release device.63 The studies mostly analyzed daily activity, bother, general health, and mental health.
Across interventions, the effect of the nonpharmacological interventions on various aspects of quality of life was mixed. Among interventions compared by more than one study, only TENS (alone) was found by all studies to be statistically significantly better than sham for any specific aspect of quality of life. TENS alone was found to result in better mental health quality of life (2 studies).35,93 All interventions evaluated by more than one study were found to have a statistically significant improvement in at least one aspect of quality of life by at least one study, except for PFMT, in which only discordant or nonsignificant results were found in daily activities and general health. Only two interventions for which any aspect of quality of life was evaluated were not found to result in better quality of life than sham: combined bladder training and PFMT, and the intravesical pressure release device. However, the data for these interventions were sparse. The two studies evaluating these two interventions each reported on only a single aspect of quality of life.
Table 15
Quality of life outcomes for nonpharmacological interventions versus sham/no treatment.
Nonpharmacological Interventions Versus Other Nonpharmacological Interventions
Nineteen nonpharmacological interventions (including combinations of interventions) were compared with other active interventions (Table 16). Forty-two studies evaluated behavioral interventions versus other behavioral interventions (or combinations of behavioral interventions), including bladder training, education, PFMT, biofeedback, electroacupuncture, weight loss, and yoga. 31,46,51,66,74,108,115–118,122–124,126,128–133,135,136,138–141,143–146,149,151–153,157–162,166,170 One study evaluated neuromodulation (electric stimulation) versus behavioral therapy,35 and ten evaluated combinations of behavioral therapy and neuromodulation versus behavioral therapy alone. 31,66,108,116,117,136,138,159,162 These studies mostly analyzed bother, daily activities, distress, general health, mental health, and sexual health.
Few interventions were compared by more than one study, and these contrasts generally were split between discordant or nonsignificant findings when analyzing bother, daily activities, or general health. Supervised PFMT was found to improve quality of life statistically significantly more than unsupervised PFMT or other specific types of exercise or physical therapy in one study for bother and in two studies each for the domains of daily activities and mental health. The other studies evaluating these comparisons reported discordant or nonsignificant differences between the interventions.46,51,74,117,118,123,124,126,128–130,133,139,141,146,149,152,153,157,170
One study reported statistically significant improvements in the daily activities domain with PFMT and biofeedback compared with PFMT alone, and one study reported significant improvements in distress for bladder training combined with PFMT and biofeedback when compared to bladder training alone or PFMT with biofeedback117,166 However, nine studies either reported discordant or nonsignificant differences across all other domains for this comparison.35,74,124,128,129,131,133,138,149
Additionally, two studies found significant improvements among the control groups for two outcomes; bladder training alone was found to have significant improvements in bother ratings over combined TENS, PFMT, and biofeedback, and electroacupuncture alone was found to have significant improvements when compared to PFMT with weights in assessing impact on daily activities.108,131
Table 16
Quality of life outcomes for nonpharmacological treatments versus other nonpharmacological treatments.
Adverse Events
Fifty-two studies reported on adverse events in studies of nonpharmacological interventions.14,29,32,46,54,60,69,92,95,98,109,115,119,120,123,126,128,129,132,133,136,138,140,144,147,149,150,154,156,157,160,161,165,168,169,171–187 The results are presented in Tables 17 and 18 (parts 1 and 2, respectively). For adverse events that were reported only by a single study (arm), the table cells show the percentage of people affected by the adverse event. Where more than one study arm reported on an adverse event, the cell gives the median and range of percentages (or just the range, if only two arms reported an adverse event). Detailed results are in Appendix F.
In general, the percentages of women with adverse events were low, but reporting was sparse with only one or two studies reporting adverse events for most of the interventions. No specific adverse event (e.g., urinary tract infection, diarrhea, dry mouth) was reported in more than one study for any specific intervention (e.g., acupuncture, bladder support). The most commonly reported adverse event was the group nonmajor or undefined adverse event. In two studies of bladder training, four studies of education, and two of three studies of magnetic stimulation (with 76 women), no adverse events were reported. The other study of magnetic stimulation reported that 3 of 60 women (5%) had an undefined adverse event. In two studies of InterStim™ (a form of sacral neuromodulation), 1 of 284 women total (0.4%) had a “serious” adverse event (an implant site erosion). In two studies of electroacupuncture (287 women) one reported no adverse events in 24 women, the other reported four adverse events in 247 women. A small study of bladder support (29 women) reported no adverse events.
Among 13 studies of TENS, nine reported no adverse events in 571 women, but two reported any or any moderate adverse event in a total of 13 of 67 women (19%). Overall, adverse events were reported in 2.8 percent of 463 women receiving TENS. In three studies of TENS, 11 percent of women (n = 199) reported urinary tract infections; however, within studies, these rates were similar to or lower than urinary tract infection rates on an anticholinergic (6%) or with BTX (35%).
Table 17
Adverse events in nonpharmacological interventions, part 1 (acupuncture to MBSR).
Table 18
Adverse events in nonpharmacological interventions, part 2 (PFMT to yoga).
Key Question 2. What are the benefits and harms of pharmacological treatments of UI in women, and how do they compare with each other?
Key Points
- Trials evaluated 7 intervention categories (including sham/no intervention) and 31 specific interventions. Trials directly compared 7 of 21 possible comparisons of intervention categories, of which 2 were comparisons between active interventions (not placebo, or no treatment). Trials directly compared 35 of 465 possible comparisons of specific interventions, of which 21 were comparisons of active interventions.
- For women with stress UI,
- The first- (or second-) line pharmacological treatments evaluated included alpha agonists and hormones with or without anticholinergics. Alpha agonists were found to be significantly more effective than hormones for improvement (OR 4.5, 95% CI 1.40 to 17.8). There were no significant findings among these interventions for cure or satisfaction.
- In studies of women with stress UI, periurethral bulking agents were evaluated as third-line pharmacological treatments. Comparisons were imprecise with wide confidence intervals.
- For women with urgency UI,
- Anticholinergics were the only first- (or second-) line nonpharmacological treatment evaluated. Compared to placebo Anticholinergics were statistically significantly more likely to result in cure (ORs 1.80, 95% CI 1.29 to 2.52) or improvement (ORs 1.79, 95% CI 1.18 to 2.7). No studies evaluated these comparisons for satisfaction.
- BTX was the only third-line nonpharmacological treatment evaluated. BTX was significantly more effective than no treatment to achieve cure (OR 4.94, 95% CI 2.82 to 8.65), or improvement (OR 7.5, 95% CI 2.88 to 19.54). No studies evaluated these comparisons for satisfaction.
- There were16 studies that reported on quality of life outcomes.
- Studies reported on 8 pharmacological interventions that were compared with placebo or no interventions. Studies were discordant (within studies among different measures of quality of life) or inconsistent (across studies).
- In 6 studies, overall anticholinergics improve quality of life compared with no treatment, but there was inconsistency both within and across studies regarding the comparative effect of anticholinergics on various aspects of quality of life.
- Other intervention categories were evaluated by only a single study each.
- There were 6 studies that compared 8 pharmacological interventions with each other. In most instances, no differences in quality of life were reported among interventions.
- There were 95 studies that reported on adverse events of pharmacological treatments.
- Serious adverse events (as defined by study authors)
- The highest rate of serious adverse events occurred with periurethral bulking agents (4.7%, 3 studies, 362 women); these adverse events included erosion and need for surgical excision of the bulking agents. The one study of a periurethral bulking agent currently available in the United States (macroplastique) reported 1.6% rate of erosion.
- In 8 studies of anticholinergics, overall 2.4% of 2,583 women had serious adverse events, although the adverse events were mostly undefined.
- In 2 studies, 0.6% of 1,390 women taking the alpha agonist duloxetine had (undefined) serious adverse events.
- For comparison, in 10 studies, 0.2% of 2,852 women taking placebo (or no treatment) had (mostly undefined) serious adverse events.
- Dry mouth
- Anticholinergics: 21 studies reported adverse events for anticholinergics. Dry mouth was the most commonly reported adverse event (oxybutynin: median 36%).
- Alpha agonists: 15 studies of the alpha agonist duloxetine reported dry mouth in a median of 13% of women.
- Placebo: In 35 studies, a median of 4% of women had dry mouth with placebo treatment; high strength of evidence.
- Other adverse events
- Alpha agonists: Other reported adverse events included nausea (23.2% (15 studies), insomnia (12.6%, 13 studies), constipation (11%, 14 studies), fatigue (10.1%, 13 studies), dizziness (10.6%, 14 studies), and headache (8.3%, 11 studies).
- BTX: the most commonly reported adverse event in 6 studies was urinary tract infection in a median of 34.6% of women (range 3.9% to 54.5%). 3 studies reported urinary retention or voiding dysfunction in 3.9%, 18.0%, and 25.5% of women.
- Periurethral bulking: the most common adverse events were urinary tract infection (median 6.6%; range 1.3% to 23.8%) and urinary retention/voiding dysfunction (median 3.8%; range 0.9% to 9.5%). The one study (122 women) that evaluated a periurethral bulking agent currently available in the United States (macroplastique) found high rates of urinary tract infection (23.8%), headache (18%), and urinary retention/dysuria (15.6%).
Findings
Table 19 repeats simplified data from Tables 7 (cure), 10 (improvement), and 13 (satisfaction) limited to pharmacological treatments (pertinent to this KQ). It summarizes the mean cure rates of the pharmacological treatments based on the network meta-analysis models of all studies and of the two subsets of studies of women with stress UI or with urgency UI.
Cure
Among pharmacological treatments, BTX had an average cure rate of 44 percent, hormones 28 percent, and anticholinergics 21 percent, compared with other pharmacological treatments and placebo, which all had cure rates less that 16 percent (Table 19). However, only BTX (OR 5.7) and anticholinergics (OR 2.0) had statistically significant greater likelihood of cure than placebo; the estimate for hormones was less precise and nonsignificant (see Table 6). Estimates from studies of women with stress UI or urgency UI were generally similar, except for an atypically low cure rate for hormones in studies of stress UI. Most comparisons across were based on indirect comparisons and resulted in imprecise OR estimates. However, based in part on a direct head-to-head comparison, BTX (3rd line therapy) was significantly more likely to result in cure than anticholinergics (2nd line therapy, OR 2.91). Among studies of women with stress UI, hormones and alpha agonists had similar cure rates (no other pharmacological treatments were compared directly or indirectly). Among studies of women with stress UI, cure rates among pharmacological treatments were mostly nonsignificantly different than each (Appendix H, Tables H-1 to H-2), except for the comparison of BTX and anticholinergics described above.
Table 19
Summary league table displaying percent of women with UI outcomes for each pharmacological treatment.
Improvement
Among pharmacological treatments, BTX, anticholinergics, periurethral bulking agents, and alpha agonists had improvement rates, across studies of 42 to 67 percent. All other pharmacological treatments and placebo had improvement rates less than 25 percent (Table 19). Improvement rates in studies of women with stress UI or with urgency UI were mostly similar for the evaluated pharmacological treatments, except it was lower with anticholinergics among women with stress UI (25%). Alpha agonists (OR 2.2), BTX (OR 6.0), and anticholinergics (OR 3.0) each had significantly higher improvement rates than placebo (see Table 9). Other pharmacological interventions had imprecise OR estimates compared with placebo. Studies restrict to women with stress UI or with urgency UI had similar findings for evaluated pharmacological treatments. (see Appendix H, Tables H-4 and H-5).
With respect to improvement, hormones were significantly less effective than other pharmacological interventions, except in comparison with the combination hormones and anticholinergics for which the estimate was imprecise. BTX (3rd line therapy) was likely more effective than alpha agonists and combination hormones and anticholinergics (both 2nd line therapy), although the differences were nonsignificant. Findings were similar across evaluated treatments for women with urgency UI. In studies of women with stress UI alpha agonists were significantly more effective than hormones (OR 4.1, see Appendix H, Tables H-4 and H-5).
Satisfaction
Among studies that reported satisfaction, the only pharmacological interventions evaluated were BTX (3rd line therapy) and anticholinergics (2nd line therapy). With BTX, 86 percent of women had satisfaction with the control of their UI (Table 19). With anticholinergics, 55 percent of women had satisfaction. These compare to placebo treatment, with which 32 percent of women had satisfaction. Both pharmacological treatments were significantly more effective than placebo (BTX OR 12.7; anticholinergics OR 2.6; see Table 12). The two pharmacological treatments were not compared directly.
Quality of Life
Sixteen RCTs reported on quality of life outcomes in pharmacological interventions versus placebo or with other pharmacological interventions.38,45,62,70,84,96,114,117,188–195 Results are given in Table 20 and Appendix E. The table cells show the number of studies and the number of people (in parentheses), followed by the number of studies with the number of studies that found statistically significant differences and which intervention was favored, the number of studies that found discordant results (that is, within the same study, significant differences favoring one intervention were found on one scale or subscale, but nonsignificant differences were found on others), and the number of studies with nonsignificant differences. Study-level details are given in Appendix E. The studies evaluated several quality of life domains, as categorized across the columns of the tables: bother, daily activities, distress, general health, mental health, and sleep/energy.
Eight pharmacological interventions were compared with placebo interventions (Table 20). Six studies evaluated anticholinergic medications,114,117,190,191,193–195 two bladder BTX,45,190 and one each an alpha agonist,84, an antiepileptic (pregabalin),192 and a periurethral bulking agent.38 Across interventions, the effects of the pharmacological interventions on various aspects of quality of life were mixed. Only two specific interventions (oxybutynin and tolterodine) were compared in more than one study, and none in more than two.
When anticholinergic medications were evaluated as a single group, four studies evaluated bother,114,117,191,194 and four studies evaluated daily activities.114,117,193,195 When these studies were significant they favored the anticholinergic medication over placebo,114,193 though most were not significant and one gave discordant results.195
Table 20
Quality of life outcomes for pharmacological interventions versus placebo/no treatment.
Eight pharmacological interventions were compared with other pharmacological interventions (Table 21). Three studies evaluated either different anticholinergics 96,189 or different doses of the same medication.190 Only one found a significant difference in one aspect of quality of life, favoring 3.9 mg of oxybutynin over 2.6 mg of the same anticholinergic for improvements in daily activities. A single study evaluated an anticholinergic compared with BTX and reported a nonsignificant difference.70 One study reported no significant difference in a comparison of an anticholinergic alone compared with an anticholinergic plus vaginal estrogen.188 One study reported nonsignificant difference of the antiepileptic pregabalin over tolterodine in the bother domain, and the same study found no significant difference in bother between pregabalin versus pregabalin plus tolterodine.192
Table 21
Quality of life outcomes for pharmacological versus pharmacological interventions.
Adverse Events
Ninety-five studies reported on adverse events in drugs.29,32,34,38,39,45,46,54,57–61,68–70,76,78,82–84,88,89,91,94,96,98,99,101,105,106,109,112,120,147,154,160,165,168,171–173,188–192,194,196–242 Results for anticholinergics are given in Table 22, and results for all other drugs, as well as for placebo arms (56 studies14,32,38,39,45,54,57–59,61,68,69,76,78,83,84,88,89,91,92,94,98,99,101,105,106,109,112,120,147,154,160,165,168,172,173,190–192,194,202,204,207–211,214,218,219,222,230,233,237,238,242), are given in Table 23. The table cells show the percentage of people affected by the adverse event if only a single study arm reported the event. Where more than one study arm reported on an adverse event, the cell gives the median and range of percentages (or just the range, if only two arms reported an adverse event). Detailed results are in Appendix F. Fifty-one studies evaluated adverse events in anticholinergics,32,34,39,46,58,60,61,68,70,88,94,96,105,112,188–192,194,196–199,201–203,205,206,209–211,213,215–218,220–222,229,231–234,236–238,240–242 but with the exception of oxybutynin and tolterodine, each specific adverse event was evaluated in only one to three studies.
For most pharmacological interventions, serious adverse events (as described by authors) were rare or did not occur; although few studies defined serious adverse events. However, with periurethral bulking agents, 4.7 percent of 362 women in three studies had serious adverse events, including erosion and need for surgical excision of the bulking agents. The one study of a periurethral bulking agent currently available in the United States (macroplastique) reported 1.6% rate of erosion. In eight studies of anticholinergics, overall 2.4 percent of 2,583 women had serious adverse events, although the adverse events were mostly undefined. In two studies, 0.6 percent of 1,390 women taking the alpha agonist duloxetine had (undefined) serious adverse events. By comparison, in 10 studies, 0.2 percent of 2,852 women taking placebo (or no treatment) had (mostly undefined) serious adverse events.
The most commonly reported adverse event across interventions was dry mouth. The median percentage of women reporting dry mouth in studies of anticholinergic medications was 24.2 percent; oxybutynin was evaluated in the most studies (21) with a median of 36.1 percent (range 0 to 100).32,34,39,58,68,105,189,190,196,198,199,201,206,215,229,232,234,238,240,242–244 A single study each evaluated dry mouth in beta agonist (0.7%),200 BTX (30.8%),70 estrogen (21.4%),243 and antiepileptic medications (10.5%).192 Fifteen studies evaluated dry mouth for duloxetine, an alpha agonist, and found a median of 12.9 percent (range 1.5 to 21.8). Placebo arms had a median of 4 percent across 29 studies (range 0 to 86.2).32,39,57–59,61,68,76,83,84,89,91,99,101,105,106,190,192,204,208–210,214,219,222,233,238,242 By comparison, in non-pharmaceutical treatments including PFMT and/or bladder training, dry mouth was reported in three studies with median rates that ranged from 0 to 34.9 percent.32,179,245
Among the other active drugs (see Table 23), duloxetine, an alpha agonist, was evaluated in the largest number of studies (16).57,59,76,83,84,89,91,99,101,106,204,208,214,219,224,230 In addition to dry mouth, discussed above, dizziness (10.6% 14 studies), gastrointestinal upset, specifically constipation (11%; 14 studies) and nausea (23.2%; 15 studies), fatigue (8.6%, 12 studies), headache (8.3%; 11 studies), and insomnia (12.6%; 13 studies) were all reported in more than ten studies.
Only six studies reported on adverse events in bladder BTX.29,45,70,78,207,227 Five reported on urinary tract infections (UTI). The percentage of women reporting UTI ranged from 33.3 to 42.9 percent in the four studies with at least 20 participants (6/11 or 55% in the fifth study), with a median of 36.4 percent.29,45,70,78,207,227 This compares to a reported percentage of 1.5 percent for mirabegron, between 1.4 and 23 percent for various anticholinergics, and 4.3 percent in placebo arms. The sixth study reported hematuria in 2.3 percent of women using bladder BTX.29,45,70,78,207,227 Two studies reported on urine retention/voiding dysfunction in10.5 and 20.5 percent of women.29,45,70,78,207,227
The most commonly reported adverse event for the periurethral bulking agents was UTI in five studies (median 6.6%; range 1.3% to 23.8%)38,223,225,226,235 and urinary retention/voiding dysfunction in seven studies (median 3.8%; range 0.9% to 9.5%).38,212,223,225,228,235,239. However, only one of these studies (122 women) evaluated a periurethral bulking agent currently available in the United States (macroplastique). This study found high rates of urinary tract infection (23.8%), headache (18%), and urinary retention/dysuria (15.6%). Serious adverse events (erosion) were low (1.6%), as were pain (5%) and yeast infection (2.5%).38 All other single and combination medications were evaluated in only one or two studies each.
Table 22
Adverse events reported for anticholinergics.
Table 23
Adverse events reported for drugs other than anticholinergics.
Key Question 3. What are the comparative benefits and harms of nonpharmacological versus pharmacological treatments of UI in women
Key Points
- Four nonpharmacological intervention categories could be compared with 6 pharmacological intervention categories and 38 specific nonpharmacological and 31 pharmacological interventions. Trials directly compared 7 of 24 possible comparisons of intervention categories. Trials directly compared 9 of 1178 possible comparisons of specific interventions.
- For women with stress UI,
- The first- (or second-) line treatments evaluated included behavioral therapy and alpha agonists. None of the interventions were found to be different in likelihood or achieving satisfaction.
- Alpha agonists were found to be less likely to result in cure (OR 0.22, 95% CI 0.06 to 0.74) or improvement (OR 0.33, 95% CI 0.14 to 0.77) than behavioral therapy.
- For improvement, hormones and combined hormones and anticholinergics were less effective than behavioral therapy (ORs 0.07 and 0.13, respectively); hormones were also less effective neuromodulation and intravesical pressure release (ORs 0.13 and 0.12, respectively).
- For third-line treatments, periurethral bulking agents were found to be less likely to result in cure than behavioral therapy (OR 0.23, 95% CI 0.06 to 0.98). Statistically nonsignificant comparisons also suggest that alpha agonists are less likely to result in cure than neuromodulation (OR 0.35, 95% CI 0.12 to 1.00) and that neuromodulation was possibly more likely to result in improvement than combination hormones and anticholinergics (OR 4.5, 95% CI 1.14 to 17.78).
- For women with urgency UI,
- The first- (or second-) line treatments evaluated included behavioral therapy and alpha agonists. For improvement, anticholinergics were significantly less effective than behavioral therapy (0.24, 95% CI 0.09 to 0.62). No statistically significant differences were found between pharmacological and nonpharmacological treatments for cure or satisfaction.
- For the third-line treatments, no statistically significant differences were found between pharmacological and nonpharmacological treatments for cure, improvement, or satisfaction.
- Four studies reported on quality of life outcomes, each comparing a unique nonpharmacological intervention to one of 4 pharmacological interventions. One study found discordant results for daily activities when comparing neuromodulation to BTX; all other results where nonsignificant.
Findings
Here we focus on comparisons of nonpharmacological and pharmacological interventions that have been compared in studies specific to stress UI or urgency UI (or that are typically used for either stress UI or urgency UI. We further focus only on comparisons of first- or second-line interventions separately from comparisons of third-line therapies, except where direct comparisons have been made within studies across lines of therapy.
Stress UI
Cure
Two pairs of first/second-line nonpharmacological versus pharmacological interventions used for stress UI have been compared. A single comparison of third-line nonpharmacological and pharmacological interventions can also be made by indirect comparisons.
Both across all studies and within studies of women with stress UI, behavioral therapy is significantly more effective than alpha agonists (OR 2.5 across all studies, Table 6; OR 4.5 within stress UI studies, Appendix H, Table H-1A). However, in both sets of studies the two interventions have not been compared directly (head-to-head).
Across all studies there is indirect evidence that behavioral therapy and hormones result do not have significantly different cure rates (Table 6). Hormones have not been evaluated for cure in stress UI studies. In addition, indirect comparisons provide only imprecise estimates of the comparative effectiveness of third-line intravesical pressure release devices and third-line periurethral bulking agents. Similarly, imprecise estimates are derived across all studies (Table 6) and studies of women with stress UI (Appendix H, Table H-1A)
Improvement
Three pairs of first/second-line nonpharmacological versus pharmacological interventions used for stress UI can be compared.
Similar to the findings for cure, both across studies and within studies of women with stress UI, behavioral therapy is significantly more effective than alpha agonists (OR 2.5 across all studies, Table 9; OR 3.3 within stress UI studies, Appendix H, Table H-4). However, in both sets of studies the two interventions have not been compared directly (head-to-head).
In contrast with the findings for cure, both across all studies and within stress UI studies, there is evidence that behavioral therapy is significant more effective to achieve improvement than hormones (OR 10.0 across all studies, Table 9; OR 14.2 within stress UI studies, Appendix H, Table H-4). Across all studies, there are direct head-to-head comparisons to support this finding; however, within stress UI studies, the comparison is indirect only.
By indirect comparison, behavioral therapy can been compared with combined hormones and anticholinergics (which are typically used for urgency UI). Behavioral therapy was likely more effective than the combination of drugs (OR 5.28; 95% CI 0.86 to 32.5; Appendix H, Table H-1A). A similar estimate was found among studies of women with stress UI.
Satisfaction
Only the comparison of anticholinergics (2nd line therapy) and behavioral therapy (1st line therapy) can be evaluated for satisfaction. Across all studies, there is direct evidence to support that behavioral therapy is significantly more effective than anticholinergics to achieve satisfaction with control of UI symptoms (OR 3.1, Table 12). Stress UI studies of anticholinergics have not reported on satisfaction.
Urgency UI
Cure
The first-line nonpharmacological treatment has only been directly compared with anticholinergics in head-to-head in studies; the comparison has been evaluated only in studies restricted to women with urgency UI.
Among all studies, behavioral therapy was significantly more likely to achieve cure than anticholinergics (OR 1.56, 95% CI 1.02 to 2.44; Table 6). The urgency UI studies found a similar, but not quite statistically significant finding (OR 1.52, 95% CI 0.90 to 2.56; Appendix H, Table H-2).
Third-line BTX has been directly compared with second-line neuromodulation both across all studies and within studies of only women with urgency UI. In both sets of studies, BTX was likely favored over neuromodulation, but the difference in effect was not quite statistically significant. Among urgency UI studies, the OR for cure was 1.68 (95% CI 0.80, 3.55; Appendix H, Table H-2). A slightly wider CI was found across all studies (Table 6).
Improvement
The same pairs of interventions evaluated for cure have evidence regarding rates of improvement. Similarly, there was direct (head-to-head) comparisons and evidence from urgency UI studies only for anticholinergics versus behavioral therapy and BTX versus neuromodulation.
There is evidence that behavioral therapy is more effective than any of the three pharmacological treatments. Compared with anticholinergics, the OR was 1.82 (95% CI 1.03 to 3.23) across all studies and 4.17 (95% CI 1.61 to 11.1) in urgency UI studies. Compared with hormones, the OR was 10.0 (95% CI 3.57 and 33.3). Compared with combination hormones and anticholinergics, behavioral therapy was likely more effective to achieve improvement (OR 5.26, 95% CI 0.86 to 33.3).
The direct comparisons between BTX and neuromodulation found no significant difference between the two interventions (Table 9 and Appendix H, Table H-5).
Satisfaction
Hormones have not been studied among studies reporting satisfaction. Therefore, there are only two evaluable comparisons.
Behavioral therapy was found to be significantly more effective than anticholinergics (OR 3.12, 95% CI 1.85 to 5.26) across all studies; however, the difference was similar but not statistically significant in the smaller number of urgency UI studies (OR 3.12, 95% CI 0.71 to 14.3). The direct comparisons between BTX and neuromodulation found no significant difference between the two interventions (Table 12 and Appendix H, Table H-8A).
Quality of Life
Four RCTs reported on quality of life outcomes in nonpharmacological interventions versus pharmacological interventions, one for each comparison against oxybutynin, trospium, and BTX; the remaining two RCTs reported comparisons against tolterodine.29,46,60,117 Results are given in Table 24 and Appendix E. The table cells show the number of studies and the number of people (in parentheses), followed by the number of studies with the number of studies that found statistically significant differences and which intervention was favored, the number of studies that found discordant results (that is, within the same study, significant differences favoring one intervention were found on one scale or subscale, but nonsignificant differences were found on others), and the number of studies with nonsignificant differences. No significant differences were seen, and one study found discordant results on daily activities when comparing neuromodulation to BTX.29
Table 24
Quality of life outcomes for nonpharmacological versus pharmacological interventions.
Key Question 4. What are the benefits and harms of combined nonpharmacological and pharmacological treatment of UI in women?
Key Points
- There were 11 studies that compared combination nonpharmacological and pharmacological interventions with other interventions; 11 report UI outcomes, 1 reports quality of life outcomes, and 7 report adverse events.
- For women with stress UI,
- The first- (or second-) line treatments evaluated included behavioral therapy with hormones, which were found to be more likely to result in cure than alpha agonists (OR 9.36 (1.19 to 73.64) or periurethral bulking agents, though this comparison was not statistically significant (OR 8.66, 95% CI 0.97 to 76.99).
- The third-line treatments evaluated included behavioral therapy with neurostimulation with or without hormones. No study found a statistically significant difference for cure, but alpha agonists (OR 0.02, 95% CI 0.04 to 0.96), hormones (OR 0.04, 95% CI 0,01, 0.14), and combined hormones and anticholinergics (OR 0.08, 95% CI 0.01 to 0.42) were less effective than triple therapy (behavioral therapy, neurostimulation, and hormones). Triple therapy was possibly more effective than periurethral bulking agents (OR 5.92, 95% CI 0.95 to 37.01).
- For women with urgency UI,
- The first- (or second-) line treatments evaluated included anticholinergics with behavioral therapy. No statistically significant differences were found for this intervention compared with any other first-line treatment for cure, but anticholinergics alone were less likely to lead to improvement than anticholinergics with behavioral therapy (OR 0.46, 95% CI 0.09 to 0.62).
- Among third-line treatments, BTX was statistically significantly more effective for improvement than the combination of anticholinergics and behavioral therapy (OR 2.17, 95% CI 1.01 to 4.67).
- One study found that addition of vaginal estrogen to a nonpharmacological intervention significantly improve UI quality of life.
Findings
All UI Outcomes
A summary of the direct comparisons between combinations intervention categories, and sham or no treatment, pharmacological and nonpharmacological intervention categories is in Table 25. Refer to Tables 6, 9, and 12 for combined direct and indirect (network meta-analysis) comparisons with all other interventions and to Tables 7, 10, and 13 for the mean event rates for the outcomes of cure, improvement, and satisfaction, respectively.
Single (separate) studies reported that combined neuromodulation, behavioral therapy, and hormones (vaginal estrogen) resulted in significantly more women cured or improved than either hormones alone82 or no treatment.42
Combined anticholinergics and behavioral therapy had similar cure and improvement rates compared with anticholinergics alone, but significantly higher rates of satisfaction.17,79 In separate studies, combined behavioral therapy and hormones had similar cure rates as vaginal estrogen alone246 and behavioral therapy alone.44
Table 25
Comparisons of cure, improvement, and satisfaction rates between intervention categories: combination versus no treatment, pharmacological, and nonpharmacological interventions.
Quality of Life
A single study of 69 people reported on quality of life in combined pharmacological and nonpharmacological versus nonpharmacological only.177 Both arms received PFMT, electrostimulation, and biofeedback, but one arm also received vaginal estrogen. The arm that received estrogen did significantly better on the Incontinence Impact Questionnaire (IIQ-7), with a net difference of −7.8 (95% CI −9.6 to −6) on the 100-point scale.
Adverse Events
Rates of adverse events from combined interventions are presented in Table 26. Each adverse event was reported in only a single study. Detailed results are in Appendix F. One study reported on a combination of PFMT and the anticholinergic medication trospium, but reported only on three adverse events in 31 people.179 In this study low percentages of women reported visual adverse events (3.2%) and discontinuation due to adverse events (3.2%), but more reported dry mouth (23%).179 Four studies reported on adverse events from estrogen combined with PFMT, pessaries, and/or transcutaneous electrical nerve stimulation (TENS).82,171,177,224 Each adverse event was evaluated by only a single study, but the percentage of women reporting these adverse events were below 4 percent.
Table 26
Adverse events in combination interventions.
- Overview of the Evidence Base Addressing All Key Questions
- Network Meta-Analyses for Urinary Incontinence Outcomes Across All Interventions
- What are the benefits and harms of nonpharmacological treatments of UI in women, and how do they compare with each other?
- What are the benefits and harms of pharmacological treatments of UI in women, and how do they compare with each other?
- What are the comparative benefits and harms of nonpharmacological versus pharmacological treatments of UI in women
- What are the benefits and harms of combined nonpharmacological and pharmacological treatment of UI in women?
- Results - Nonsurgical Treatments for Urinary Incontinence in Women: A Systematic...Results - Nonsurgical Treatments for Urinary Incontinence in Women: A Systematic Review Update
Your browsing activity is empty.
Activity recording is turned off.
See more...
![This is the topology map of the network meta-analysis of all the treatments that evaluated improvement. Treatments are displayed by circles representing individual treatments or combinations of treatments, grouped together by treatment type. The interventions categories and individual interventions are: alpha agonist (duloxetine, midodrine), onabotulinum toxin A, anticholinergic (oxybutynin, solifenacin, tolterodine, tropsium, fesoterodine, flavoxate, phenylpropanolamine, propantheline, propiverine), hormones (vaginal estrogen, oral estrogen, subcutaneous estrogen, transdermal estrogen, raloxifene), neuromodulation (electroacupuncture, InterStim, magnetic stimulation, transcutaneous electrical nerve stimulation [TENS]), behavioral therapy (bladder training, education, heat therapy, pelvic floor muscle training [PFMT], bladder support, biofeedback, mindfulness-based stress reduction [MBSR], yoga, vaginal weights), periurethral bulking (polyacrylamide, collagen, autologous fat, carbonated beads, polydimethylsiloxane, porcine collagen, dextranomer hyaluronate), intravesical pressure release, and sham/no treatment/placebo/placebo. Combinations of aforementioned individual treatments are displayed, and are generally combinations between two or three treatments. Many of the combination treatments are within the behavioral therapy and neuromodulation categories. Lines between treatment circles indicate a direct study comparison of the treatments, and several of the treatment circles connect to the central sham/no treatment/placebo circle. Not all of the treatment circles are connected to each other within the treatment categories or across categories. There are also two connected subgraphs: one that connects polyacrylamide, collagen, carbonated beads, and dextranomer hyluronate; and all remaining nodes.](/books/NBK534614/bin/ch5f7.gif)
![This is the topology map of the network meta-analysis of all the treatments that evaluated satisfaction. Treatments are displayed by circles representing individual treatments or combinations of treatments, grouped together by treatment category. The interventions categories and individual interventions are: alpha agonist (duloxetine, midodrine), onabotulinum toxin A, anticholinergic (oxybutynin, solifenacin, tolterodine, tropsium, fesoterodine, flavoxate, phenylpropanolamine, propantheline, propiverine), hormones (vaginal estrogen, oral estrogen, subcutaneous estrogen, transdermal estrogen, raloxifene), neuromodulation (electroacupuncture, InterStim, magnetic stimulation, transcutaneous electrical nerve stimulation [TENS]), behavioral therapy (bladder training, education, heat therapy, pelvic floor muscle training [PFMT], bladder support, biofeedback, mindfulness-based stress reduction [MBSR], yoga, vaginal weights), periurethral bulking (polyacrylamide, collagen, autologous fat, carbonated beads, polydimethylsiloxane, porcine collagen, dextranomer hyaluronate), intravesical pressure release, and sham/no treatment/placebo. Combinations of aforementioned individual treatments are displayed, and are generally combinations between two or three treatments. Many of the combination treatments are within the behavioral therapy, neuromodulation, and anticholinergic categories. Several of the treatment circles connect to the central sham/no treatment/placebo circle, and several also connect to other treatment circles. Not all of the treatment circles are connected to each other within the treatment categories or across categories. There are also two connected subgraphs: onabotulinum toxin A and InterStim; and all remaining nodes.](/books/NBK534614/bin/ch5f9.gif)