NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Chou R, McDonagh MS, Nakamoto E, et al. Analgesics for Osteoarthritis: An Update of the 2006 Comparative Effectiveness Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 Oct. (Comparative Effectiveness Reviews, No. 38.)
Overview
For the original comparative efectiveness review (CER), searches identified 2,789 publications: 1,522 from the Cochrane Central Register of Controlled Trials, 68 from the Cochrane Database of Systematic Reviews, 1,015 from MEDLINE and 184 from the combination of other sources listed above. There were also 59 studies not previously reviewed for inclusion that were suggested through peer review or public comment or published after the searches were conducted. Following application of inclusion criteria, 321 publications were included in the original CER.
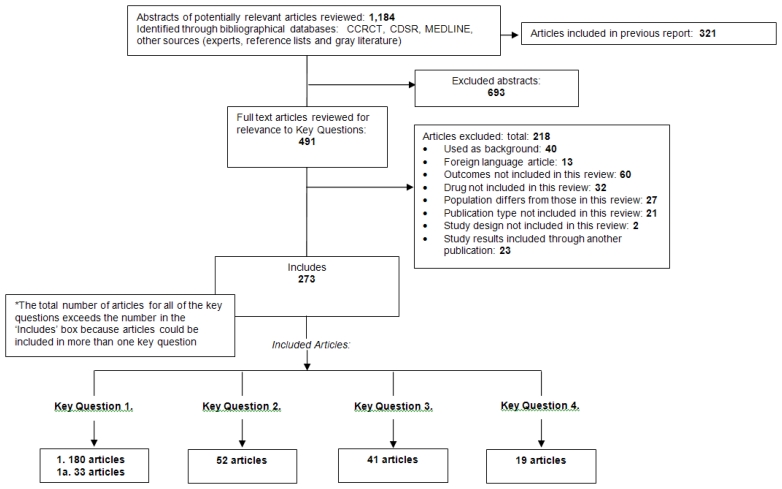
For the update, searches identified 93 citations from the Cochrane Central Register of Controlled Trials, 52 from the Cochrane Database of Systematic Reviews, 579 from MEDLINE and 139 from other sources (including suggestions from experts, gray literature searches, and reviewing reference lists). We combined the publications found in the update searches with the publications included from the original report in the EndNote library. A total of 491 full-text articles were reviewed for inclusion in this update, with 273 publications determined to be eligible. There were 180 articles included in Key Question 1, 33 articles for Key Question 1a, 52 articles for Key Question 2, 41 articles for Key Question 3, and 19 articles for Key Question 4. Reasons for exclusion of studies can be found in the literature flow diagram (Figure 2) and a list of excluded studies can be found in Appendix E.
Few randomized trials met criteria to be considered effectiveness studies.48 Almost all trials applied numerous exclusion criteria, used rigid dosing regimens. In addition, most trials were relatively short term. An exception was a new trial of topical versus oral ibuprofen that randomized patients to advice to use topical or oral ibuprofen without a fixed dosing regimen and followed patients through one year.49 A number of large observational studies were population-based or evaluated patients followed in large practice databases and met many criteria for effectiveness studies.
Key Question 1. What are the Comparative Benefits and Harms of Treating Osteoarthritis With Oral Medications or Supplements?
Summary of Evidence
Benefits:
- Celecoxib versus nonselective nonsteroidal anti-inflammatory drugs (NSAIDs):
- There were no clear differences between celecoxib and various nonselective NSAIDs in efficacy for pain relief or withdrawals due to lack of efficacy.
- Partially selective NSAIDs versus nonselective NSAIDs:
- Meloxicam, etodolac, and nabumetone were associated with no clear differences in efficacy compared to nonselective NSAIDs in patients with osteoarthritis.
- Nonselective NSAIDs versus nonselective NSAIDs:
- There were no clear differences in efficacy between various non-aspirin, nonselective NSAIDs
- Aspirin or salsalate versus other NSAIDs:
- Sparse evidence of no difference in efficacy between aspirin and salsalate. No trials compared aspirin or salsalate versus other NSAIDs
Harms: gastrointestinal (GI) and cardiovascular (CV)
- Celecoxib:
- In systematic reviews of arthritis trials, most of which evaluated short-term use, celecoxib was associated with fewer ulcer complications than nonselective NSAIDs.
- It is not clear whether celecoxib is associated with fewer serious GI harms than nonselective NSAIDs when used longer than 3–6 months. In the only large, long-term trial (CLASS) designed to assess ulcer complications (perforation, obstruction, or bleeding), celecoxib at 800 mg daily did not decrease predefined ulcer complications compared with diclofenac and ibuprofen at 12 months; the risk of ulcer complications at 6 months was lower with celecoxib than with ibuprofen, but not diclofenac, in patients who did not use aspirin; and there was no reduction in ulcer complications at 12 months. The overall rate of serious adverse events with celecoxib was similar to the rate with ibuprofen and diclofenac.
- Celecoxib was associated with an increased risk of CV events or trend towards increased risk (CV death, myocardial infarction, stroke, heart failure, or thromboembolic events) relative to placebo in systematic reviews of randomized controlled trials (RCTs). Most of the CV events with celecoxib were reported in two large polyp-prevention trials evaluating 200 mg or 400 mg twice daily, or 800 mg once daily.
- –
One additional CV event occurred for about every 270 patients treated for one year with celecoxib compared to placebo.
- –
Systematic reviews found no clear difference between celecoxib and nonselective NSAIDs in risk of CV events.
- Partially selective NSAIDs:
- Meloxicam (relative risk [RR] 0.53, 95% confidence interval [CI] 0.29 to 0.97) and etodolac (RR 0.32, 95% CI 0.15 to 0.71) were associated with a lower risk of ulcer complications or symptomatic ulcers compared to nonselective NSAIDs in a systematic review of randomized, but differences in risk of ulcer complications alone did not reach statistical significance.
- There was insufficient evidence to make reliable judgments about GI harms of nabumetone relative to nonselective NSAIDs, or CV harms of any partially selective NSAID.
- Nonselective NSAID versus nonselective NSAID or any cyclooxygenase (COX)-2 selective NSAID:
- No clear difference in GI safety was found among nonselective NSAIDs at commonly used doses.
- COX-2 selective NSAIDs as a class were associated with similar, lower risks of ulcer complications relative to naproxen (RR 0.34, 95% CI 0.24 to 0.48), ibuprofen (RR 0.46, 95% CI 0.30 to 0.71), and diclofenac (RR 0.31, 95% CI 0.06 to 1.6).
- The CV safety of naproxen appeared moderately superior to that of any COX-2 selective NSAID in two systematic reviews of RCTs.
- –
In a large systematic review of RCTs, one additional myocardial infarction occurred for about every 300 patients treated for 1 year with a COX-2 selective NSAID instead of naproxen.
- Most observational studies showed similar estimates of CV risk for naproxen, COX-2 selective NSAIDs, and other nonselective NSAIDs.
- The CV safety of nonselective NSAIDs other than naproxen (data primarily on ibuprofen and diclofenac) was similar to that of COX-2 selective NSAIDs in a large systematic review of randomized trials.
- In two systematic reviews that included indirect analyses of randomized trials, naproxen was the only nonselective NSAID associated with neutral CV risk relative to placebo (RR 0.92, 95% CI 0.67 to 1.3 and RR 1.2, 95% CI 0.78 to 1.9).
- Aspirin:
- Aspirin is associated with a lower risk of serious cardiovascular events (0.51% aspirin vs. 0.57% control per year, p=0.0001 for primary prevention 6.7% vs. 8.2%, p<0.0001 for secondary prevention) and a higher risk of major GI and other extracranial bleeds (0.10% vs. 0.07%, p<0.0001) compared to placebo when given at long-term, primarily lower prophylactic doses.
- There is insufficient evidence to assess the balance of GI and CV safety of higher dose aspirin as used for pain relief compared with nonaspirin NSAIDs.
- Salsalate:
- Salsalate was associated with a lower risk of adverse events than other selective and nonselective NSAIDs using broad composite endpoints in older, poor-quality observational studies.
- No randomized trial or observational study evaluated risk of serious GI or CV harms associated with salsalate.
Harms: mortality
- Individual trials and systematic reviews have recorded too few events to detect differences in mortality between different NSAIDs.
- In one fair-quality cohort study, nabumetone was associated with a lower risk of all-cause mortality compared with diclofenac and naproxen, but this finding has not been replicated.
Harms: hypertension, congestive heart failure (CHF), or impaired renal function
- All COX-2 selective and nonselective NSAIDs can cause or aggravate hypertension, congestive heart failure, and impaired renal function.
- Short-term trials showed that, on average, nonselective NSAIDs raised mean blood pressure by about 5.0 mm Hg (95% CI 1.2 to 8.7).
- There was no clear evidence of clinically relevant, consistent differences between celecoxib, partially selective, and nonselective NSAIDs in risk of hypertension, congestive heart failure, or impaired renal function.
Harms: hepatotoxicity
- Clinically significant hepatotoxicity was rare.
- Among currently marketed NSAIDS, diclofenac was associated with the highest rate of hepatic laboratory abnormalities (78/1,000 patient-years with diclofenac vs. 16 to 28/1,000 for other NSAIDs in one systematic review; 3.6% vs. <0.43% in another systematic review).
Tolerability
- Relative to nonselective NSAIDs, COX-2 selective and partially selective NSAIDs were better or similarly tolerated.
- There were no clear differences in tolerability between nonselective NSAIDs.
- Two of three short-term trials found salsalate less well tolerated than nonselective NSAIDs, but older, flawed observational studies found salsalate better tolerated than nonselective NSAIDs.
Other oral agents: benefits and harms
- Acetaminophen
- Acetaminophen was modestly inferior to NSAIDs for pain and function in four systematic reviews.
- –
Pain severity ratings averaged less than 10 points higher for acetaminophen compared to NSAIDs on 100-point visual analogue scales.
- Compared with NSAIDs, acetaminophen had fewer GI side effects (clinical trials data) and serious GI complications (observational studies).
- Acetaminophen may be associated with modest increases in blood pressure and renal dysfunction (observational studies).
- One good-quality, prospective observational study found an increased risk of CV events with heavy use of acetaminophen that was similar to the risk associated with heavy use of NSAIDs.
- Acetaminophen may cause elevations of liver enzymes at therapeutic doses even in healthy persons.
- Glucosamine and chondroitin
- Seven randomized trials showed no clear difference between glucosamine and oral NSAIDs for pain or function.
- One randomized trial showed no clear difference between chondroitin and an oral NSAID for pain or function.
- A systematic review including recent, higher-quality trials found glucosamine associated with statistically significant but clinically insignificant beneficial effects on pain (−0.4 cm on a 10 cm scale) and joint space narrowing (−0.2 mm, 95% CI −0.3 to 0.0) compared to placebo.
- Similar results were reported for chondroitin.
- Glucosamine and chondroitin were tolerated similarly to placebo and no serious adverse events were reported in randomized trials.
Detailed Analysis
Benefits
Celecoxib
Two systematic reviews included in the original CER evaluated the efficacy of celecoxib versus nonselective NSAIDs.50, 51 We identified two fair-quality head-to-head trials of celecoxib versus diclofenac (n=925 and n=249) published since the original CER (Appendix H).52, 53
A good-quality systematic review (published in 2002) funded by the makers of celecoxib found similar effects on Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores associated with celecoxib and nonselective NSAIDs based on data from published and unpublished randomized trials of at least 12 weeks’ duration in patients with either osteoarthritis (OA) or rheumatoid arthritis (RA).50 A more recent systematic review (published in 2005) with access to all unpublished manufacturer-held clinical trial reports found celecoxib at doses of 200–400 mg associated with slightly higher rates of withdrawals due to lack of efficacy compared to nonselective NSAIDs (RR 1.1; 95% CI 1.0, 1.2), based on data from 31 primarily short-term (≤12 weeks) trials.51
The two largest head-to-head trials of celecoxib versus nonselective NSAIDs are the Celecoxib Long-term Arthritis Safety Study (CLASS)54 and the Successive Celecoxib Efficacy and Safety Study-1 (SUCCESS-1).55 Both systematic reviews included CLASS (n=7,968), a pivotal, long-term (6 to 13 months) trial of celecoxib versus the nonselective NSAIDs ibuprofen or diclofenac for rheumatoid and osteoarthritis.54 The nonselective NSAIDs were associated with a slightly higher (but statistically significant) likelihood of withdrawal due to lack of efficacy compared to celecoxib (15% vs. 13%, p=0.005). CLASS focused on assessment of adverse events rather than efficacy, and other efficacy results were not reported. The Moore et al. systematic review51 included the large (n=13,274), Successive Celecoxib Efficacy and Safety Study (SUCCESS-1), which found no clinically meaningful (and mostly statistically nonsignificant) differences after 12 weeks in efficacy (pain, global assessment of arthritis, or WOMAC total score) between celecoxib 100 mg or 200 mg twice daily and the nonselective NSAIDs diclofenac and naproxen in patients with osteoarthritis.55 Withdrawals due to lack of efficacy were not reported.
A new, fair-quality trial (high loss to followup) found no differences between celecoxib 200 mg once daily and diclofenac 50 mg twice daily in pain scores, global assessment of arthritis, or patient satisfaction through 52 weeks of followup in older (≥60 years) patients (n=925) with osteoarthritis.52 Withdrawals due to adverse events were slightly less frequent with celecoxib compared with diclofenac, but the difference was not statistically significant (27% vs. 31%, RR 0.87, 95% CI 0.71–1.1). Another new, fair-quality trial (high loss to followup, allocation concealment method not described, failure to report intention-to-treat analysis) reported results inconsistent with other trials.53 It failed to demonstrate noninferiority of celecoxib 200 mg compared to diclofenac 50 mg three times daily on pain at 6 weeks (mean difference between drugs in change from baseline 12 mm on a 0 to 100 visual analogue scale (VAS), 95% CI 5.8 to 18) or 12 weeks (10 mm, 95% CI 2.8 to 17) in patients with hip osteoarthritis requiring joint replacement surgery. Withdrawals due to lack of efficacy were similar (13 percent vs. 11 percent).
Partially Selective NSAIDs
Three systematic reviews included in the original CER evaluated the efficacy of the partially selective NSAIDs etodolac or nabumetone versus nonselective NSAIDs.9, 56, 57 One new, good-quality systematic review evaluated comparative efficacy of the partially selective NSAIDs for osteoarthritis or rheumatoid arthritis (Appendix H).58 We identified no new head-to-head trials of partially selective NSAIDs versus nonselective NSAIDs published since the original CER.
Eleven randomized, double-blinded trials of meloxicam 7.5 mg, 15 mg, or 25 mg versus other NSAIDs for osteoarthritis found no clear or consistent differences in efficacy.59–69 In two of the trials, meloxicam was associated with a greater likelihood of withdrawal due to lack of efficacy than nonselective NSAIDs.63, 68 The new systematic review, which included trials of patients with osteoarthritis or rheumatoid arthritis, found meloxicam associated with lower efficacy compared to nonselective NSAIDs for pain (difference 1.7 points on a 10-point VAS pain scale, 95% CI 0.8 to 2.7) and withdrawals due to lack of efficacy (RR 1.5, 95% CI 1.2 to 1.7).58
The original CER included several good-quality Cochrane systematic reviews of randomized trials that found no difference between etodolac and various nonselective NSAIDs for OA of the hip (trials published through 1994),70 back (through 1998),9 or knee (through 1997).57 In seven trials published after or not included in the Cochrane reviews, there were also no differences between sustained-release etodolac and diclofenac71 or tenoxicam;72 or between standard-formulation etodolac and piroxicam (2 trials73, 74), naproxen (2 trials75, 76), or nimesulide77 for OA of the knee, hip, or foot. The new systematic review found no differences between etodolac and various nonselective NSAIDs for pain (mean difference 2.1, 95% CI −2.1 to 6.2) or withdrawals due to lack of efficacy (RR 1.0, 95% CI 0.85 to 1.2) in patients with osteoarthritis or rheumatoid arthritis.58
The Cochrane review of NSAIDs for knee osteoarthritis found nabumetone similar in efficacy to the nonselective NSAIDs diclofenac SR78 and etodolac79 in two 4-week trials.57
Nonselective NSAIDs
The original CER included several good-quality systematic reviews by the Cochrane Collaboration of trials that compared various nonselective NSAIDs for OA of the hip (trials published through 1994),70 back (through 1998),9 or knee (through 1997).57 These reviews found no clear differences in efficacy between non-aspirin, primarily nonselective NSAIDs. We identified no new head-to-head trials comparing efficacy of one non-aspirin, nonselective NSAID versus another. The large SUCCESS-1 trial included diclofenac and naproxen arms, but only reported combined efficacy results for these two nonselective NSAIDs.55
Aspirin or Salsalate
We identified no new head-to-head trials comparing efficacy of aspirin or salsalate versus other NSAIDs. A head-to-head trial included in the original CER found salsalate 3 g once daily and aspirin 3.6 g once daily associated with similar efficacy in patients with OA after 2 weeks of treatment.80
Safety: Serious Gastrointestinal and Cardiovascular Harms
Randomized Controlled Trials
Celecoxib: GI Harms
One systematic review of randomized trials of serious GI harms associated with celecoxib versus nonselective NSAIDs was included in the original CER (Appendix H).51 We included another fair-quality systematic review that only had preliminary results available at the time of the original CER (Appendix H).81 We identified one new pooled analysis of three similarly designed, 12-week trials of celecoxib versus diclofenac82 and one other new head-to-head trial of celecoxib versus diclofenac,52 but they either did not report serious GI events82 or reported too few events (two GI ulcers in nearly 1,000 patients)52 to affect the conclusions of the systematic reviews.
The systematic reviews both included the pivotal CLASS trials (n=7,968),54 which compared the risk of serious GI harms associated with celecoxib versus nonselective NSAIDs for osteoarthritis or rheumatoid arthritis. CLASS was designed as two trials with separate patient recruitment and randomization procedures: one compared celecoxib 400 mg twice a day with ibuprofen 800 mg three times a day and the other compared celecoxib 400 mg twice a day with diclofenac 75 mg twice a day. The prespecified primary outcome was ulcer-related complications, defined as gastric or duodenal perforation, gastric outlet obstruction, or upper GI bleeding (POBs).83 Another prespecified outcome was ulcer related complications plus symptomatic ulcers (PUBs). The planned maximum duration of the trials were 15 and 12 months, respectively, or until at least 20 ulcer-related complications occurred in each trial, or 45 in both trials combined.84 The prespecified criteria to conclude superiority of celecoxib was statistically significant differences between celecoxib and each of the comparators, as well as between celecoxib versus the comparator groups combined.
CLASS was stopped early after reaching a predefined threshold of ulcer complications. The main publication in the Journal of the American Medical Association (JAMA) reported 6-month results even though the median duration of followup was 9 months (the rationale for reporting truncated data was high attrition), and combined the ibuprofen and diclofenac results without reporting the results of the two trials separately.54 Additional details of the study were subsequently made public on the Food and Drug Administration (FDA) Web site.84
CLASS randomized 3,987 subjects to celecoxib and 3,981 subjects to nonselective NSAIDs. The JAMA article reported celecoxib associated with fewer PUBs (a secondary outcome) compared to the combined nonselective NSAIDs (32/3,987 vs. 51/3,981, annualized incidence rates 2.1% vs. 3.5%, p=0.02),54 while the rates of POBs (the primary outcome) were not significantly different (13/3,987 vs. 22/3,981, annualized incidence rates 0.76% vs. 1.4%, p=0.09). By 12 months, according to FDA documents (see Table 13, FDA Medical Officer Review)84 there was no longer a trend favoring celecoxib for POBs (17/3987 [0.43%] events with celecoxib vs. 21/3,981 [0.53%] with the nonselective NSAIDs,84 relative risk 1.1, 95% CI 0.47 to 2.685, 86, also see Figure 4, Scheiman review87). For the individual comparisons between celecoxib and ibuprofen or diclofenac, which were not reported in the JAMA article, there was no difference in the rate of ulcer complications at either 6 months or the end of followup.85 For the secondary outcome of PUBs, celecoxib was superior to ibuprofen, but not to diclofenac at 6 months and the end of followup.85 Celecoxib was also associated with a lower risk of hemoglobin (>2 g/dL) and/or hematocrit drops (≥0.10), among all patients (2.4% vs. 4.4% and 5.7% for celecoxib, diclofenac, and ibuprofen, respectively.84
About 20 percent of the patients in the CLASS trial took aspirin in addition to their study NSAID. When patients taking aspirin were excluded from the analysis, there were fewer confirmed serious ulcer complications in the celecoxib group than in the ibuprofen group (p=0.03).84, 85 However, serious ulcer complications were equivalent for celecoxib and diclofenac after exclusions of patients taking aspirin.
The new, fair-quality, nonmanufacturer-funded systematic review found celecoxib associated with a lower risk of POBs compared to nonselective NSAIDs (3 trials, RR 0.23, 95% CI 0.07 to 0.76) as well as a lower risk of PUBs (4 trials, RR 0.39, 95% CI 0.21–0.73).81 Use of 12-month instead of 6-month CLASS data did not significantly alter the pooled estimates. The systematic review also found selective COX-2 inhibitors as a class associated with lower risk of GI adverse events and withdrawal due to GI adverse events compared to nonselective NSAIDs, but did not report separate analyses for celecoxib.
The largest study in the Rostom et al. review was a manufacturer-funded combined analysis by Goldstein et al. of 14 randomized controlled trials (RCTs) of celecoxib (not including CLASS) versus placebo or nonselective NSAIDs (usually naproxen).88 The trials ranged in duration from 2 to 24 weeks, with most lasting 6 or 12 weeks. The definition of ulcer complications (POBs) was similar to the one used in CLASS, and in all trials a blinded Safety Committee adjudicated potential ulcer complications. Not all of the included trials have been published, and their quality was not assessed by Goldstein et al. In addition, data were pooled across trials without regard to randomization, duration of therapy, or which comparator NSAID was evaluated. In the 14 trials, there were 2 POBs among 6,376 patients in the celecoxib group (3 per 10,000) and 9 among 2,768 in the NSAIDs group (33 per 10,000). This corresponded to annual rates of 2 per 1,000 patient-years for celecoxib and about 17 per 1,000 patient-years for NSAIDs (p=0.002). Rostom et al. found that excluding this study eliminated heterogeneity from the pooled analyses, but celecoxib was still associated with a lower risk of POBs (RR 0.42, 95% CI 0.22 to 0.80) and PUBs (RR 0.34, 95% CI 0.22 to 0.80) compared to nonselective NSAIDs.81
A systematic review by Moore et al. included in the original CER was funded by Pfizer and the Oxford Pain Relief Trust.51 The authors obtained a declaration from Pfizer that they had received information on all completed clinical trials of celecoxib and could publish whatever results they found, but much of the data on which this meta-analysis was based is not publicly accessible. Thus, although the meta-analysis methods appeared appropriate, it is impossible to verify the reproducibility of the meta-analysis. Rather than including the pooled analysis by Goldstein et al., 88 Moore et al. appeared to have access to the individual trial methods and data.
All 18 trials of celecoxib versus nonselective NSAIDs included in the systematic review were rated 5 out of 5 on the Jadad quality scale, and 16 out of 16 on an 8-item validity scale.51 Only 2 of the 31 trials were longer than 12 weeks in duration. Although POBs was not evaluated as an outcome, celecoxib was associated with a lower risk of clinical ulcers and bleeds than nonselective NSAIDs in 18 trials (RR 0.61, 95% CI 0.46 to 0.81). When the analysis was limited to trials evaluating doses of 200 or 400 mg daily of celecoxib (excluding CLASS), the benefit was more pronounced (RR 0.35, 95% CI 0.22 to 0.56). The meta-analysis also found celecoxib associated with a lower risk of hemoglobin fall of 20 g/L or more (RR 0.72, 95% CI 0.56 to 0.92) and hematocrit fall of 5% or more (RR 0.78, 95% CI 0.69 to 0.89) compared with nonselective NSAIDs.51
In addition to having access to the individual trials included in Goldstein et al., another difference between the systematic review by Moore et al. and the one by Rostom et al. is that the latter did not include results of SUCCESS-1, the largest (N=13,274) randomized controlled trial of celecoxib.55 SUCCESS-1 found celecoxib associated with a lower risk of POBs than naproxen or diclofenac after 12 weeks in patients with osteoarthritis (0.1% vs. 0.8%, odds ratio [OR] 0.14, 95% CI 0.03 to 0.68). Post hoc analysis of nonaspirin users found nonselective NSAIDs associated with a significantly higher risk of ulcer complications compared to celecoxib, though the estimate was very imprecise (OR 12, 95% CI 1.4 to 100).55
There are several possible reasons why the results of the systematic reviews differed from those of CLASS, which did not clearly show a decreased risk of POBs for celecoxib compared to nonselective NSAIDs. First, the incidence of POBs in CLASS was relatively high.54 In the CLASS trials, the annualized rate of POBs was 0.8/100 patient-years for celecoxib and 1.4 per 100 patient-years for nonselective NSAIDs,54 compared to 0.1/100 patient-years and 0.8/100 patient-years, respectively, in SUCCESS-1.55 The high rate of POBs in the CLASS trials could be due in part to enrollment of a higher-risk population, the use of concomitant medications, or other factors. In CLASS, 20 percent of patients randomized to celecoxib were on aspirin and 31 percent on corticosteroids,54 whereas in SUCCESS-1, 7 percent were on aspirin and corticosteroid use was not permitted.55 In addition, antiulcer medications (except for occasional antacids) were prohibited in CLASS, but used in 16 percent of celecoxib patients in the Goldstein et al. combined analysis.88 Another potential explanatory factor is that the high dose of celecoxib used in CLASS—400 mg twice daily—was evaluated in few other trials, and could be associated with an increased risk of bleeding compared to lower doses. Finally, different comparator NSAIDs could be associated with different risks of GI complications. Pooling data from trials evaluating different comparator NSAIDs could obscure differential effects on GI safety if they were present.
Partially Selective NSAIDs
Five systematic reviews included in the original CER evaluated the comparative risks of serious GI harms associated with partially selective compared to nonselective NSAIDs.89–93 We identified one new systematic review (Appendix H).58 We identified no new head-to-head trials comparing serious GI harms of partially selective versus nonselective NSAIDs.
Four systematic reviews of short-term trials reported PUBs associated with meloxicam.58, 91–93 The meta-analyses mainly included in the same trials, and reported fairly consistent results. A new, good-quality systematic review, funded by UK Health Technology Assessment Programme, found meloxicam (primarily at a dose of 7.5 mg/day) associated with a lower risk for PUBs compared to various nonselective NSAIDs (6 trials, RR 0.53, 95% CI 0.29 to 0.97, p for heterogeneity=0.77), but the difference in risk of POBs did not reach statistical significance (6 trials, RR 0.56, 95% CI 0.27 to 1.2, p for heterogeneity=0.95).58 Results were mainly driven by short-term (4 week) trials of low-dose (7.5 mg) meloxicam. An earlier systematic review of 10 trials found the risk of PUBs reduced with meloxicam (OR 0.52, 95% CI 0.28 to 0.96) compared to nonselective NSAIDs.92 The third meta-analysis was funded by the manufacturer of meloxicam and used manufacturer-held documents from 28 trials.93 It found a dose-response relationship between meloxicam and PUBs (ascertained by a blinded, external adjudication committee). Meloxicam 7.5 mg was associated with lower PUB rates during the first 60 days compared to diclofenac, piroxicam, or naproxen, but the 15 mg dose was only associated with lower PUB rates than piroxicam. Finally, a good-quality systematic review found meloxicam associated with no increased risk of a composite GI outcome (including GI tolerability, PUBs, GI hospitalization, or GI-related death) compared to nonuse (RR 1.2, 95 % CI 0.98 to 1.6), and a similar risk compared to nonselective NSAIDs.91 Estimates for GI hospitalizations or GI-related deaths alone were not reported.
The new systematic review found etodolac (primarily at a dose of 600 mg/day) associated with a lower risk of PUBs compared to various nonselective NSAIDs (9 trials, RR 0.32, 95% CI 0.15 to 0.71, p for heterogeneity=0.87).58 The difference in risk of POBs was not statistically significant (6 trials, RR 0.39, 95% CI 0.12 to 1.2) but the number of events was very small (1 in the etodolac arms and 7 in the nonselective NSAID arms).
For nabumetone, a fair-quality meta-analysis included in the original CER of 6 short-term (3 to 6 months) studies (5 published and 1 abstract) found 1 PUB event among 4,098 patients taking nabumetone versus 17 events among 1,874 nonselective NSAID patients; this difference was highly statistically significant.89 The absolute PUB rates were about 2 versus 6 per 1,000 patient-years. For comparison, in a similar meta-analysis, the PUB rates per 1,000 patients per year were 13 for rofecoxib and 26 for NSAIDs.90 It is not clear why the rates of PUBs were so much lower in the nabumetone trials. There was also a significant reduction in treatment-related hospitalizations in the nabumetone group (6.4 per 1,000 patient-years versus 20 per 1,000 patients-years). Risks of POBs were not reported. A problem in interpreting these results is that the methods used to ascertain the endpoints in the trials were not described in enough detail to determine whether they were accurate or applied consistently.
Nonselective NSAIDs
Two systematic reviews evaluated comparative risks of serious GI harms associated with nonselective NSAIDs.91, 94 One was included in the original CER.91 We also included final results from a fair-quality systematic review which only had preliminary results94 at the time of the original CER (Appendix H).81 It found COX-2 inhibitors as a class (celecoxib, rofecoxib, valdecoxib, lumiracoxib, and meloxicam) associated with a similarly decreased risk of POBs compared to naproxen (RR 0.34, 95% CI 0.24 to 0.48), ibuprofen (RR 0.46, 95% CI 0.30 to 0.71), and diclofenac (RR 0.31, 95% CI 0.06 to 1.6).81 The systematic review did not include the large SUCCESS-1 study, which found no statistically significant difference in risk of POBs between naproxen (4 events, 1.83/100 patient-years) and diclofenac (3 events, 0.41/100 patient-years), though analyses were limited by the small number of events.55
The results of the new systematic review are consistent with a previous meta-analysis which found similarly increased risks of GI complications (major plus minor) for different NSAIDs relative to nonuse: indomethacin (RR 2.2, 95% CI 1.0 to 5.1), naproxen (RR 1.8, 95% CI 1.2 to 2.7), diclofenac (RR 1.7, 95% CI 1.2 to 2.5), piroxicam (RR 1.7, 95% CI 1.1 to 2.4), tenoxicam (RR 1.4, 95% CI 0.40 to 5.1), and ibuprofen (RR 1.2, 95% CI 0.93 to 1.5).91
Aspirin and Salsalate
We identified no new trials or systematic reviews on risk of ulcer complications in patients prescribed aspirin or salsalate at doses effective for analgesia. As noted in the original CER, randomized controlled trials assessing the risk of upper GI bleeding with aspirin have mainly been conducted in populations receiving aspirin as prophylaxis for thrombotic events. The populations evaluated in these trials may differ in bleeding risk compared to patients who take aspirin for arthritis. In these studies, the dose of aspirin varied widely and was generally lower (75 mg to 500 mg daily in most trials) than the doses considered effective for analgesia and anti-inflammatory effects, and patients typically received aspirin for prolonged periods. In a good-quality meta-analysis of 24 randomized trials with nearly 66,000 participants, the risk of GI hemorrhage was 2.5 percent with aspirin compared with 1.4 percent with placebo (OR 1.7, 95% CI 1.5 to 1.9), based on an average of 28 months therapy.95 A good-quality collaborative meta-analysis of individual patient data from randomized trials (over 110,000 participants) found aspirin associated with increased risk of GI and other extracranial bleeding when given for primary prevention (RR 1.5, 95% CI 1.3 to 1.8, absolute risk 0.10% vs. 0.07%) or secondary prevention (RR 2.7, 95% CI 1.2 to 5.8; absolute difference not estimated due to incomplete reporting).96
No randomized trial reported risk of ulcer complications associated with salsalate.
Observational Studies
One new systematic review97 and five systematic reviews10, 98–101 included in the original CER evaluated serious GI harms associated with various NSAIDs.
The new, fair-quality (did not assess quality of included studies) systematic review (by Massó González et al.) found celecoxib associated with an increased risk of upper GI bleeding or perforation compared to nonuse (four studies, RR 1.4, 95% CI 0.85 to 2.4), but the risk was lower than for nonselective NSAIDs as a group (eight studies, RR 4.5, 95% CI 3.8 to 5.3) as well as for individual nonselective NSAIDs, though confidence interval estimates overlapped in some cases (Table 3, Appendix H).97
Meta-analyses of observational studies included in the original CER reported similar findings. In a collaborative meta-analysis of cohort and case-control studies published between 1985 and 1994, use of all nonselective NSAIDs were associated with significantly increased risks of peptic ulcer complication hospitalizations relative to nonuse.100 As in the Massó González et al. review, ibuprofen was associated with the lowest risk of peptic ulcer complication-related hospitalizations compared to other nonselective NSAIDs.95 In two other meta-analyses of cohort and case-control studies published between 1990 and 1999, however, risk of upper GI bleeds was no lower for ibuprofen compared to any other non-aspirin, nonselective NSAID when results were stratified by low to medium (RR 2.1 vs. nonuse, 95% CI 1.6 to 2.7) or high dose (RR 5.5 vs. nonuse, 95% CI 3.0 to 10) (Table 4).98, 101 A systematic review of observational studies published through 2002 also found GI bleeding risk increased for all nonselective NSAIDs, with risk appearing related more to dose than to the specific drug evaluated.10
Eight large case-control (>1,000 cases) or cohort (n>100,000) studies reported risks of serious upper GI complications associated with various NSAIDs (Table 4, Appendix H).98, 102–108 Two of the studies were published after the original CER,102, 108 and all but two were included in the new systematic review.103, 108 Three studies used a cohort design106–108 and the remainder used a case-control (or nested case-control) design. Two case-control studies were rated good quality102, 104 and the remainder of the observational studies rated fair quality (Appendix G). The most common methodological shortcomings in the fair-quality case-control studies were failure to report the proportion of patients who met inclusion criteria who were excluded from the study and unclear accuracy of methods used to ascertain exposures and potential confounders. The most common methodological shortcomings in the fair-quality cohort studies were noncomparability of groups at baseline, unclear blinding status of outcomes assessors and data analysts, and failure to report attrition from a defined inception cohort. Four of the observational studies found celecoxib associated with an no increased risk of upper GI complications compared to nonuse103, 104, 106 or acetaminophen use.108 A fifth study found celecoxib associated with an increased risk of upper GI perforation or bleeding compared to nonuse, but risk estimates were similar or lower than those for nonselective NSAIDs.102
The partially selective NSAID meloxicam was evaluated in four of the large observational studies.98, 102, 104, 105 Meloxicam was associated with a risk of upper GI bleeding relative to nonuse of NSAIDs that was generally in the midrange of risks reported for various nonselective NSAIDs. Only one study reported risks associated with other partially selective NSAIDs, and estimates were imprecise.98
For various nonselective NSAIDs, the observational studies generally showed increased risk of GI bleeding relative to nonuse.98, 102, 103, 105–107 Naproxen was associated with a higher risk than ibuprofen in seven studies,98, 102–105, 107, 108 though the risk estimates were relatively close in two of them.103, 107 Comparative data for other nonselective NSAIDs was less consistent. For example, diclofenac was associated with similar or lower risk compared to ibuprofen in three studies, 103, 104, 108 but higher in four others.98, 102, 105, 107
The risk of upper GI bleeding was similar with aspirin compared to non-aspirin, nonselective NSAIDs in one large nested case-control study.103 Systematic reviews of observational studies included in the original CER found that aspirin increases risk of serious GI events relative to placebo or nonuse, at a rate similar to that of other nonselective NSAIDs.99, 100
Serious GI event rates (bleeding, perforation, obstruction) associated with salsalate were reported in one smaller cohort study (n=1,198) of long-term care residents in Indiana.109 The number of cases of GI-related hospitalizations associated with salsalate (1, 5.9 percent) after 14 months was similar to that of other selective and nonselective NSAIDs.
Cardiovascular Harms
Randomized Controlled Trials
Celecoxib
Four systematic reviews or meta-analyses included in the original CER (one as an earlier version available only as an FDA briefing document110) evaluated risk of serious CV events in randomized controlled trials of celecoxib (Table 5, Appendix H).51, 111–113 Two new systematic reviews were identified for this update (Table 5, Appendix H).114, 115 We identified one new placebo-controlled Chinese trial of celecoxib for prevention of gastric cancer that reported serious CV events,116 and one head-to-head trial of celecoxib versus diclofenac for osteoarthritis.52
The systematic reviews all included CLASS.54 Six-month data from CLASS showed no association between celecoxib and risk of myocardial infarction or any CV event (stroke, myocardial infarction, or angina) compared with the nonselective NSAIDs (myocardial infarctions 0.3% [10/3987] vs. 0.3% [11/3981]).54 A subsequent analysis based on complete followup data also showed no differences in the rates of any significant CV event for the overall sample (0.5% [19/3987] vs. 0.3% [13/3981]) or for the subgroup who did not use aspirin.117 Approximately 2,770 subjects in CLASS (about one-third of the sample) had at least 9 months of followup, and 1,126 had at least 12 months of followup.
Three systematic reviews provided the best information on CV risks associated with long-term use of celecoxib.111, 114, 115 All included preliminary or published results from trials of celecoxib for prevention of colon polyps or Alzheimer’s disease (Adenoma Prevention with Celecoxib trial [APC], Alzheimer’s Disease Anti-Inflammatory Prevention Trial [ADAPT], Prevention of Colorectal Sporadic Adenomatous Polyps [PreSAP]). Two systematic reviews were rated fair-quality due to failure to adequately assess trial quality111, 114 or report statistical heterogeneity.114 The third systematic review was rated good quality.115 All of the meta-analyses excluded a number of short-term trials,111, 114, 115 one of the meta-analyses excluded trials that did not have at least two arms with at least 100 patient years of followup,115 and one of the meta-analyses111 excluded trials without publicly available information on CV events. Although excluding short-term trials limited conclusions regarding short-term risks, data on long-term harms may be more relevant for patients using NSAIDs for chronic conditions such as osteoarthritis.
One of the two systematic reviews was a new study which limited inclusion to randomized, double-blind, placebo-controlled trials with planned followup of at least 3 years.114 It included 6 trials (3,664 people randomized to celecoxib), none of which evaluated patients with osteoarthritis. Three trials evaluated celecoxib for colon polyp prevention (APC, PreSAP, and the Celecoxib/Selenium trial), one for prevention of Alzheimer’s disease (ADAPT), one for prevention of recurrent breast cancer (MA27), and one for treatment of retinopathy (CDME). Relative to placebo, the overall risk of a CV event (CV death, myocardial infarction, stroke, heart failure, or a thromboembolic event) in patients randomized to celecoxib at any dose was increased (hazard ratio [HR] 1.6, 95% CI 1.1 to 2.3). The absolute difference in risk of a CV event was 3.7/1000 patient-years (11.2/1000 patient-years with celecoxib vs. 7.5/1000 patient-years with placebo), or 1 additional CV event for about every 270 patients treated with celecoxib instead of placebo for 1 year. However, the risk appeared to vary at different doses, and was lowest for celecoxib 400 mg once daily (HR 1.1, 95% CI 0.6 to 2.0), intermediate for celecoxib 200 mg twice daily (HR 1.8, 95% CI 1.1 to 3.1), and highest for celecoxib 400 mg twice daily (HR 3.1, 95% CI 1.5 to 6.1). In subgroup analyses, patients at higher baseline risk were at disproportionately increased risk of CV events compared to those at lower baseline risk (p-value for interaction 0.003).
The second systematic review, which was also a new study, limited inclusion to trials with at least 100 patient years of followup and performed a network analysis to incorporate indirect evidence into pooled estimates.115 It included 31 trials of various NSAIDs versus placebo or other NSAIDs, with 6 trials of celecoxib versus placebo (12,799 patient years), including ADAPT, APC, and PreSAP (these three trials accounted for 6,801 patient-years of celecoxib exposure). It found celecoxib associated with a nonstatistically significant trend toward increased risk of myocardial infarction (RR 1.4, 95% CI 0.7 to 2.7) and composite cardiovascular events (nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death, RR 1.4, 95% CI 0.94 to 2.2). Results were insensitive to a variety of sensitivity analyses based on methodological factors (such as use of independent adjudication of harms) or dose. There was no difference in risk of myocardial infarction between celecoxib and naproxen, ibuprofen, or diclofenac.
The third systematic review, which was included in the original CER, limited its analysis to trials that were at least 6 weeks in duration and reported CV events in published articles or publicly available material.111 It found the risk of myocardial infarction increased in three trials (APC, ADAPT, PreSAP; none evaluated arthritis patients) that compared celecoxib to placebo (OR 2.3, 95% CI 1.0 to 5.1) and in five trials (APC, CLASS, ADAPT, PreSAP, VACT; the latter two evaluated arthritis patients) that compared celecoxib to placebo, diclofenac, ibuprofen, or paracetamol (OR 1.9, 95% CI 1.2 to 3.1). No heterogeneity was present. There was no association between celecoxib use and either cerebrovascular events, CV death, or composite CV events. The meta-analysis did not include the large (N=13,274), 12-week SUCCESS-I Study, which reported results consistent with its findings (10 myocardial infarctions or 0.55/100 patient-years in the combined celecoxib arms versus 1 myocardial infarction or 0.11/100 patient-years in the combined nonselective NSAID arms).55
Neither of the systematic reviews included a new, fair-quality head-to-head trial (n=916) that found no difference in risk of myocardial infarction after 1 year in 916 patients randomized to celecoxib versus diclofenac for osteoarthritis (0.9% vs. 1.3%, RR 0.67, 95% CI 0.19 to 2.35),52 or a new, fair-quality Chinese trial (n=1,024) that found no difference in risk of CV events (defined as fatal or nonfatal myocardial infarction, and ischemic or hemorrhagic stroke) between celecoxib 200 mg twice daily and placebo after 1.5 years in patients at high risk for gastric cancer (0.86% vs. 1.1%, OR 0.84, 95% CI 0.23 to 3.2).116 In both trials, the number of events was small (9 or 10 total), and it was unclear if myocardial infarctions were subject to blinded adjudication.
Three meta-analyses included in the original CER found no increased risk of serious CV events with celecoxib versus placebo.51, 112, 113 However, these meta-analyses did not include trials completed after 2004, including two large, long-term trials of colon polyp prevention (APC and PreSAP).118, 119 These two trials account for a high proportion of the myocardial infarctions in the celecoxib trials (70 events in persons randomized to celecoxib, compared with 31 in one of the meta-analyses113). The pooled relative risk from these trials for celecoxib versus placebo was 1.9 (95% CI 1.1 to 3.1, no heterogeneity) for the composite outcome of CV death, nonfatal myocardial infarction, nonfatal stroke, or heart failure.120 Rates of fatal or nonfatal myocardial infarction were 1.6 percent (22/1356) versus 0.4 percent (3/679) in the APC trial and 9/933 (1.0 percent) versus 4/628 (0.6 percent) in PreSAP. The meta-analyses also focused almost exclusively on short-term trials, with the proportion 12 weeks or shorter in duration ranging from 87 percent to 94 percent.51, 112, 113 In addition, two of the meta-analyses were rated poor quality, in part due to failure to assess study quality and because they pooled raw event rates for a particular drug and dose across studies,112, 113 resulting in loss of randomization effects, and making it impossible to evaluate heterogeneity across studies.
A meta-analysis121 that was included in the original report was excluded from this section because it pooled risks of different COX-2 selective NSAIDs together. Based on published and unpublished data from 121 RCTs, including the polyp prevention trials previously mentioned, the relative risk for any vascular event with COX-2 selective NSAIDs as a class compared to placebo was 1.4 (95% CI 1.1 to 1.8). Much of the association appeared to be related to an increased risk of myocardial infarction (RR 1.9, 95% CI 1.3 to 2.6), with no increased risk of stroke (RR 1.0, 95% CI 0.71 to 1.5). From 41 trials, the raw event rate for myocardial infarction in patients randomized to celecoxib was 0.5 percent (44/8976 person-years) compared to 0.2 percent (9/4953 person-years) in those randomized to placebo. Based on the forest plot presented with the meta-analysis, the point estimate for celecoxib was similar to the overall pooled estimate for all COX-2 selective NSAIDs, and just met criteria for statistical significance. A trend towards increased risk of vascular events (p=0.03) with higher doses of celecoxib was observed, but nearly all of the events at the highest (800 mg daily) dose occurred in the polyp prevention trials. Analyses on the effects of duration and independent event adjudication were not stratified by specific COX-2 inhibitor, nor were estimates of CV risk with specific COX-2 inhibitors relative to naproxen or nonnaproxen NSAIDs.
In summary, celecoxib appears to be associated with an increased risk of myocardial infarctions or thromboembolic CV events compared to placebo. Much of the evidence for increased CV risk comes from two large, long-term polyp prevention studies that compared celecoxib 200 or 400 mg twice daily, or 400 mg once daily, to placebo.
Other NSAIDs
One systematic review included in the original CER evaluated risk of serious CV events associated with nonselective NSAIDs.121 We identified one new systematic review115 Two trials included in the original CER and not included in the systematic review also reported serious CV events in patients prescribed naproxen.55, 122
A new, good-quality systematic review by Trelle and colleagues of 31 trials (with at least two arms with at least 100 patient-years of followup) compared CV risks associated with various nonselective NSAIDs, based on a network analysis.115 It found ibuprofen associated with increased risk of composite cardiovascular outcomes (nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death) compared with placebo (RR 2.3, 95% CI 1.1 to 4.9) and diclofenac associated with a trend towards increased risk (RR 1.6, 95% CI 0.85 to 3.0). Among the nonselective NSAIDs, with respect to specific CV outcomes, diclofenac was associated with the highest risk of stroke (RR 2.9, 95% CI 1.1 to 8.4) and cardiovascular death (RR 4.0, 95% CI 1.5 to 13). Naproxen was associated with only a slight, nonsignificant trend toward increased risk (RR 1.2, 95% CI 0.78 to 1.9). There were no statistically significant differences in risk of composite CV outcomes between naproxen, ibuprofen, and diclofenac.
A fair-quality systematic review included in the original CER by Kearney and colleagues of 91 trials (mostly ranging from 4 to 13 weeks in duration) evaluated risks associated with any nonselective NSAID (33,260 person-years of exposure) compared to any COX-2 selective NSAID (23,325 person-years of exposure).121 Most of the trials evaluated naproxen (42 trials), ibuprofen (24 trials), and diclofenac (26 trials); only 7 evaluated other nonselective NSAIDs. Generalizability to usual practice could be limited because the majority of the trials evaluated higher than standard doses of NSAIDs. Much of the data regarding CV event rates were obtained by requesting unpublished data from trial sponsors.
Table 6 shows estimates of risk for different CV outcomes with COX-2 inhibitors relative to nonselective NSAIDs. Risk of myocardial infarction was similar with COX-2 inhibitors and nonnaproxen NSAIDs, but about twofold as great for COX-2 inhibitors compared with naproxen (0.6% or 99/16360 vs. 0.3% or 30/10,978, RR 2.0, 95% CI 1.4 to 3.0). This is equivalent to about 1 additional myocardial infarction for every 300 patients treated for 1 year with a COX-2 inhibitor instead of naproxen. COX-2 inhibitor use was also associated with a lower risk of stroke relative to nonnaproxen NSAIDs (RR 0.62, 95% CI 0.41 to 0.95). In subgroup analyses of specific nonselective NSAIDs (ibuprofen, diclofenac, other nonselective NSAIDs), the difference in stroke risk was only observed with diclofenac, which was usually evaluated at high doses (RR 0.48, 95% CI 0.27 to 0.83). There was insufficient data to analyze the effects of lower doses on estimates of risk.
Kearney et al. found insufficient data to directly estimate risks of nonselective NSAIDs from placebo-controlled trials. Indirect analyses (based on trials of nonselective NSAIDs versus COX-2 inhibitors and trials of COX-2 inhibitors vs. placebo) suggested an increased risk of vascular events with ibuprofen (RR 1.5, 95% CI 0.96 to 2.4) and diclofenac (RR 1.6, 95% CI 1.1 to 2.4) relative to placebo, but not with naproxen (RR 0.92, 95% CI 0.67 to 1.3). However, indirect analyses should be interpreted with caution because they can give discrepant results compared to head-to-head comparisons.123
The Kearney meta-analysis did not include results of the large SUCCESS-1 trial, which reported 0.61 MIs/100 patient-years with naproxen (n=905), and no cases of MI in diclofenac users (n=3489).55 It also didn’t include the Alzheimer’s Disease Anti-Inflammatory Prevention Trial (ADAPT), which was terminated early in December 2004 because of an “apparent increase in CV and cerebrovascular events among the participants taking naproxen when compared with those on placebo.”124 Results from ADAPT showed a nonsignificant increased in risk of CV deaths (HR 1.5, 95% CI 0.30 to 7.3), myocardial infarction (HR 1.5, 95% CI 0.69 to 3.2), or stroke (HR 2.1, 95% CI 0.81 to 5.6).122 Naproxen was associated with an increased risk based on the composite outcome of CV death, myocardial infarction, stroke, congestive heart failure, or transient ischemic attack (HR 1.6, 95% CI 1.0 to 2.6). The decision to terminate ADAPT has been criticized because rigorous stopping protocols were not used, the increased risk associated with naproxen for individual and most composite CV outcomes did not reach statistical significance, the events were not adjudicated, and the number of events was small.125
Aspirin and Salsalate
Aspirin is known to be protective against occlusive vascular events because of its irreversible antiplatelet effects. In a collaborative meta-analysis of infidel patient data from 22 randomized trials (over 110,000 participants), lower doses of aspirin (primarily less than 325 mg daily) were associated with decreased risk of serious vascular events when given for primary prevention (0.51% aspirin vs. 0.57% control per year, p=0.0001) or secondary prevention (6.7% vs. 8.2%, p<0.0001).96 The populations evaluated in these trials probably varied substantially from trials of patients with arthritis.
Observational Studies
Three systematic reviews evaluated CV risk associated with various NSAIDs.126–128 Two were included in the original CER and focused on risks associated with naproxen.126, 127 The third was a new, good-quality systematic review of CV risk (primarily myocardial infarction) from 23 observational studies that was published too late to be included in the original CER, though results were summarized in a brief addendum (Appendix H).128 We also identified four large observational studies not included in the original CER.108, 129–131
The new systematic review included a total of 23 observational studies (16 case-control and 7 cohort studies).128 It found diclofenac associated with the highest risk, followed by indomethacin and meloxicam (Table 7). Celecoxib, naproxen, piroxicam, and ibuprofen were not associated with increased risks. For all NSAIDs, increases in risk were modest (RR <1.5), and all of the main analyses were characterized by substantial between-study heterogeneity.
Nineteen large observational studies (case-control studies with >1000 cases or cohort studies with >100,000 subjects) evaluated risk of CV events associated with various NSAIDs (Table 8, Appendix H).108, 129–146 All of these studies except for four108, 129–131 were included in the original CER. Seven studies108, 129–132, 141, 146 not included in the systematic review of observational studies.128 Six studies used a cohort design108, 130, 131, 140, 146, 147 and the remainder a case-control (or nested case-control) design. Three studies evaluated the UK General Practice Research Database133, 134, 143 and three evaluated the same Canadian (Quebec) database.108, 130, 141 Only one study was rated good quality,139 the remainder were rated fair quality. The most common methodological shortcoming in the fair-quality case-control studies was failure to report the proportion of patients who met inclusion criteria who were excluded from the study. The most common methodological shortcomings in the fair-quality cohort studies were unclear blinding status of outcomes assessors and data analysts, and failure to report attrition from a defined inception cohort. Interpretation of the studies was complicated by the use of different study designs, adjustment for different numbers and types of confounders, and evaluation of different populations and outcomes.
Sixteen observational studies evaluated risk of serious CV events (primarily myocardial infarction) associated with celecoxib.108, 129–132, 135–140, 145–149 Three studies found celecoxib associated with similar risk of CV events compared to naproxen, ibuprofen, or diclofenac.130, 145, 146 A fourth study found ibuprofen (OR 1.3, 95% CI 1.0 to 1.6) and naproxen (OR 1.4, 95% CI 1.1 to 1.8) associated with a higher risk of acute MI requiring admission or sudden cardiac death than celecoxib.135 Twelve studies found no increased risk of serious CV events with celecoxib relative to nonuse of NSAIDs.108, 129, 131, 135, 136, 138–140, 145–148 Three studies found current (RR 1.6, 95% CI 1.2 to 2.0),132 new (RR 2.1, 95% CI 1.4 to 3.1),137 or any (HR 1.5, 95% CI 0.99 to 2.2)149 use of celecoxib associated with increased risk compared to nonuse of NSAIDs. In these studies, the increased MI risk was either time-137 or dose-dependent.132
The nonselective NSAID naproxen has received additional scrutiny since the VIGOR trial17 showed an increased risk of CV events with rofecoxib versus naproxen, due to the hypothesis that naproxen might be protective against myocardial infarction. In addition, a systematic review121 of randomized trials (described earlier) found that naproxen was not associated with the same increased in CV risk as other nonselective and selective NSAIDs. In addition to the new systematic review of observational studies described above (which found a neutral effect of naproxen on CV risk),128 two systematic reviews included in the original CER specifically focused on CV risks associated with naproxen.126, 127 The first, a meta-analysis of 11 observational studies of naproxen (four based on the General Practice Research Database) found naproxen associated with a small cardioprotective effect (OR 0.86, 95% CI 0.75 to 0.99), with Merck-funded studies reporting larger effect sizes.127 Nine observational studies published after this systematic review showed no cardioprotective effect associated with naproxen,108, 129, 132, 135–137, 139, 148, 149 though one other study showed a modest protective effect (HR 0.79, 95% CI 0.67 to 0.93).131 An FDA review included in the original CER concluded no cardioprotective effect of naproxen after taking into account various methodological issues.126
Large observational studies found no other nonselective NSAID consistently associated with increased risk of CV events compared to nonuse of NSAIDs.129, 131–137, 139, 140, 142–144 For example, ibuprofen was associated with a modest increased risk (OR 1.4, 95% CI 1.3 to 1.6 and OR 1.2, 95% CI 1.1 to 1.4) of serious CV events compared to nonuse of NSAIDs in two129, 136 studies, but no increased risk in nine others.131–135, 143, 144, 147, 149
Partially selective NSAIDs have not been well studied in large observational studies. Three studies found no increased risk of serious CV events with meloxicam compared to nonuse.129, 134, 139 One study found no increased risk of acute myocardial infarction with use of etodolac or nabumetone versus nonuse of NSAIDs, but estimates were imprecise.133
In April 2005, after reviewing the available observational data, the FDA issued a Public Health Advisory stating, “Long-term controlled clinical trials have not been conducted with most of these (nonselective) NSAIDs. However, the available data suggest that use of these drugs may increase CV risk. It is very difficult to draw conclusions about the relative CV risk among the COX-2 selective and nonselective NSAIDs with the data available. All sponsors of nonselective NSAIDs will be asked to conduct and submit to FDA a comprehensive review and analysis of available controlled clinical trial databases pertaining to their NSAID product(s) to which they have access to further evaluate the potential for increased CV risk.”150 The FDA also required labeling changes to both prescription and nonprescription nonselective NSAIDs warning about potential CV risks.
Overall Rate of Serious Adverse Events
Because use of different NSAIDs could be associated with different tradeoffs for serious CV and GI harms (for example, reducing serious GI harms but increasing serious CV harms), analyses that evaluate the risk of all serious harms simultaneously could be helpful for understanding overall comparative risks. However, not all serious adverse events are equal in importance to patients and physicians. A reduction in the rate of one kind of adverse event might be considered more important than an increase in another one.
Analyses of all serious adverse events in CLASS were included in the original CER. A Canadian analysis used data from FDA documents84 to analyze serious adverse events, defined as death, hospitalization, or “any life-threatening event, or event leading to severe disability.151 It found similar rates of all serious adverse events between celecoxib and ibuprofen or diclofenac (6.8 percent vs. 5.8 percent). An FDA analysis of CLASS found 12 serious adverse events/100 patient-years for celecoxib; 10/100 patient-years for diclofenac, and 11/100 patient-years for ibuprofen, a difference that was not statistically significant.84
A fair-quality retrospective cohort study not included in the original CER evaluated risk of first hospitalization for acute myocardial infarction or GI bleeding in a Canadian cohort of patients 65 years or older.108 For the combined outcome, naproxen use was associated with the largest risk compared to acetaminophen use (HR 1.6, 95% CI 1.3 to 1.9). Celecoxib (HR 0.93, 95% CI 0.83 to 1.0) and ibuprofen (HR 1.0, 95% CI 0.74 to 1.5) were associated with neutral risk, and diclofenac with an intermediate but nonstatistically significant increased risk (HR 1.2, 95% CI 0.99 to 1.4)
Other Adverse Events Associated With Selective and Nonselective NSAIDs
Mortality
We identified no new studies evaluating mortality associated with different NSAIDs. Large clinical trials included in the original CER did not show differences in mortality between different NSAIDs.54, 152 In CLASS, mortality rates were 0.47%, 0.37%, and 0.45% for celecoxib, diclofenac, and ibuprofen, respectively.84 In SUCCESS-1, 5 deaths (0.06 percent) were observed after 12 weeks in the celecoxib group and 5 (0.11 percent) in the nonselective NSAIDs group.55 A meta-analysis that included unpublished company clinical trial data (including CLASS and SUCCESS-1) found no significant difference in rates of death in patients randomized to celecoxib compared with nonselective NSAIDs, though there were few events (0.03% or 6/18,325 in the celecoxib arms vs. 0.11% or 14/12,685 in the NSAID arms).51
One retrospective cohort study of Saskatchewan health-services databases that followed patients from 6 months following prescription until death found nabumetone associated with significantly lower rates of all-cause mortality compared with diclofenac (OR 2.0; 95% CI 1.2 to 3.1) and naproxen (OR 3.0, 95% CI 1.9 to 4.6).153 However, we found no other studies that replicated this finding.
Hypertension, CHF, Edema, and Renal Function
Six systematic reviews or meta-analyses included in the original CER evaluated comparative risks of hypertension, CHF, edema, and renal function associated with various NSAIDs.19, 51, 110, 154–156 A seventh systematic review was published too late to be fully included in the original CER, but described in an appendix.157 It was rated fair quality because it did not assess the quality of included studies. Two new observational studies evaluated risk of congestive heart failure in high risk patients.158
All NSAIDs appear to be associated with increases in blood pressure. However, evidence regarding differential effects of specific NSAIDs is somewhat conflicting. One meta-analysis included in the original CER found that nonselective NSAIDs raised mean blood pressure by an average of about 5.0 mm Hg (95% CI 1.2 to 8.7).154 Piroxicam produced the most marked elevation in blood pressure compared to placebo. In head-to-head trials, there were no significant differences between indomethacin and sulindac (10 trials), indomethacin and salicylate (1 trial), diclofenac and sulindac (1 trial), ibuprofen and sulindac (1 trial), and naproxen and sulindac (3 trials). Another meta-analysis found that piroxicam and ibuprofen had negligible effects on blood pressure, and that indomethacin and naproxen were associated with the largest increases.155 In both meta-analyses, aspirin and sulindac were associated with minimal hypertensive affect. More than half of the published NSAID trials did not report hypertension rates as an outcome.155
Several meta-analyses of celecoxib included in the original CER found no increased risk of hypertension compared to nonselective NSAIDs.19, 51, 110 A fair-quality meta-analysis found celecoxib (dose not specified) not associated with an increased risk of hypertension compared to either placebo (RR 0.81, 95% CI 0.13 to 5.21) or nonselective NSAIDs (RR 0.82, 95% CI 0.68 to 1.00).19 A Pfizer-funded meta-analysis submitted to the FDA found an increased risk of developing hypertension with celecoxib at any dose compared to placebo (1.1% vs. 0.7%, p=0.02), though the risk was lower than for nonselective NSAIDs (1.5% vs. 2.0%, p=0.002).110 A third meta-analysis, funded in part by the manufacturer, reported similar findings for risk of hypertension (celecoxib vs. nonselective NSAID, RR 1.1, 95% CI 0.90 to 1.3).51 The fourth meta-analysis, which was included as an appendix in the original CER, found celecoxib associated with slightly lower risk of hypertension (RR 0.83, 95% CI 0.71 to 0.97) compared with control treatments (placebo, other NSAID, or mixed/other).157 Most of the trials included in the meta-analyses were short-term and only one meta-analysis51 evaluated the quality of the trials.
Results from large trials of celecoxib are mostly consistent with the meta-analyses. In CLASS (median duration of followup 9 months), celecoxib was associated with a similar rate of hypertension (new-onset and aggravated preexisting) compared with diclofenac (2.7 percent vs. 2.6 percent), and a lower rate compared with ibuprofen (2.7 percent vs. 4.2 percent).117 In the shorter-term (12 weeks) SUCCESS-I trial (N=13,274), rates of hypertension were similar with celecoxib 100 or 200 mg twice a day compared with either diclofenac or naproxen (RR 0.86, 95% CI 0.62 to 1.20).55 The APC polyp prevention trial found celecoxib associated with greater systolic blood pressure elevations compared to placebo at 1 and 3 years at either 200 mg twice daily (2.0 mm Hg at 1 year and 2.6 mm Hg at 3 years) and 400 mg twice daily (2.9 mm Hg at 1 year and 5.2 mm Hg at 3 years).120 On the other hand, the PreSAP polyp prevention trial found no difference in systolic blood pressure increases between celecoxib 400 mg once daily and placebo.120
With regard to renal dysfunction, it is unclear whether COX-2 selective NSAIDs as a class are associated with clinically important differences in risk compared to nonselective NSAIDs. A systematic review included in the original CER of five small (sample size range 15 to 67), short-term (28 days or less) trials found that COX-2 selective NSAIDs had similar effects on glomerular filtration rate and creatinine clearance compared to nonselective NSAIDs in three trials, and were modestly superior in two.156 The clinical effects of the modest differences observed in the latter two trials were unclear. Another systematic review found no difference in risk of creatinine increase greater than 1.3 times the upper limit of normal with celecoxib at 200 to 400 mg compared with nonselective NSAIDs (RR 0.78, 95% CI 0.46 to 1.3).51 CLASS showed no differences in the risk of experiencing an increase in serum creatinine >1.0 mg/dl with celecoxib (0.2 percent), diclofenac (0.1 percent), or ibuprofen (0.2 percent), though the nonselective NSAIDs were associated with slightly greater increases in serum creatinine, particularly in patients with prerenal azotemia at baseline.159 A systematic review of randomized trials included as an appendix in the original CER found celecoxib associated with lower risk of renal dysfunction (RR 0.61, 95% CI 0.40 to 0.94) compared to control treatments (placebo, other NSAID, or mixed/other), but no difference for composite renal events (RR 0.97, 95% CI 0.84 to 1.1).157
Two systematic reviews of randomized controlled trials included in the original CER found no clear difference between celecoxib and nonselective NSAIDs in risk of heart failure. In one systematic review, heart failure was more frequent with celecoxib than with placebo (13 of 8,405 vs. 1 of 4,057, p=0.05), though not compared with nonselective NSAIDs (0.1% vs. 0.2%, p=0.06).110 A second meta-analysis also found no significant difference between celecoxib and nonselective NSAIDs in risk of heart failure (RR 0.70, 95% CI 0.43 to 1.1).51 Similar results were observed in large trials of celecoxib. In CLASS, CHF rates were similar with celecoxib versus ibuprofen or diclofenac (0.3 percent vs. 0.3 percent).117, and withdrawals due to heart failure rare with all three NSAIDs (0.1% vs. <0.1% vs. 0.3%).159 The APC polyp prevention trial found no difference in rates of heart failure between celecoxib versus placebo, though event rates were low (five cases of heart failure among 1,356 subjects).119
The risks of hypertension and heart failure with celecoxib and nonselective NSAIDs were evaluated in several observational studies. A new Danish cohort study of patients who had been hospitalized for congestive heart failure found use of celecoxib, ibuprofen, diclofenac, or naproxen at any dose associated with similar risk of hospitalization due to congestive heart failure (HR estimates ranged from 1.2 to 1.4), though celecoxib and diclofenac were associated with greater risk of death (HR 1.8, 95% CI 1.6 to 1.9 and HR 2.1, 95% CI 2.0 to 2.2, respectively) compared with ibuprofen and naproxen (HR 1.3, 95% CI 1.2 to 1.4 and HR 1.2, 95% CI 1.1 to 1.4, respectively).160 A new nested case-control study found indomethacin associated with increased risk of heart failure compared to celecoxib (OR 2.0, 95% CI 1.2–3.6) in patients older than 66 years recently hospitalized for heart failure.158 There was no difference in risk of heart failure between other nonselective NSAIDs (diclofenac [OR 0.82, 95% CI 0.51 to 1.3] and ibuprofen [OR 1.5, 0.66 to 3.2]) or acetaminophen (OR 1.2, 95% CI 0.92 to 1.4) relative to celecoxib. A retrospective cohort study included in the original CER based on the same Canadian database found nonselective NSAIDs associated with an increased risk of death (HR 1.5, 95% CI 1.2 to 2.0), recurrent heart failure (HR 1.2, 95% CI 0.92 to 1.6), or either (HR 1.3, 95% CI 1.0 to 1.6) in similarly high risk patients.161 Another retrospective cohort study included in the original CER found nonselective NSAIDs (RR 1.4, 95% CI 1.0 to 1.9) but not celecoxib (RR 1.0, 95% CI 0.8 to 1.3) associated with increased risk of heart failure admission compared to nonuse.162 A case-control study based on data from the General Practice Research Database found nonselective NSAIDs associated with an increased risk of newly diagnosed heart failure compared to nonuse of NSAIDs (RR 1.6, 95% CI 1.2 to 2.1).163
A fair-quality systematic review included as an appendix in the original CER found no difference between celecoxib and controls (placebo, other NSAIDs, or mixed/other) in risk of arrhythmia, but the number of events was small (RR 0.84, 95% CI 0.45 to 1.6) and most trials didn’t report arrhythmias.157
Hepatotoxicity
One systematic review164 included in the original CER and one new meta-analysis165 evaluated randomized controlled trials reporting hepatotoxicity associated with various NSAIDs. Another systematic review included in the original CER evaluated observational studies.166 We identified one new randomized controlled trial of celecoxib versus diclofenac that reported rates of hepatic adverse events,52 and a report of hepatotoxicity from the diclofenac arm of a large randomized trial.167
The new meta-analysis included 41 randomized trials involving celecoxib.165 It found risk of hepatobiliary abnormalities (clinical or laboratory) similar for celecoxib (276/24933 or 1.1 percent), ibuprofen (38/2484 or 1.5%, p=0.06 vs. celecoxib), and placebo (36/4057 or 0.89%, p=0.21 vs. celecoxib); slightly lower rate for naproxen (0.68%, p=0.03 vs. celecoxib); and slightly higher for diclofenac (324/2618 or 4.24%, p<0.0001 vs. celecoxib). No patient randomized to an NSAID met Hay’s rule (elevation of alanine aminotransferase ≥3 times the upper limit of normal with an elevation of bilirubin ≥2 times the upper limit of normal), and no cases of liver failure or drug-related liver transplant were reported. The rate of alanine aminotransferase (ALT) abnormalities was higher with diclofenac (78/1000 patient-years) compared with the other NSAIDs or placebo (16 to 28/1000 patient-years). Four deaths occurred (2 in patients randomized to celecoxib, 1 naproxen, and 1 diclofenac), but none were considered related to drug treatment. A systematic review included in the original CER reported similar findings.164 Based on 67 published articles and 65 studies accessible from the FDA archives, it found diclofenac (3.6%, 95% CI 3.1% to 4.0%) associated with higher rates of aminotransferase elevations greater than 3 times the upper limit of normal compared with placebo (0.29%; 95% CI 0.17% to 0.51%) and other NSAIDs (all ≤ 0.43 percent), and a higher rate of liver-related discontinuations compared to placebo (2.2%, 95% CI 1.8 to 2.6). Serious complications related to liver toxicity were rare: only one liver-related hospitalization (among 37,671 patients) and death (among 51,942 patients) occurred in a patient on naproxen in a trial of rofecoxib versus naproxen. Data from the diclofenac arm (n=17,289) of a randomized trial showed similar results.167 The rate of aminotransferase elevation greater than three times the upper limit of normal was 3.1 percent, with four cases of liver-related hospitalizations (0.023 percent) and no cases of liver failure, death, or transplant.
Large trials that have evaluated diclofenac also suggested an increased risk of hepatotoxicity compared to other NSAIDs. In CLASS, celecoxib was associated with a lower risk of elevation in serum ALT (0.6 percent vs. 2.2 percent), serum AST (0.5 percent vs. 1.8 percent), and withdrawals due to hepatic enzyme elevations (<0.1 percent vs. 1.2 percent) compared to diclofenac or ibuprofen.54 In SUCCESS-1, rates of increase in ALT levels were 0.5 percent with celecoxib versus 1.3 percent with diclofenac or naproxen (p<0.001).55 A smaller (n=916), new trial comparing celecoxib versus diclofenac also found a lower risk of hepatic function abnormalities with celecoxib compared to diclofenac (0.6 percent vs. 3.5 percent).52
A systematic review of seven population-based epidemiological studies found a similarly low risk of serious hepatic toxicity associated with NSAIDs.166 In those studies, the excess risk of liver injury associated with current NSAIDs ranged from 4.8 to 8.6/100,000 person-years of exposure compared with past use. There were zero deaths from liver injury associated with NSAIDs in more than 396,392 patient-years of exposure. A recent cohort study from Italy found that nimesulide, an NSAID not available in the United States, was associated with a higher incidence of serious liver injury compared with other NSAIDs.168 None of the other NSAIDs, including celecoxib, were associated with an increased risk of serious liver injury. An earlier review of five population-based studies found sulindac associated with a five- to tenfold higher incidence of hepatic injury compared with other NSAIDs.169 Diclofenac was associated with higher rates of aminotransferase elevations compared with users of other NSAIDs, but not with a higher incidence of serious liver disease.
Tolerability
Celecoxib
Two systematic reviews50, 51 included in the original CER and one new systematic review58 evaluated the relative tolerability of celecoxib compared to nonselective NSAIDs (Table 9). We also identified one new pooled analysis of randomized trials from the Pfizer registry,170 one randomized trial not included in the original CER,52 and one pooled analysis of three similarly designed trials.82
The new systematic review found no differences between celecoxib and nonselective NSAIDs in the risk of any adverse event (RR 0.96, 95% CI 0.91 to 1.0), GI adverse events (RR 0.90, 95% CI 0.78 to 1.0), or withdrawals due to adverse events (RR 0.86, 95% CI 0.73 to 1.0).58 However, celecoxib was associated with a lower likelihood of withdrawals due to GI adverse events (RR 0.45, 95% CI 0.35 to 0.56). A systematic review included in the original CER reported found celecoxib associated with decreased risk of withdrawal due to adverse events (RR 0.86, 95% CI 0.81 to 0.91), withdrawal due to GI adverse events (RR 0.75, 95% CI 0.70 to 0.80), or any GI adverse event (RR 0.85, 95% CI 0.82 to 0.88).51 The risk of serious adverse events (RR 1.0, 95% CI 0.91 to 1.2) and any adverse event (RR 0.96, 95% CI 0.94 to 0.98) were similar. An older systematic review reported results consistent with the other two systematic reviews.50 All of the systematic reviews included the large and longer-duration CLASS trial, which reported lower risks of withdrawal due to adverse events (18 percent vs. 21 percent) and withdrawal due to GI adverse events (8.7 percent vs. 11 percent) with celecoxib compared to diclofenac or ibuprofen.54
A meta-analysis of 21 randomized trials from the Pfizer registry reported results that were generally consistent with the systematic reviews.170 It found celecoxib associated with lower risk of GI adverse events (20 percent) compared to naproxen (32 percent), ibuprofen (31 percent), or diclofenac (24 percent), as well as lower likelihood of withdrawal due to GI adverse events (4.2% vs. 5.0% to 8.5%). However, this study was rated poor quality, in part because it did not assess study quality and because raw event rates were pooled across studies, resulting in loss of randomization.
One new randomized trial (n=925) found celecoxib and diclofenac associated with no difference in risk of withdrawal due to adverse events (27% vs. 31%, respectively, p=0.22) or withdrawal due to GI adverse events (15 percent vs. 14 percent).52 A pooled analysis from three similarly designed trials of patients in Asia (total n=880) found no difference between celecoxib and diclofenac in risk of withdrawal due to adverse events (3.4% vs. 6.1%, p>0.05).82
Partially Selective NSAIDs
Two systematic reviews89, 92 of randomized trials included in the original CER and one new systematic review58 evaluated the tolerability of meloxicam, etodolac, or nabumetone compared to nonselective NSAIDs. The new systematic review found meloxicam associated with decreased risk of any adverse event (RR 0.91, 95% CI 0.84 to 0.99), any GI adverse event (RR 0.31, 95% CI 0.24 to 0.39), and withdrawals due to GI adverse events (RR 0.61, 95% CI 0.54 to 0.69), though there was no difference in the risk of withdrawal for any adverse event (RR 0.92, 95% CI 0.66 to 1.3).58 The median Jadad quality score for the trials included in the systematic review was three (maximum five), indicating moderate overall quality. A meta-analysis of meloxicam studies included in the original CER reported similar findings, with lower risks of any GI event (OR 0.64; 95% CI 0.59 to 0.69) and withdrawals due to GI events (OR 0.59; 95% CI 0.52 to 0.67) with meloxicam compared with nonselective NSAIDs.92
The new systematic review also evaluated tolerability of etodolac.58 It found etodolac associated with a lower risk of any adverse event (RR 0.83, 95% CI 0.70 to 0.99) compared to nonselective NSAIDs, but there was no difference in risk of GI adverse events (RR 0.77, 95% 0.55 to 1.1), withdrawal due to adverse events (RR 0.93, 95% CI 0.77 to 1.1), or withdrawal due to GI adverse events (RR 0.95, 95% CI 0.54 to 1.6). Only two of 29 trials of etodolac scored 5 out of 5 on the Jadad quality scale; 7 received only 2 points.
In a meta-analysis included in the original CER, the incidence of GI adverse events was slightly but statistically significantly lower with nabumetone compared to nonselective NSAIDs (25 % vs. 28%, p=.007), corresponding to about one fewer event for every 34 patients treated with nabumetone.89
Nonselective NSAIDs
A Cochrane review included in the original CER evaluated the tolerability of different NSAIDs.56 The only relatively consistent finding was that indomethacin was associated with higher rates of toxicity than other NSAIDs, but it was not clear if these differences were statistically significant.
Aspirin and Salsalate
Five randomized trials (all included in the original CER) evaluated the efficacy or safety of aspirin or salsalate compared with nonaspirin NSAIDs in patients with arthritis.80, 171–174 All were short-term (≤ 12 weeks) and involved a total of 471 patients; of the subjects enrolled, only 4 had osteoarthritis of the hip/knee for every 100 patients with rheumatoid arthritis. Aspirin was associated with higher incidence of overall adverse events than salsalate (70% vs. 40%, p<0.05)80 and diclofenac (61% vs. 46%; p<0.05);173 these led to higher rates of withdrawals due to adverse events for aspirin compared with diclofenac (23% vs. 6%; p<0.05). Salsalate was associated with a higher incidence of overall adverse events compared to other nonselective NSAIDs in two171, 174 of three trials, but the actual rates were not reported.
The overall safety profile of salsalate has also been evaluated in the rheumatoid arthritis population using the Arthritis, Rheumatism, and Aging Medical Information System (ARAMIS) databases. These studies reported summary measures of drug toxicity based on tabulations of mean frequencies of overall adverse events per patient years, weighted by severity, and adjusted for differences in demographic factors. Numerically larger index scores indicate greater levels of toxicity. The summary index score takes into account symptoms from all body systems, laboratory abnormalities, and all-cause hospitalizations.175–178 Symptoms were assessed every 6 months using patient self-report in response to open-ended questions. Hospitalizations and deaths were ascertained from discharge summaries and death certificates. Descriptions of study methods varied, but the ARAMIS studies were somewhat vague with regard to patient selection and ascertainment methods; adverse events were not clearly defined or prespecified; exposure duration and length of followup were unclear; and adjustments were made only for demographic factors such as age and gender. Because the results of these studies are more subject to recall bias and had other methodological shortcomings, the findings that aspirin, salsalate, and ibuprofen were the least toxic among the NSAIDs studied (Table 10) are less convincing than if they were reported in more rigorously designed observational studies.
Acetaminophen
Four systematic reviews included in the original CER evaluated the efficacy and safety of acetaminophen compared with NSAIDs (selective or nonselective) for osteoarthritis.179–182 We identified no new systematic reviews. One new randomized trial compared acetaminophen versus naproxen for osteoarthritis.183 One new observational study evaluated risk of acute myocardial infarction associated with various NSAIDs compared to acetaminophen.108
The systematic reviews generally met all criteria for good-quality systematic reviews, except that three180–182 did not provide sufficient detail about trials that were excluded. The overall conclusion from the reviews was that NSAIDs were modestly superior to acetaminophen for general or rest pain (Table 11). For pain on motion and overall assessment of clinical response, NSAIDs also appeared modestly superior, though the differences were not always statistically significant.180, 181 Only two reviews assessed functional disability; neither found clear differences.180, 181
The risk of adverse events with acetaminophen versus NSAIDs was assessed in three systematic reviews (Table 12).179, 180, 182 In two reviews, there were no differences in withdrawal due to any adverse event.179, 180 Acetaminophen was associated with lower risk of GI adverse events compared with nonselective NSAIDs in two systematic reviews (though not compared with coxibs)180, 182 and lower risk of withdrawals due to GI adverse events in one systematic review.180 One systematic review found no difference between NSAID and acetaminophen in serious GI, renal, or CV harms, but found few events in the primarily small, short-term trials (data not provided).180
A new, fair-quality (high loss to followup) randomized trial found no differences in withdrawal due to lack of efficacy or WOMAC scores between acetaminophen 4 g once daily and naproxen 750 mg once daily for osteoarthritis after 6 months (n-=105) or 1 year (n=476).183 Acetaminophen and naproxen were also associated with similar rates of withdrawal due to adverse events (25% vs. 22%, NS), serious adverse events (3.5 percent vs. 2.5 percent), any adverse event (72 percent vs. 74 percent), renal adverse events (three total), or hepatic enzyme increases (three in acetaminophen group vs. zero in the naproxen group). Naproxen was associated with an increased risk of constipation (9.9% vs. 3.1%, p<0.002) and peripheral edema (3.9% vs. 1.0%, p<0.033) compared to acetaminophen.
Clinical trials of acetaminophen have not been large enough to assess serious but less common complications such as PUBs, myocardial infarction, acute renal failure, or hypertension. Several observational studies included in the original CER provide some additional information about the safety of acetaminophen relative to NSAIDs. A fair-quality nested case-control study of 1,197 cases and 10,000 controls from a population-based cohort of 458,840 people in the General Practice Research Database found current acetaminophen use associated with a lower risk for symptomatic peptic ulcer (adjusted RR 1.9, 95% CI 1.5 to 2.3) than NSAID use (adjusted RR 4.0, 95% CI 3.2 to 5.1) when each was compared with nonuse.184 There was no clear relationship between higher acetaminophen dose and increased risk for symptomatic ulcers. An earlier analysis on the same database also found current acetaminophen use associated with a lower risk for upper GI bleeds or perforations (adjusted RR 1.3, 95% CI 1.1 to 1.5) than current NSAID use (adjusted OR 3.9, 95% CI 3.4 to 4.6), each compared with nonuse.98 A retrospective cohort study of elderly patients found that patients using lower doses of acetaminophen (<2,600 mg once daily) had lower rates of GI events (defined as GI-related hospitalizations, ulcers, and dyspepsia) compared with users of NSAIDs (RR 0.73, 95% CI 0.67 to 0.80 for 1,951 to 2,600 mg once daily), but the risks were similar at higher doses (RR 0.93 to 0.98).185 Although GI hospitalization rates were not reported separately, the authors noted that dyspepsia was responsible for most of the increase in GI events in the high-dose acetaminophen groups. A meta-analysis on individual patient data from three earlier retrospective case-control studies (2472 cases) was consistent with the above studies.186 It found acetaminophen associated with a minimal increase in the risk for serious upper GI bleeding (OR 1.2, 95% CI 1.1 to 1.5). By contrast, nonselective NSAIDs were associated with higher risks, though estimates of risk varied considerably for different NSAIDs (OR 1.7 for ibuprofen to 35 for ketoprofen).
No randomized trial evaluated the association between acetaminophen use and myocardial infarction or other thromboembolic CV events. An analysis from the large, prospective Nurses’ Health Study found heavy use of acetaminophen (more than 22 days/month) associated with an increased risk of CV events (RR 1.4, 95% CI 1.1 to 1.6) similar to that with heavy use of NSAIDs (RR 1.4, 95% CI 1.3 to 1.6).187 Dose-and frequency-dependent effects were both significant. A new retrospective cohort study found no difference in risk of acute myocardial infarction between celecoxib, ibuprofen, diclofenac, or naproxen versus acetaminophen (Table 13, Appendix H).108
The association between renal failure and acetaminophen use was evaluated in several case-control studies included in the original CER. Interpretation of these studies is difficult because many had important flaws (such as failure to identify patients early enough in the course of their disease to ensure that the disease had not led to a change in the use of analgesics, failure to specify diagnostic criteria, failure to adjust for the use of other analgesics, incompleteness of data on exposure, and use of proxy respondents) in the collection or analysis of data.188 The largest (926 cases) case-control study was designed to try to avoid many of these flaws, though results remain susceptible to confounding by indication.189 It found regular use of acetaminophen associated with an increased risk for chronic renal failure (Cr >3.8 for men and >3.2 for women) compared with nonuse (OR 2.5, 95% CI 1.7 to 3.6). Use of NSAIDs was not associated with an increased risk (OR 1.0). A prospective cohort study of 1,697 women in the Nurses’ Health Study found increased lifetime acetaminophen exposure associated with a higher risk of decline in glomerular filtration rate of 30% or greater (p<0.001), though NSAIDs were not (p=0.88).190 The absolute risk of renal function decline, however, was modest, even in women reporting high amounts of lifetime acetaminophen use. Compared with women consuming less than 100 g of cumulative acetaminophen, the odds of a decline in GFR of at least 30 mL/min per 1.73 m2 for women consuming more than 3,000 g was 2.04 (95% CI, 1.28 to 3.24). By contrast, analyses of men in the Physicians’ Health Study found no association between acetaminophen or NSAIDs and change in kidney function.191, 192
The risk of heart failure associated with acetaminophen has not been well studied. In a single study using the General Practice Research Database, current use of acetaminophen was associated with a higher risk of newly diagnosed heart failure compared with nonuse (RR 1.3, 95% CI 1.1 to 1.7), though the risk was lower compared with current use of NSAIDs (RR 1.6, 95% CI 1.2 to 2.0).163
The risk of hypertension has been evaluated using data from the Nurses’ Health Studies193–195 and the Physicians’ Health Study.196 In the Nurses’ Health Studies, acetaminophen and NSAIDs were associated with similar increases in risk of incident hypertension (Table 13). In the Physicians’ Health Study, there was no association between NSAID or acetaminophen use and hypertension.
Glucosamine and Chondroitin
Five new systematic reviews (Appendix I)197–201 and four systematic reviews 202–205 included in the original CER evaluated benefits and harms of glucosamine and chondroitin. New trials identified for this update include one trial of glucosamine versus acetaminophen and placebo,206 two trials of glucosamine versus placebo,207, 208 four trials of chondroitin versus placebo,209–212 and one trial of the combination of glucosamine and chondroitin versus placebo (Table 14, Appendix I).213 We also identified two followup analyses from the previously included Glucosamine/chondroitin Arthritis Intervention Trial (GAIT).214, 215
Glucosamine
The most promising results for glucosamine have been reported in trials evaluating a pharmaceutical grade glucosamine not available in the United States, and sponsored by its European manufacturer (the Rotta Corporation). Because the content and purity of over-the-counter glucosamine preparations vary substantially, the results of trials that evaluated pharmaceutical grade glucosamine may not be directly applicable to over-the-counter preparations available in the U.S.216
The original CER included a good-quality Cochrane review (searches through November 2004) with four short-term (4 to 8 weeks) head-to-head trials of glucosamine versus an oral NSAID (ibuprofen or piroxicam).205 Two of the trials were rated 5 out of 5 on the Jadad scale, and the other two were rated 3 or 4 out of 5. Three of the trials were sponsored by the European manufacturer; the fourth217 was also conducted in Europe, but funding information was not reported. One of the trials has only been published as an abstract,218 with analyses based on data from an unpublished manuscript. Two of the four trials found glucosamine superior to oral NSAIDs for efficacy,217, 218 and two found no difference.219, 220 In pooled analyses, glucosamine was superior to an oral NSAID for improving pain (three trials, standardized mean difference −0.40, 95% CI −0.60 to −0.19), but not for improving function measured with the Lequesne Index (two trials, standardized mean difference [SMD] −0.36, 95% CI −1.07 to 0.35). Glucosamine was also associated with fewer adverse events (RR 0.29, 95% CI 0.19 to 0.44) and withdrawals due to toxicity (RR 0.06, 95% CI 0.01 to 0.25).
Three head-to-head trials221–223 included in the original CER were not included in the Cochrane review. The large, (n=1,583), NIH-funded, good-quality GAIT trial compared glucosamine versus celecoxib, as well as placebo, chondroitin, and the combination of glucosamine plus chondroitin (Tables 14 and 15, Appendix I).221 GAIT evaluated pharmaceutical-grade glucosamine hydrochloride (over-the-counter supplements commonly available in the United States are typically glucosamine sulfate) and chondroitin sulfate under an investigational new drug application. It found no differences between glucosamine and celecoxib in the proportion of responders defined by those with at least a 20 percent decrease in WOMAC pain score (70% vs. 64%, RR 0.91 [95% CI 0.82 to 1.02]), or as defined using Outcomes Measures in Arthritis Clinical Trials-Osteoarthritis Research Society International (OMERACT-OARSI) criteria (67% vs. 61%, RR 0.90 [95% CI 0.80 to 1.01]). There were also no differences in change from baseline on WOMAC scores, SF-36 Mental or Physical Component summary scores, or the Health Assessment Questionnaire. The number of withdrawals due to adverse events was similar (2.8 percent vs. 2.2 percent), with no serious GI adverse events or deaths in either group. One patient randomized to glucosamine had chest pain and one patient randomized to celecoxib had a stroke. The celecoxib group experienced a nonsignificant but higher incidence of “cardiac” events compared to patients randomized to other treatments, though these were predominantly arrhythmias (palpitations and atrial fibrillation) rather than ischemic events (data not reported). Two small (n=40 and n=45), 12-week Canadian trials (not funded by the European manufacturer of pharmaceutical grade glucosamine) found no differences between glucosamine and ibuprofen for general osteoarthritis pain222 or temporomandibular joint osteoarthritis.223 Only limited details of the study design were reported for the first trial, though the second met all criteria for a good-quality study.
One new, fair-quality trial sponsored by the European manufacturer of pharmaceutical grade glucosamine found no difference between glucosamine and acetaminophen in improvements from baseline on the Lequesne Index (−3.1 [95% CI −3.8 to −0.8) vs. −2.7 [95% CI −3.3 to −2.1], respectively), the WOMAC total score (−13 [95% CI −16 to −10] vs. −12 [95% CI −15 to −10.0]), the WOMAC pain score (−2.7 [95% CI −3.3 to −2.1] vs. −2.4 [−3.0 vs. −1.8]), and the WOMAC function score (−9.2 [−11 vs. −7.2] vs. −8.7 [−11 vs. −6.8]).206 There was also no difference in the proportion of responders based on OARSI-A criteria (40 percent vs. 33 percent). Adverse events were similar.
Four systematic reviews not included in the original CER focused on evaluations of glucosamine versus placebo (Appendix I).197, 198 The first, fair-quality systematic review, by Bjordal et al., was based on 7 randomized trials (sample size range 10 to 126, median 46, total n=401).197 It found glucosamine associated with a statistically significant but clinically nonsignificant beneficial effect on pain compared to placebo (mean difference 4.7 points on a 100-point scale, 95% CI 0.3 to 9.1). The second, good-quality systematic review, by Wandel et al., differed from the first in that in focused on larger (n>200) randomized trials (7 trials of glucosamine, 1,939 patients randomized to glucosamine vs. placebo), included more recently published trials, and conducted network analysis to incorporate indirect evidence.198 It also found a statistically significant but clinically nonsignificant beneficial effect of glucosamine on pain (−0.4 cm on a 10 cm scale, 95% credible interval −0.7 to −0.1) and joint space narrowing (−0.2 mm, 95% CI −0.3 to 0.0) compared to placebo. No differences were found when trials were stratified according to whether they evaluated glucosamine hydrochloride or sulfate. There was no difference between glucosamine and placebo in withdrawals due to adverse events. A third, good-quality systematic review (by Vlad et al.) of 15 randomized trials (sample size range 24 to 630, median 155, total n=2,613) found glucosamine associated with an SMD of 0.35 (95% CI 0.14 to 0.56), based on the primary outcome reported in each study.201 However, the overall estimate was associated with substantial statistical heterogeneity (I2=80 percent). In stratified analyses, there was no statistically significant effect and heterogeneity was absent in subgroups of trials without industry funding (four trials, SMD 0.05, 95% CI −0.32 to 0.41), those that did not evaluate a Rottapharm produce (seven trials, SMD 0.11, 95% CI −0.16 to 0.38), and those with adequate allocation concealment (five trials, SMD 0.09, 95% CI −0.24 to 0.42). A fourth, fair-quality systematic review by Lee et al. found glucosamine associated with decreased joint space narrowing (SMD 0.43, 95% CI 0.23 to 0.63) and decreased risk of >0.5 mm joint space narrowing (OR 0.36, 95% CI 0.20 to 0.64), but results were based on only two trials.200
Other systematic reviews202, 204, 205 included in the original CER are now outdated, as they excluded recent good-quality and relatively large trials. The Cochrane review included in the original CER found higher trial quality and evaluation of non-Rotta brand glucosamine associated with lower estimates of benefits.205 Other older systematic reviews also found important methodological flaws in the glucosamine trials that could have exaggerated estimates of effect.202, 204
The previously described, good-quality GAIT trial is the largest trial of glucosamine.224 It found no difference between glucosamine and placebo in the likelihood of experiencing a >20% improvement in WOMAC pain score after 24 weeks (64% vs. 60%, RR 1.1 [95% CI 0.94 to 1.2]), or various WOMAC, SF-36, and Health Assessment Questionnaire scores. There was also no difference in joint space width narrowing,214 likelihood of achieving a 20 percent reduction in WOMAC pain (OR 1.2, 95% CI 0.65 to 2.0), or improvement in WOMAC function after 24 months.215
Three trials of glucosamine versus placebo have been published since the original CER.206–208 All except one208 were included in the Wandel et al. systematic review.198 Of the three new trials, two were rated good quality.207, 208 Both found no differences on outcomes related to pain, function, or (in one trial) radiographic narrowing between glucosamine and placebo for hip osteoarthritis207 or low back pain with degenerative osteoarthritis.208 The hip osteoarthritis trial also found no differences in efficacy in subgroups defined by radiographic severity, type of osteoarthritis (localized or generalized), level of pain, and other factors.207 The third, fair-quality trial (high attrition) found glucosamine more effective than placebo in improving the Lequesne score (difference in mean change from baseline −1.2 [95% CI −2.3 to −0.8] on a 24 point scale), WOMAC Function score (difference −3.7 [95% CI −6.9 to −0.5] on a 68-point scale), and in the proportion experiencing an OARSI response (40% vs. 21%, RR 1.9 [95% CI 1.2 to 2.9]).206 In all three trials, withdrawals due to adverse events and specific adverse events were similar with glucosamine and placebo.
Chondroitin
The only trial that compared chondroitin to an NSAID was the GAIT trial.224 It found no difference between chondroitin and celecoxib in the proportion of patients with a 20 percent decrease in WOMAC pain score (65% vs. 70%, RR 0.93 [95% CI 0.84 to 1.0]), response based on OMERACT-OARSI criteria, or mean changes in WOMAC, SF-36, or Health Assessment Questionnaire Scores.
For chondroitin versus placebo, a good-quality systematic review by Wandel et al. found chondroitin associated with a borderline statistically significant (but clinically insignificant) effect on pain versus placebo (−0.3 cm on a 10 cm scale, 95% CI −0.7 to 0.0).198 There was no effect on radiological joint space narrowing (mean difference −0.1 mm, 95% CI −0.3 to 0.1). The analysis was restricted to trials with sample sizes >200 subjects (four studies). There was no difference between chondroitin and placebo in withdrawals due to adverse events. A fair-quality systematic review by Hochberg et al. that focused on effects of chondroitin versus placebo on joint space narrowing in trials with longer (2 years) followup reported a point estimate similar to Wandel et al., but result were statistically significant (3 trials, mean difference 0.13 mm, 95% CI 0.06 to 0.19 mm). Another fair-quality systematic review also found chondroitin associated with less joint space narrowing compared to placebo (2 trials, SMD 0.26, 95% CI 0.13 to 0.39).199, 200
Systematic reviews202, 204, 225 included in the original CER are now outdated as they do not include several recently published, larger trials.202, 204, 225 In addition, two of the systematic reviews did not evaluate effects of trial quality,204, 225 and one did not evaluate effects of chondroitin separately from glucosamine.204 One of the systematic reviews found that lower quality and smaller trials reported larger effects compared to higher quality and larger trials.202
The good-quality, large GAIT trial (included in the original CER and the Wandel et al. systematic review) provides the strongest evidence on efficacy of chondroitin versus placebo.224 It found no differences between chondroitin and placebo for experiencing a >20% improvement in WOMAC Pain score after 24 weeks (65% vs. 60%, RR 1.1, 95% CI 1.0 to 1.2) or various WOMAC, SF-36, and Health Assessment Questionnaire scores. There was also no difference in joint space width narrowing,214 WOMAC pain, or WOMAC function after 24 months.215 Adverse events with chondroitin and placebo were similar.
Three new fair-quality trials included in the Wandel et al. systematic review198 (unclear allocation concealment methods in all three trials,209–211 and high attrition in two of the three trials209, 211) found no clear benefit from chondroitin versus placebo for knee osteoarthritis on most clinical outcomes (pain or function) for knee osteoarthritis, though the two trials209, 211 that evaluated radiographic outcomes found chondroitin associated with less joint space narrowing. One of the trials found chondroitin associated with no benefit on the primary outcomes of pain and function, but a higher likelihood of an OMERACT-OARSI response (68% vs. 56%, RR 1.2 [95% CI 1.0 to 1.5]).210 In all three trials, adverse events with chondroitin and placebo were similar.209–211 One other new fair-quality trial not included in the Wandel et al. systematic review found chondroitin associated with decreased pain (mean difference −12 on a 0 to 100 mm VAS, 95% CI −20 to −3.7) and improved Lequesne index (mean difference −1.7 points on a 0 to 24 scale, 95% CI −3.0 to −0.4) compared to placebo in patients with osteoarthritis of the knee and psoriasis.212 There were no differences in SF-36 physical or mental component scores.
Glucosamine Plus Chondroitin
The GAIT trial also evaluated the combination of glucosamine plus chondroitin.224 It found no differences between the combination and placebo in the likelihood of achieving a clinical response after 24 weeks. Followup analyses at 24 months found no differences between the combination and placebo in joint space narrowing,214 WOMAC pain, or WOMAC function.215 In a post hoc analysis, the combination was superior to placebo for achieving a clinical response at 24 weeks in an analysis of a small (20 percent of enrollees) subgroup of patients with moderate to severe (WOMAC 301 to 400 mm) baseline pain (79% vs. 54.3%, RR 1.5 [95% CI 1.1 to 1.9]). The authors postulated that the lack of effect in the mild baseline pain group could have been due in part to floor effects. Adverse events were similar in the combination and placebo groups. A new, fair-quality trial (unclear randomization and allocation concealment methods) found no difference between the combination of glucosamine and chondroitin in patients with osteoarthritis of the knee, in combination with exercise or as a standalone treatment.213 Adverse events were not reported.
Key Question 1a. How do These Benefits and Harms Change With Dosage and Duration of Treatment, and What is the Evidence That Alternative Dosage Strategies, Such as Intermittent Dosing and Drug Holidays, Affect the Benefits and Harms of Oral Medication use?
Summary of Evidence
- Higher doses of NSAIDs were associated with greater efficacy for some measures of pain relief, and in some trials with greater withdrawals due to adverse events.
- A meta-analysis of 41 randomized trials found no clear association between duration of therapy with COX-2 selective NSAIDs and risk of CV events.
- The meta-analysis found higher doses of celecoxib associated with increased risk of CV events.
- Almost all of the CV events in trials of celecoxib were reported in long-term trials of colon polyp prevention that used higher, twice-daily dosing.
- For nonselective NSAIDs, large observational studies showed no association between higher dose and longer duration of NSAID therapy and increased risk of CV events.
- Many studies found that risk of GI bleeding increases with higher doses of nonselective NSAIDs, but no clear association with duration of therapy.
- One small trial found continuous celecoxib slightly more effective than intermittent use on pain and function, and similar rates of withdrawals due to adverse events. No trial has been designed to assess serious GI or CV harms associated with intermittent dosing strategies.
Detailed Analysis
Eight systematic reviews10, 95, 99, 101, 114, 121, 186, 226 included in the original CER and one new systematic review97 evaluated effects of dose and duration on benefits and harms of NSAIDs. We identified one new trial that compared continuous to intermittent use of celecoxib for osteoarthritis.227
One good-quality systematic review of eight trials included in the original CER found higher doses of nonselective and partially selective NSAIDs associated with greater efficacy for some measures of pain relief when directly compared to lower doses.226 Higher doses also were associated with greater withdrawals due to adverse events in two of four trials.
Evidence on the association between dose of NSAID or duration of therapy and risk of CV events is mixed. A meta-analysis of 41 randomized trials included in the original CER found that risk of CV events with COX-2 inhibitors did not vary according to duration of treatment.121 For celecoxib specifically, evidence of an association with CV events largely comes from long-term trials.114 The 33-month APC polyp prevention trial was the first to show an increased risk of CV events relative to placebo.119 The lack of an association in CLASS54 and other shorter term trials could be due to a duration-dependent effect, or lack of power due to small numbers of events in the shorter trials.
The meta-analysis121 also found higher doses of celecoxib associated with greater CV risks relative to placebo (p=0.03). Most of the events at the highest dose (800 mg once daily) came from the two long-term polyp prevention trials.119, 228 Large observational studies showed no association between higher doses of various celecoxib and various nonselective NSAIDs132, 133, 139, 143, 144, 146 or longer duration of therapy129, 130, 132, 133, 143, 144, 146 and increased risk of CV events. However, one new cohort study of patients following an index hospitalization for heart failure found higher doses of celecoxib, ibuprofen, diclofenac, and naproxen associated with increased risk of death compared to lower doses, though there was no dose-dependent effect on risk of subsequent hospitalization due to heart failure of myocardial infarction.160
Evidence on the association between dose of NSAID therapy and risk of ulcer complications is more consistent, though the association between duration and risk of ulcer complications is less clear. CLASS found celecoxib more effective than nonselective NSAIDs at reducing GI events at 6 months compared with longer duration of exposure, though interpretation of final results is difficult due to high withdrawal rates.54, 85 A new systematic review of observational studies found higher doses of selective and nonselective NSAIDs (RR 5.4 compared to nonuse of NSAIDs, 95% CI 4.6 to 6.3) consistently associated with greater risk of upper GI bleeding or perforation compared to lower or medium doses (RR 2.8, 95% CI 2.2 to 3.6).97 There was no clear association with duration of therapy. Similar findings were reported in older systematic reviews of observational studies included in the original CER.10, 101, 186 In three studies98, 102, 104 included in the new systematic review, slow-release formulations of NSAIDs (RR 5.9, 95% CI 4.7 to 7.3) and NSAIDs with a half-life longer than 12 hours (RR 5.7, 95% CI 3.6 to 9.2) were also associated with a greater risk of upper GI bleeding or perforation compared to NSAIDs with a half-life shorter than 12 hours (RR 3.1, 95% CI 2.4 to 4.1).97
For aspirin, a systematic review of randomized trials included in the original CER found no association between higher dose and increased risk of upper GI bleeding.95 Modified release formulations did not attenuate the risk for bleeding. In a fair-quality meta-analysis of 31 randomized trials with more than 190,000 subjects, the risk of major bleeding was 1.6 percent with doses <100 mg once daily, 1.5 percent with 100–200 mg once daily, and 2.3 percent with >200 mg once daily.229 Although the difference between doses >200 mg once daily and <100 mg once daily was statistically significant, the absolute difference were small. A systematic review of observational studies found that most (but not all) studies found a dose-dependent effect of aspirin on risk of upper GI complications.99
The risk of bleeding associated with acetaminophen was not clearly associated with increased dose in a meta-analysis of three case-control studies included in the original CER,186 though there was a modest dose response in one other case-control185 and one retrospective cohort study108 of older adults.
Few studies have evaluated risks associated with lower over-the-counter doses of NSAIDs. Based on data from the ARAMIS database, the risk of GI hospitalizations associated with over-the-counter doses of aspirin, acetaminophen, and ibuprofen were similar to background rates in patients with rheumatoid arthritis or osteoarthritis.230 A systematic review of observational studies found use of aspirin and nonaspirin NSAIDs at over-the-counter doses associated with an increased risk of GI bleeding, though the risk was lower than observed at prescription doses (approximately twofold greater risk at over-the-counter doses and sixfold or higher increases at heavy prescription levels.10 One recent analysis of the Nurses’ Health Study found that the risk of CV events was dose-related for both NSAIDs and acetaminophen.187
Data on effects of intermittent dosing or frequency of dosing is sparse. One new (n=123) randomized trial found continuous celecoxib 200 mg once daily slightly more effective than intermittent use on the WOMAC total score (difference from baseline 38 vs. 25, scale not reported, p<0.05) and associated with a smaller percentage of days using medications for flares (48% vs. 53%, p=0.03) after 24 weeks in patients with osteoarthritis of the knee or hip.227 Continuous and intermittent dosing were associated with similar rates of withdrawal due to adverse events, but the trial was not designed to assess serious harms such as ulcer complications or myocardial infarction. One difference between the APC trial (which found an increased risk of CV events with celecoxib) and the PreSAP trial (which reported no association) was twice-daily (APC) versus once-daily (PreSAP) dosing.120 However, no study has directly compared such dosing strategies. Furthermore, other studies of twice-daily dosing with celecoxib (such as CLASS54 and ADAPT122) reported no increase in CV risk.
Key Question 2. Do the Comparative Benefits and Harms of Oral Treatments for Osteoarthritis Vary for Certain Demographic and Clinical Subgroups?
Summary of Evidence
- Age, sex, and race
- The absolute risk of serious GI and CV complications increases with age.
- Large observational studies have not consistently shown increased relative risks of serious GI or CV complications with older age.
- Because the absolute risk of serious GI and CV complications increases with older age, more complications occur even with similar relative risks.
- Evidence on effects of sex and race on comparative benefits and harms associated with oral treatments for osteoarthritis is very sparse.
- History of bleeding ulcer
- Risk of GI bleeding is higher in patients with prior bleeding
- Two trials found high rates of recurrent ulcer bleeding in patients randomized either to celecoxib (4.9% to 8.9% with 200 mg twice daily) or a nonselective NSAID + PPI (6.3 percent).
- One trial found the combination of celecoxib with higher dose PPI associated with lower risk of recurrent bleeding compared with celecoxib alone (0% vs. 8.9%; p=0.0004).
- Underlying CV or renal risk
- A systematic review of randomized trials of celecoxib found risk of CV events doubled in patients at moderate versus low risk (HR 2.0, 95% CI 1.5 to 2.6) and doubled again in patients at high risk (HR 3.9 for high risk vs. low risk, 95% CI 2.3 to 6.7).
- Most large observational studies found an association between increased CV risk and increased risk of CV events in persons using NSAIDs.
- Following hospitalization for heart failure, one large observational study found celecoxib and diclofenac associated with a higher risk of death compared to ibuprofen or naproxen, and another large observational study found an increased risk of repeat heart failure admission with indomethacin compared to other nonselective NSAIDs, ibuprofen, acetaminophen, or celecoxib.
- Concomitant use of anticoagulants and analgesics
- Concomitant use of anticoagulants and nonselective NSAIDs increase the risk of GI bleeding three-to sixfold compared with anticoagulant use without NSAIDs.
- The risk with concomitant celecoxib is not clear due to conflicting findings among observational studies, but may be increased in older patients.
- Reliable conclusions about the comparative safety of nonselective, partially selective and selective NSAIDs with concomitant anticoagulants could not be drawn due to small numbers of studies with methodological shortcomings.
- Warfarin plus low-dose aspirin increased the risk of bleeding compared with warfarin alone in patients with indications for antithrombotic prophylaxis.
- Acetaminophen can increase International Normalized Ratio (INR) levels, but effects on bleeding rates have not been studied.
- Concomitant use of prophylactic dose aspirin
- Concomitant use of aspirin appears to attenuate the GI benefits of COX-2 selective NSAIDs, resulting in risk similar to nonselective NSAIDs.
- Addition of a PPI may reduce the risk of GI harms in persons using either celecoxib or nonselective NSAIDs and low-dose aspirin.
- Evidence regarding the effects of concomitant aspirin use on CV risk associated with selective or nonselective NSAIDs is limited, though three polyp prevention trials of COX-2 selective NSAIDs found that concomitant aspirin use did not attenuate the observed increased risk of CV events.
- Observational studies did not find increased CV risk with the addition of nonselective NSAIDs as a class to low-dose aspirin.
- Limited evidence suggests an increased risk of mortality with aspirin and concomitant ibuprofen compared to aspirin alone among high risk patients (HR 1.9, 95% CI 1.3 to 2.9), but studies on effects of ibuprofen added to aspirin on MI risk in average risk patients were inconsistent and did not clearly demonstrate increased risk.
Detailed Analysis
Demographic Subgroups Including Age, Sex, and Race
In general, the risk of CV, cardiorenal, and GI adverse events associated with NSAIDs increase with age.12 In one United Kingdom population, for example, the risk of adverse GI outcomes in patients taking selective or nonselective NSAIDs was 1.4 per 1,000 patient-years for all patients 25 years or older, but 4.0 per 1,000 patient-years in patients aged 65 or more.103 Similarly, the risk of myocardial infarction was 1.7 per 100 person-years for all patients 25 years or older, but 4.6 per 100 person-years for those 65 or older.136 We found no trial designed to assess whether the relative harms and benefits associated with different NSAIDs for osteoarthritis vary according to age. Large observational studies that have stratified subjects by age have not showed a consistent increase in relative estimates of risk associated with NSAIDs in older compared to younger age strata for ulcer complications107, 133 or myocardial infarction.130, 132 However, even if the relative benefits and harms associated with different drugs are consistent across age groups, the absolute effects would increase with age because of greater baseline CV and GI risk. In one observational study, the CV event rate in older adults (mean age 80 years) was 12/100 patient-years for ibuprofen overall, and 18/100 patient-years in people 80 years and older.114
Studies that evaluated the efficacy and safety of selective and nonselective NSAIDs in average-risk elderly patients have generally reported similar findings compared with studies in populations with younger adults. An individual patient data meta-analysis of three celecoxib trials, for example, found effects of celecoxib 200 mg once daily or 400 mg once daily and naproxen 1,000 mg once daily similar in elderly patients when evaluating WOMAC and SF-36 scores.231 For the SF-36, there were no statistically significant differences: naproxen scored better than celecoxib 200 mg on 4 of 10 components of the SF-36, while celecoxib 200 mg scored better on 6, including general health. Celecoxib 200 mg was significantly better than placebo on nine of the 10 components, while naproxen was significantly better than placebo on seven. The study also confirmed that the overall incidence of GI adverse events was lower with celecoxib; the difference was about 1 event in 20 patients for celecoxib 200 mg and 1 in 10 for celecoxib 400 mg. Another meta-analysis found that trials of NSAIDs in patients over the age of 60 reported similar risks for GI complications compared to trials of patients under the age of 60.91
Data suggesting differential effects of oral medications for osteoarthritis according to gender, ethnicity, or race remain scant. In most of the published trials, a majority of subjects were women. As noted in the discussion of acetaminophen, results from the Nurses’ Health Studies suggest that acetaminophen is associated with modest reductions in renal function in women,195 but results from the Physicians’ Health Study have found no association between acetaminophen use and renal dysfunction in men.196 The effects of different NSAIDs in specific ethnic minorities have only been evaluated in small studies. In a randomized crossover study of 25 black and Hispanic patients on ACE inhibitors, peak increases in blood pressure were similar in patients on diclofenac compared with celecoxib.232 We did not find any other publications focusing on the differential efficacy or safety of coxibs in African-Americans, Hispanics, or other ethnic minorities.
Coexisting Diseases Including History of Previous Bleeding Ulcer Due To NSAIDs: Hypertension, Edema, Ischemic Heart Disease, and Heart Failure
Previous Bleeding Ulcer
Two randomized trials included in the original CER233, 234 and one new trial235 compared the risk of GI harms in patients with a recent bleeding ulcer randomized to celecoxib versus the combination of celecoxib plus a PPI (Table 16).
In two fair-quality, 24-week trials (total n=529) included in the original CER of patients with a history of a recent bleeding ulcer, rates of recurrent bleeding were similar for celecoxib (200 mg daily 3.7 percent and twice a day 4.9 percent) and the combinations of extended-release diclofenac 75 mg twice a day plus omeprazole 20 mg daily (6.3 percent)233 or naproxen 250 mg three times a day plus lansoprazole 30 mg a day (6.3 percent)234 (differences not statistically significant). There were also no differences between celecoxib and either combination therapy in GI, renal, and CV adverse events or in rates of withdrawal due to adverse events. One exception was that celecoxib 200 mg daily was associated with a higher rate of dyspepsia than naproxen 250 mg three times a day plus lansoprazole 30 mg daily in one trial.236
A new, fair-quality, 12-month trial (n=273) of patients with recently healed GI bleeding (following cessation of NSAID therapy and treatment with a PPI for 8 weeks) found celecoxib 200 mg twice daily plus esomeprazole 20 mg twice daily associated with significantly fewer ulcer bleeding recurrences compared with celecoxib alone.235 After a median of 13 months, zero events occurred in the combined treatment group, compared with 12 (8.9 percent) in the celecoxib alone group (p=0.0004). Similar results were found among those taking low-dose aspirin (0% vs. 19%; p=0.03). Other adverse events and rates of discontinuations were similar between groups.
Underlying Cardiovascular or Renal Risk
We found no randomized trials designed to assess whether the relative harms and benefits associated with different oral treatments for osteoarthritis vary according to underlying CV or renal risk. A new systematic review of long-term celecoxib trials found that risk of CV events doubled between patients at low and moderate baseline CV risk (HR 2.0, 95% CI 1.5 to 2.6) and doubled again in patients at high baseline risk (HR, high risk to low risk, 3.9, 95% CI 2.3 to 6.7).114 Most132, 133, 145, 237 but not all134 large observational studies also found a history of coronary heart disease, coronary heart disease risk factors, or categorization as high CV risk associated with increased risk estimates with NSAIDs as a group. A good-quality, population-based study of a very high-risk group of 58,000 Danish patients with previous myocardial infarction found hazard ratios for death of 2.6 (95% CI 2.2 to 3.1) for celecoxib, 1.5 (95% CI 1.4 to 1.7) for ibuprofen, 2.4 (95% CI 2.1 to 2.8) for diclofenac, and 1.3 (95% CI 1.2 to 1.4) for other NSAIDs compared to nonuse of NSAIDs.238 Based on the rates of death in this population (95 per 1,000 person-years in those not using NSAIDs), the estimated number of patients needed to treat with an NSAID for one year to cause one additional death was 14 (95% CI 10 to 24) for celecoxib, 45 (95% CI 29 to 102) for ibuprofen, and 24 (95% CI 16 to 45) for diclofenac.
We found no trials evaluating comparative risks of different oral medications in patients with known congestive heart failure. A new cohort study found celecoxib, ibuprofen, diclofenac, and naproxen associated with similar risk of hospitalization due to acute myocardial infarction (HR estimates ranged from 1.3 to 1.5) or hospitalization due to congestive heart failure (HR estimates ranged from 1.2 to 1.4) following an index hospitalization for congestive heart failure, though celecoxib (HR 1.8, 95% CI 1.6 to 1.9) and diclofenac (HR 2.1, 95% C I 2.0 to 2.2) were associated with greater risk of death compared to ibuprofen (HR 1.3, 95% CI 1.2 to 1.4) or naproxen (HR 1.2, 95% CI 1.1 to 1.4).162 A new nested case-control study found indomethacin associated with increased risk of heart failure compared to celecoxib (OR 2.0, 95% CI 1.2 to 3.6) in patients older than 66 recently hospitalized for heart failure.158 There was no difference in risk of heart failure between other nonselective NSAIDs (diclofenac [OR 0.82, 95% CI 0.51 to 1.3] and ibuprofen [OR 1.5, 0.66 to 3.2]) or acetaminophen (OR 1.2, 95% CI 0.92 to 1.4) relative to celecoxib. A retrospective cohort study included in the original CER based on the same Canadian database found nonselective NSAIDs associated with an increased risk of death (HR 1.5, 95% CI 1.2 to 2.0), recurrent heart failure (HR 1.2, 95% CI 0.92 to 1.6), or either (HR 1.3, 95% 1.0 to 1.6) in similarly high risk patients.161
One new trial (n=88) compared ibuprofen, acetaminophen, and piroxicam in hypertensive patients on lisinopril/hydrochlorothiazide or amlodipine.239 Both NSAIDs blunted the effects of the antihypertensive drugs, with the lisinopril/hydrochlorothiazide combination more affected. Acetaminophen had almost no effect on blood pressure.
Concomitant Anticoagulants
Nonselective NSAIDs
Concomitant use of anticoagulants and nonselective NSAIDs increase the risk of GI bleeding three-to sixfold compared to anticoagulants alone.240, 241 Three observational studies included in the original CER evaluated risk of bleeding in patients on an NSAID plus anticoagulants versus an anticoagulant alone.242–244 We identified no new studies.
A good-quality nested case-control study of elderly (>66 years old) patients on warfarin in Ontario, Canada, evaluated the association between hospitalization for upper GI bleeding (361 cases) and use of selective or nonselective NSAIDs.242 It found that after adjustment for potential confounders (antiplatelet agents, hypoglycemic agents, glucocorticoids, gastroprotective agents, history of previous bleed, and comorbidities), recent use of nonselective NSAIDs (OR 1.9, 95% CI 1.4 to 3.7), and celecoxib (OR 1.7, 95% CI 1.2 to 3.6) were associated with increased and overlapping risks for upper GI bleeding, compared with nonuse. Because this study relied on pharmacy databases to identify exposures prior to hospitalization, it could not assess the confounding effects of over-the-counter use of aspirin, other NSAIDs, or acid suppressive medications. It also was unable to control for variations in INR level and the risk for bleeding.
In a fair-quality cohort study of patients enrolled in an anticoagulation clinic, 1,145 patients who were receiving warfarin (INR ≥1.4) but not aspirin, acetaminophen, or other nonselective NSAID were indentified retrospectively. 243 Eleven percent (n=123) were taking celecoxib concurrently with warfarin during the study period. The risk of major bleeding events (requiring hospitalization, transfusion or resulting death) was not significantly elevated in the celecoxib group (RR 1.0, 95% CI 0.14 to 7.8).
A smaller, fair-quality nested retrospective study of patients in the Netherlands evaluated the risk of bleeding in anticoagulated patients receiving partially selective (meloxicam or nabumetone) or nonselective NSAIDs.244 This study differed from the others in that it included all cases of bleeding, including minor visible bleeding, hematoma, or black tarry stools. Patients were identified as having exposure to anticoagulation by being enrolled in a pharmacy-based anticoagulation program. Bleeding events were identified through the pharmacy clinic records, and discharge diagnosis records (national database). Patient questionnaires were sent out to those identified as having a bleeding event, to assess exposure status and comorbidities. Patients were interviewed over the phone if answers were incomplete or unclear. The response rates were significantly higher in the cases (approximately 70 percent) compared with controls (approximately 31 percent). The study found that nonselective NSAIDs were associated with an increased risk of bleeding compared with partially selective NSAIDs after adjustment for duration of use and INR level (OR 3.1, 95% CI 1.2 to 8.0).
Aspirin and Anticoagulation
In the original CER, we found no studies evaluating risks and benefits of concomitant anticoagulants and aspirin in patients with arthritis. No new studies were identified for this update. Combination therapy has been studied in patients with indications for thromboembolic prophylaxis. However, the results of those studies are not directly applicable to patients with arthritis because of important differences in the populations (particularly with regard to CV risk), and because aspirin was used in lower, prophylactic doses (rather than anti-inflammatory and analgesic doses). One fair-quality meta-analysis (did not evaluate quality of included trials) found major bleeding risk increased with warfarin plus aspirin versus warfarin alone (at the same intensity) in patients with mechanical heart valves (three trials, RR 1.58, 95% CI 1.02 to 2.44).245 In patients with recent myocardial infarction or atrial fibrillation (one trial each), the increase in risk was not statistically significant (RR 3.07, 95% CI 0.33 to 28.38 and RR 2.13, 95% CI 0.20 to 23.03, respectively). In patients with mechanical heart valves, the increase in bleeding risk was offset by a reduction in thromboembolic events (RR 0.33, 95% CI 0.19 to 0.58), and there was no difference in all-cause mortality (RR 0.78, 95% CI 0.29 to 1.83). Other evidence on the risks and benefits of combination therapy has focused on comparing warfarin plus aspirin to aspirin alone. A good-quality meta-analysis of 10 trials found the combination of warfarin plus aspirin increased the risk of major bleeding compared with aspirin alone following myocardial infarction or the acute coronary syndrome (RR 2.5, 95% CI 1.7 to 3.7).246 However, the increase in bleeding risk was offset by lower risks for myocardial infarction, ischemic stroke, and revascularization. Mortality did not differ.
Other Analgesics
No study evaluated risk of bleeding in anticoagulated patients on acetaminophen compared with those on NSAIDs. A small, randomized controlled trial found acetaminophen associated with greater increases in INR levels compared with placebo.247 Several observational studies have also found an association between excess anticoagulation and use of acetaminophen.248, 249 However, changes in INR are not the only important factor for predicting increased risk of bleeding. NSAIDs, for example, also affect platelet function and disrupt the gastric mucosal lining. Studies evaluating actual bleeding complications are necessary to better assess the comparative risks from acetaminophen and other NSAIDs.
No studies evaluated risk of bleeding in anticoagulated patients on glucosamine, chondroitin, or topical agents.
Concomitant Aspirin: Gastrointestinal Harms
Celecoxib Plus Aspirin Compared With Nonselective NSAID Plus Aspirin
Beneficial effects of COX-2 selective inhibition on GI complication rates could be attenuated or eliminated by the concomitant use of aspirin. The original CER included two large trials (CLASS54 and SUCCESS-155) and a systematic review51 that reported rates of ulcer complications associated with celecoxib and nonselective in subgroups of patients also using aspirin. Two new observational studies also compared risks of serious GI harms with celecoxib and nonselective NSAIDs in aspirin users.250, 251
In the 20 percent of patients in CLASS who took aspirin in addition to their study drug, there was no difference in rates of ulcer complications (2.0% vs. 2.1%, p=0.92) or ulcer complications plus symptomatic ulcers (4.7% vs. 6.0%, p=0.49) in patients randomized to celecoxib versus those randomized to diclofenac or ibuprofen.54, 252 There were also no differences when celecoxib was compared to diclofenac and ibuprofen separately. In SUCCESS-1, among the 7 percent of the study population on aspirin, only four ulcer complications occurred, resulting in imprecise estimates (OR 2.0, 95% CI 0.14 to 27).55 The systematic review found that use of aspirin increased the rate of endoscopic ulcers by about 6 percent in patients randomized to celecoxib (4.2% without aspirin and 9.9% with aspirin) or those randomized to a nonselective NSAID (18% and 24%, respectively).51 Celecoxib (any dose) was associated with a lower risk of endoscopic ulcers in aspirin users (RR 0.48, 95% CI 0.28 to 0.83), but ulcer complications were not reported in this subgroup.
The two new, fair-quality retrospective cohort studies were conducted by the same authors and evaluated the same Quebec health services administrative databases (Appendix H).250, 251 One study found use of celecoxib plus aspirin associated with a lower risk of hospitalizations due to ulcer complications compared with use of a nonselective NSAID plus aspirin (HR 0.62, 95% CI 0.48 to 0.80) among patients 65 years or older.250 The second study found that in patients 50 years and older, users of celecoxib plus aspirin had a lower risk of hospitalization for GI complications (HR 1.8, 95% CI 1.5 to 2.1) than users of diclofenac plus aspirin (HR 2.8, 95% CI 2.2 to 2.8, 95% CI 2.2 to 3.5), though estimates for ibuprofen plus aspirin (HR 1.4, 95% CI 0.8 to 2.7), naproxen plus aspirin (HR 2.2, 95% CI 1.6 to 3.0), and piroxicam plus aspirin (HR 2.0, 95% CI 0.8 to 5.4) were similar (each compared with users of acetaminophen without aspirin).251
Impact of Concomitant PPI use on GI Risk With a Celecoxib or Nonselective NSAID
Subgroup analyses from four randomized controlled trials (reported in three publications) provided evidence on the effects of adding a PPI to celecoxib or nonselective NSAIDs in aspirin users.235, 253, 254 Only one fair-quality trial reported ulcer complications. 235 In patients (n=273) at very high risk for rebleeding (recently healed GI bleed) enrolled in this study, low-dose aspirin was started during the trial period in 43 patients. The rate of recurrent bleeding in this subgroup was 0 percent with celecoxib 200 mg twice daily plus esomeprazole 20 mg twice daily group compared with 19 percent in the group taking celecoxib alone (p=0.03).235
In two similarly designed, fair-quality trials (reported in one publication, total n=861) the pooled rate of endoscopically proven gastric ulcer in the subgroup also taking low-dose aspirin (n= 201) was significantly lower with naproxen plus a PPI (3 percent) compared with enteric coated naproxen alone (28%; p<0.001).253 A large, fair-quality trial of celecoxib versus naproxen plus lansoprazole in low-dose aspirin users (n = 1,045) found no difference in risk of endoscopically proven gastric or duodenal ulcers (9.9% vs. 8.9%, p=0.65).254 Post-hoc analyses showed no effect based on aspirin dose (81 mg or 325 mg daily).
Concomitant Aspirin: Cardiovascular Harms
Celecoxib
The original CER included a systematic review, a randomized trial not included in the systematic review, and two large observational studies on effects of aspirin on CV harms associated with celecoxib use. We identified no new studies.
A systematic review of 84 placebo-controlled trials of celecoxib that permitted aspirin use found a very similar risk of vascular events among aspirin users (RR 1.6, 95% CI 0.90 to 2.7) and aspirin nonusers (RR 1.5, 95% CI 1.1 to 2.0), though the absolute rate of events was higher in aspirin users (1.9 percent/year vs. 1.1 percent/year), perhaps due to higher baseline risk.121 In a large celecoxib polyp prevention trial not included in the systematic review, use or nonuse of low-dose aspirin did not affect the observed increased risk of thrombotic events.255 Consistent with these findings, two large observational studies found no significant interaction between concurrent NSAID and aspirin use and risk of myocardial infarction.134, 136
Nonselective NSAIDs
It has been suggested that some nonselective NSAIDs may reduce or eliminate the CV benefits associated with low-dose aspirin.36 In particular, ibuprofen is thought to be associated with unique pharmacokinetic and pharmacologic properties that could interfere with aspirin. Six observational studies108, 133, 256–259 and one subgroup analysis from a randomized trial260 evaluated effects of concomitant NSAIDs on CV risk in aspirin users (Table 17, Appendix H). The studies used heterogeneous designs, outcome measures, and methods of analysis, making it difficult to reach firm conclusions about comparative risks.
Three observational studies found no increase in risk of mortality257 or myocardial infarction133, 256 in users of a nonselective NSAID plus aspirin versus aspirin alone (Table 17). A subgroup analysis from a randomized trial also found no increased risk with short-term (<60 days) use of a nonselective NSAID plus aspirin compared to aspirin alone.260 The estimate for longer term use suggested increased risk, but was imprecise and not statistically significant.
For the effect of adding ibuprofen to aspirin, one fair-quality study of patients recently discharged from the hospital for a CV disease diagnosis found an increased risk of overall and CV mortality with the combination of ibuprofen and aspirin compared to aspirin alone (Table 17).258 The study did not report baseline characteristics of patients, although the analysis did control for potential confounders.
Two other observational studies evaluated risk of acute MI with ibuprofen plus aspirin versus aspirin alone.256, 259 A fair-quality case-control study found the risk of first nonfatal MI was elevated in those using ibuprofen plus aspirin compared with those using only aspirin, while a retrospective cohort study (also fair quality) found that adding ibuprofen to aspirin resulted in decreased risk of myocardial infarction (Table 17).256, 259 These studies used different methods, which could account for their discrepant findings. The case-control study identified controls from the community, used telephone interviews to collect exposure and covariate data, and considered the patient to be exposed to ibuprofen or aspirin if they reported using the drug(s) in the week prior to the event.256 Recall bias is a major concern with this study, and differences between groups suggest potentially important differences in baseline risk. The cohort study used Veterans Affairs prescription and medical records to identify regular users of ibuprofen and aspirin or aspirin alone with matching based on age, race, sex, and cholesterol levels.259 The study did not measure potential confounders or conduct adjusted analyses, and very limited information was provided about the patients’ comparability at baseline.
Two other observational studies found no statistically significant differences in risk between ibuprofen plus aspirin versus ibuprofen alone108 or use of ibuprofen plus aspirin versus nonuse of NSAIDs (including aspirin),133 but were not designed to assess risk associated with addition of ibuprofen to aspirin.
Combined Cardiovascular and Gastrointestinal Harms
A large retrospective cohort study of patients 65 years and older evaluated risk of hospitalizations due to upper GI bleeding or acute myocardial infarction associated with various NSAIDs and acetaminophen in low-dose aspirin users (n=112,141), compared to use of acetaminophen alone.108 The adjusted odds of GI bleeding or acute myocardial infarction relative to acetaminophen alone were similar for celecoxib, ibuprofen, diclofenac, naproxen, and acetaminophen (RR range 1.3 to 1.7), with overlapping confidence intervals.
Key Question 3. What are the Comparative Effects of Coprescribing of H2-Antagonists, Misoprostol, or Proton Pump Inhibitors (PPIs) on the Gastrointestinal Harms Associated With NSAID use?
Summary of Evidence
- Misoprostol was the only gastroprotective agent to reduce risk of serious ulcer complications (perforation, obstruction, or bleeding) compared with placebo in patients with average risk of GI bleeding prescribed nonselective NSAIDs, but was also associated with a high rate of withdrawals due to adverse GI symptoms.
- Celecoxib alone was associated with fewer decreases in hemoglobin (> 2 g/dl) without overt GI bleeding compared with diclofenac plus a proton pump inhibitor (PPI).
- Coprescribing of PPIs, misoprostol, and H2-antagonists all reduced the risk of endoscopically detected gastric and duodenal ulcers compared to placebo in patients prescribed a nonselective NSAID.
- In direct comparisons, coprescribing of PPIs in patients prescribed a nonselective NSAID was associated with a lower risk of endoscopically detected duodenal ulcers compared to misoprostol or H2-antagonists, a lower risk of endoscopically detected gastric ulcers compared to H2-antagonsits, and a similar risk of endoscopically detected gastric ulcers compared to misoprostol.
- In direct comparisons, coprescribing of misoprostol was associated with a lower risk of endoscopically detected gastric ulcers compared to ranitidine, and a similar reduction in risk of endoscopically detected duodenal ulcers.
- Compared to placebo, double (full) dose H2-antagonists may be more effective than standard dose for reducing endoscopically detected gastric and duodenal ulcers.
- Celecoxib plus a PPI may reduce the risk of endoscopic ulcers and ulcer complications compared to celecoxib alone in average risk persons (see Key Question 2 for high-risk people).
Detailed Analysis
Nonselective NSAIDs Plus Misoprostol, H2 Antagonists, or PPIs Versus NSAIDs Alone
Two good-quality systematic reviews259,260 and one fair-quality systematic reviews261 included in the original CER evaluated effects of coprescribing gastroprotective agents (H2-antagonists, misoprostol, and PPIs) with NSAIDs versus placebo or against one another on GI harms. We identified four new fair-quality trials (reported in two publications) of an NSAID plus a PPI versus an NSAID alone.253, 261
The three systematic reviews (published in 2002 and 2004)262–264 included numerous randomized controlled trials8, 264–293 of patients with osteoarthritis or rheumatoid arthritis prescribed NSAIDs. They found misoprostol, standard-and double-dose H2 blockers and PPIs all effective in reducing risk of endoscopic gastric and duodenal ulcers relative to placebo in patients prescribed nonselective NSAIDs (Table 18).262–264 Misoprostol (RR 0.36, 95% CI 0.20 to 0.67) and PPIs (RR 0.09, 95% CI 0.02 to 0.47) also reduced NSAID-associated symptomatic ulcers, and serious GI complications. None of the coprescribed drugs affected mortality.262 The reduction in serious complications with misoprostol was in large part due to one large, good-quality trial (MUCOSA).290 In this study, misoprostol was associated with a rate of definite ulcer complications of 25/4,404 (0.6 percent) compared with 44/4,439 (0.9 percent) with placebo (p=0.05).290 However, misoprostol was also the only gastroprotective agent associated with an increased risk of withdrawal due to nausea (RR 1.3, 95% CI 1.1 to 1.6), diarrhea (RR 2.4, 95% CI 2.0 to 2.8), and abdominal pain (RR 1.4, 95% CI 1.2 to 1.6).
The new trials were consistent with the systematic reviews (Appendix H).253, 261 Two similarly designed, fair-quality trials (total n=861) reported in one publication found naproxen 500 mg plus esomeprazole 20 mg combination tablet associated with better scores on patient-reported ratings of gastric symptoms and fewer endoscopic ulcers compared to enteric coated naproxen alone (RR 0.24, 95% CI 0.15 to 0.39 for endoscopic gastric ulcers and RR 0.14, 95% CI 0.04 to 0.46 for endoscopic duodenal ulcer).253 The studies reported no serious GI complications in either group over 6 months. Two other similarly designed, fair-quality trials evaluated esomeprazole 20 mg or 40 mg daily added to an NSAID compared to the NSAID alone in 1,378 patients.261 In one trial, about two-thirds of patients were on a nonselective NSAID, and in the other, about 85 percent. In a pooled analysis stratified by type of NSAID, esomeprazole was associated with decreased risk of endoscopic ulcers in patients on nonselective NSAIDs (6.8% with esomeprazole 20 mg, 4.8% with esomeprazole 40 mg, 17% with placebo, RR 0.29, 95% CI 0.17 to 0.49 for esomeprazole 40 mg versus placebo, RR 0.41, 95% CI 0.26 to 0.64 for esomeprazole 20 mg vs. placebo) after 6 months. Both trials reported more patients in the esomeprazole 20 mg daily group had no sleep disturbance, acid regurgitation, or heartburn after one month of treatment compared with placebo, but results were not stratified by NSAID type. There was no difference in the proportion without nausea or upper abdominal bloating, and the 40 mg daily dose was significantly better than placebo only in the proportion of patients without heartburn in both studies, and acid regurgitation in one study. Rates of withdrawal due to adverse events and overall adverse event were similar across groups. Two patients in the nonselective NSAID alone group (0.4 percent) had GI bleeds during the study, compared with none in the nonselective NSAID plus esomeprazole groups.
A systematic review included in the original CER included five trials279, 281, 286, 291, 293 that directly compared one gastroprotective agent with another when coprescribed with a nonselective NSAID.263 The systematic review found both misoprostol and omeprazole superior to ranitidine for prevention of endoscopic gastric ulcers, and omeprazole and lansoprazole superior to misoprostol and ranitidine for prevention of duodenal ulcers. Other outcomes were not reported. We identified no new head-to-head trials (Table 19).
COX-2 Inhibitors Alone Compared With Nonselective NSAIDs Plus a PPI
The original CER included a good-quality systematic review of 26 trials that compared coprescribing of a PPI with a nonselective NSAID versus a COX-2 selective NSAID on GI harms.294 We identified two new trials254, 295 and two new observational studies.296, 297
The systematic review found coadministration of a PPI with a nonselective NSAID associated with a lower risk of dyspepsia, epigastric pain, and nausea compared with a selective COX-2 inhibitor alone, when each was compared to a nonselective NSAID alone (relative risk reduction 66 percent and absolute risk reduction 9 percent for the PPI + nonselective NSAID vs. RRR 12 percent and ARR 3.7 percent with COX-2 inhibitor).294 The systematic review did not assess endoscopic ulcers, symptomatic ulcers, or ulcer complications.
One large (n=4,484), new, good-quality trial was designed to assess ulcer complications.295 It found diclofenac slow release 75 mg twice a day plus omeprazole 20 mg once a day associated with a higher risk of clinically significant upper and lower GI events (bleeding, obstruction or perforation in the upper and lower GI tract, decrease in hemoglobin ≥ 2 g/dL and/or hematocrit ≥ 10%) compared with celecoxib 200 mg twice daily after 6 months in patients with osteoarthritis or rheumatoid arthritis (3.8% vs. 0.9%, HR 4.3, 95% CI 2.6 to 7.0). An independent, blinded expert panel adjudicated adverse events and categorized anemia as of GI origin or presumed occult GI origin. Most of the GI events were decreases in hemoglobin or hematocrit without overt bleeding. Five patients in the celecoxib group and four in the diclofenac plus omeprazole group experienced GI hemorrhage; no cases of perforation or obstruction were reported in either group. Of those allocated to celecoxib, 114 (6 percent) patients withdrew early because of GI adverse events versus 167 (8 percent) allocated diclofenac SR plus omeprazole (p=0.0006). Another new, fair-quality trial (n=1,045) found no difference in risk of endoscopic gastric or duodenal ulcers in patients with osteoarthritis using low-dose aspirin after 12 weeks between celecoxib alone compared with naproxen plus lansoprazole.254 Only one GI complication (0.1 percent) was reported.
Two new, large observational studies found the risk of GI complications similar with a nonselective NSAID plus a PPI compared with celecoxib alone.296, 297 A fair-quality retrospective cohort study found similar reduction in risk of a hospitalization due to a GI adverse event (peptic ulcer, gastritis with hemorrhage, or any GI hemorrhage) for a COX-2 selective NSAID alone (RR 0.60, 95% CI 0.46 to 0.77) and a nonselective NSAID plus PPI (RR 0.46, 95% CI 0.28 to 0.73), when each was compared to a nonselective NSAID alone.297 A fair-quality retrospective cohort study found a similar risk of hospitalization due to perforated or bleeding ulcer in older patients using an NSAID plus a PPI versus celecoxib alone (HR 0.98, 95% CI 0.67 to 1.4).296
COX-2 Inhibitors Alone Compared With COX-2 Inhibitors Plus a PPI
The original CER found no studies on GI harms associated with use of a COX-2 selective NSAID plus a PPI versus a COX-2 selective NSAID alone. Two new, similarly designed fair-quality trials (reported in one publication) reported GI harms associated with an NSAID plus esomeprazole versus an NSAID alone.261 Although most patients in this trial used nonselective NSAIDs, some results were stratified according to the type of NSAID (nonselective or celecoxib, see section on nonselective NSAIDs plus a PPI versus a nonselective NSAID alone for details). Another new, fair-quality trial evaluated patients at very high risk due to recent GI bleeding and is discussed in Key Question 2.235 The two new observational studies that evaluated GI harms with a nonselective NSAID plus a PPI versus celecoxib alone also evaluated risks associated with celecoxib plus a PPI versus celecoxib alone.296, 297
A pooled analysis of 400 patients from two fair-quality trials found celecoxib plus esomeprazole associated with fewer endoscopic ulcers compared to celecoxib plus placebo (0.9 percent for esomeprazole 20 mg once daily [p<0.001 vs. placebo], 4.1 percent for esomeprazole 40 mg once daily [p=0.002 vs. placebo], 16 percent for placebo). 261 Two upper GI bleeds were reported, both in the placebo groups. Other GI harms were not reported in the subgroup of patients on celecoxib.
The two large observational studies also found some benefit from adding a PPI to celecoxib.296, 297 A fair-quality retrospective cohort study found celecoxib plus a PPI associated with a lower risk of hospitalizations related to perforated or bleeding ulcer of the stomach compared to celecoxib alone (HR 0.69, 95% CI 0.52 to 0.93) among older (age greater than 65 years) adults.296 In stratified analyses, the benefit was observed in patients 75 years and older, with no benefit in those 66 to 74 years old. A good-quality retrospective cohort study found celecoxib plus a PPI (RR 0.18, 95% CI 0.09 to 0.37) associated with a lower risk of hospitalization due to GI bleeding compared to celecoxib alone (RR 0.34, 95% CI 0.24 to 0.49) when each was compared to naproxen alone, though confidence intervals overlapped.297
Key Question 4. What are the Comparative Benefits and Harms of Treating Osteoarthritis With Oral Medications as Compared With Topical Preparations?
Summary of Evidence
- The only FDA-approved topical NSAIDs are formulations with diclofenac.
- Three head-to-head trials found topical diclofenac similar to oral NSAIDs for efficacy in patients with localized osteoarthritis.
- Topical NSAIDs were associated with a lower risk of GI adverse events and higher risk of dermatologic adverse events compared to oral NSAIDs.
- There was insufficient evidence to evaluate comparative risks of GI bleeding or CV events.
- No head-to-head trials compared topical salicylates or capsaicin to oral NSAIDs for osteoarthritis.
- Topical salicylates were no better than placebo in two trials of patients with osteoarthritis, and associated with increased risk of local adverse events.
- Topical capsaicin was superior to placebo for pain relief (NNT 8.1) in a systematic review of trials and subsequent randomized trial, but associated with increased local adverse events and withdrawals due to adverse events (13% vs. 3%, RR 4.0, 95% CI 2.3 to 6.8).
Detailed Analysis
Topical Compared With Oral NSAIDs: Benefits
Eight trials directly compared topical and oral NSAIDs for osteoarthritis (Table 20 and Appendix J).49, 298–304 Four trials published since the original CER evaluated topical diclofenac,301 topical ketoprofen,299 or topical ibuprofen 302 versus an oral NSAID (one trial each), or advice to use topical ibuprofen versus advice to use oral ibuprofen (one trial).49 The original CER included four other trials of topical diclofenac (two trials), topical eltenac (one trial), and topical piroxicam (one trial), each versus an oral NSAID.298, 300, 303, 304 Of the eight trials, we rated three good quality299, 301, 303 and four fair quality.49, 298, 300, 302 We could not rate the eighth trial304 because it was not published in English, though a systematic review305 gave it the maximum 5 points on the Jadad scale. The original CER included two systematic reviews305, 306 that would now be considered outdated since they included only three of the eight currently available trials. We identified no new systematic reviews that met inclusion criteria.
The only topical NSAIDs approved by the FDA as of late 2010 are diclofenac-based formulations. Three trials of topical versus oral diclofenac found no differences in efficacy for localized osteoarthritis.301, 303, 304 Two good-quality trials (n=622303 and n=305301) the latter new for this update) found no clinically or statistically significant differences at 12 weeks in WOMAC Pain or Stiffness scores or patient global assessment scores (Table 20 and Appendix J). Both trials evaluated topical diclofenac in a DMSO-based carrier. In one of the trials, topical diclofenac was slightly inferior to oral diclofenac on the WOMAC Physical Function score, but the difference was not clinically significant (difference of 90 mm on a 1,700 mm scale, with 255 mm thought to be clinically significant).303 This trial also reported a similar proportion of responders (as defined by the Outcomes Measures in Arthritis Clinical Trials and the Osteoarthritis Research Society VI recommendations) with topical or oral diclofenac (66 percent vs. 70 percent, p=0.37). A third, non-English language trial (n=321) found no difference between diclofenac 1 percent gel versus oral ibuprofen at 3 weeks in the proportion of patients with hand osteoarthritis with a ≥40 percent improvement in pain on a 100 mm VAS (40% vs. 34%, RR 1.20, 95% CI 0.88 to 1.60; data as reported in a systematic review).304,305
The other trials evaluated topical NSAIDs not approved by the FDA. None found any differences between a topical and oral NSAID in efficacy. One new, fair-quality trial (n=282) found no differences in WOMAC Pain, Stiffness, Physical Function, or Global scores through 12 months between advice to use topical or oral ibuprofen in patients with knee osteoarthritis (Table 20).49 A new, small (n=20) trial of topical versus oral ibuprofen found no differences in WOMAC or SF-36 scores.302 Another new, fair-quality (n=270) trial of topical ketoprofen 110 mg in 4.8 g transferone carrier versus oral celecoxib found no difference in WOMAC Pain or Stiffness scores; patient global assessment scores; or the proportion of OMERACT-OARSI responders (69 percent vs. 64 percent).299 One fair-quality trial included in the original CER found no difference between piroxicam 0.5% and oral ibuprofen in the proportion of patients reporting a “good” or “excellent” response.298
A fair-quality trial of eltenac 1 percent gel was included in the original CER but is of limited relevance since it is no longer being investigated for use in humans.300
Topical Compared With Oral NSAIDs: Harms
Eight head-to-head trials reported adverse events associated with topical versus oral NSAIDs. Three trials evaluated topical versus oral diclofenac.301, 303, 304 In two good-quality trials (one published since the original CER301), rates of withdrawal due to adverse events were similar (21 percent vs. 25 percent303 and 10 percent vs. 13 percent,301 respectively). Topical diclofenac was associated with fewer GI, systemic, and laboratory adverse events but more dermatologic adverse events compared to oral diclofenac (Table 21, Appendix J). The risk of GI events with topical and oral NSAIDS was 35 percent versus 48 percent303 and 6.5 percent versus 24 percent,301 respectively. One trial that categorized adverse event severity also found topical diclofenac associated with a lower risk of serious GI events (7.4 percent vs. 21 percent).303 A similar pattern was observed for specific GI adverse events (dyspepsia, nausea, diarrhea, abdominal pain, abnormal liver function tests). Topical NSAIDs were also associated with smaller increases in serum creatinine and smaller decreases in hemoglobin compared with oral NSAIDs. Topical NSAIDs were associated with an increased risk of dry skin, rash, and pruritus. A third, non-English language trial304 found topical diclofenac associated with a lower risk of withdrawal due to adverse events compared to oral ibuprofen (data as reported in Mason et al.305).
Other trials evaluated topical NSAIDs not approved by the FDA. A new, good-quality trial found topical ketoprofen associated with similar withdrawal due to adverse events compared to oral celecoxib, fewer GI adverse events, and more skin adverse events.299 A new, fair-quality trial on advice to use topical ibuprofen versus advice to use oral ibuprofen found few differences in GI adverse events, perhaps because the dosing regimen was not fixed and may have resulted in less consistent or lower doses.49 The exception was for respiratory events, which favored topical NSAIDs, due to a greater risk of a decrease in peak expiratory flow in the oral NSAIDs group. A third, fair-quality trial was too small to draw reliable conclusions about comparative harms.302
A fair-quality trial included in the previous systematic review found no clear differences in adverse events between topical piroxicam and oral ibuprofen.298 Another trial evaluated topical eltenac, a drug no longer being investigated for use in humans.300
No RCT was adequately designed to assess risks for serious but uncommon adverse events such as myocardial infarction, renal failure, or GI bleeding. In one new trial, only one serious adverse event (postpolypectomy lower GI bleed) was observed with either topical or oral diclofenac.301
Two case-control studies included in the original CER evaluated the risk of GI bleeding with topical and oral NSAIDs. A nested case-control study of the General Practice Research Database found topical NSAID use was not associated with symptomatic peptic ulcer (RR=1.0 vs. nonuse, 95% CI 0.6 to 1.7), though oral NSAID use was associated with increased risk (RR=4.0, 95% CI 3.2 to 5.1).184 Similarly, a study (1,103 cases) found no association between exposure to topical NSAIDs within 45 days and risk of hospital admission for upper GI bleeding and perforation after adjusting for the confounding effects of exposure to oral NSAIDs and ulcer healing drugs (OR 1.45, 95% CI 0.84 to 2.50 with community controls and OR 1.06, 95% CI 0.60 to 1.88 with hospital controls).307 By contrast, oral NSAIDs were associated with increased risk (OR 2.59, 95% CI 2.12 to 3.16 for community controls and 2.00, 95% CI 1.60 to 2.50 for hospital controls).
One case-control study of similar design included in the original CER found exposure to topical NSAIDs not associated with acute renal failure (adjusted OR 1.33, 95% CI 0.79 to 2.24 using community controls and 1.04, 95% CI 0.60 to 1.83 using hospital controls).308 Recent exposure to oral NSAIDs was associated with increased risk of renal failure using either community (adjusted OR 2.20, 95% CI 1.49 to 3.25) or hospital (adjusted OR 1.84, 95% CI 1.15 to 2.93) controls.
We identified no studies comparing the risk of CV events in persons on topical versus oral NSAIDs.
Topical Salicylates and Capsaicin
We identified no trials comparing topical salicylates to oral or topical NSAIDs for osteoarthritis. We also identified no new trials comparing topical salicylates to placebo. A systematic review30 included in the original CER has been updated.309 It included only two trials of topical salicylates versus placebo for osteoarthritis.310, 311 Both trials found topical salicylates associated with no greater likelihood of clinical success (50 percent reduction in chronic pain) compared with placebo (RR 1.2, 95% CI 0.59 to 2.7310 and RR 1.0, 95% CI 0.63 to 1.6).311 The systematic review also found topical salicylates associated with increased risk of local adverse events compared to placebo, when used for any acute or chronic pain condition (RR 2.2, 95% CI 1.1 to 4.1).
We identified no trials comparing topical capsaicin to oral or topical NSAIDs for osteoarthritis. We also identified one new trial comparing topical capsaicin to placebo.312 A systematic review included in the original CER found that for chronic musculoskeletal pain, capsaicin was superior to placebo for achieving clinical success (defined as approximately a 50 percent reduction in pain), with a relative benefit of 1.5 (three trials, 95% CI 1.1 to 2.0) and number needed to treat of 8.1 (4.6 to 34).313 About 54 percent of patients had local adverse events with capsaicin, compared with 15 percent with placebo (relative risk 3.6, 95% CI 2.6 to 5.0). Withdrawals due to adverse events were also significantly more likely with capsaicin (13% vs. 3%, relative risk 4.0, 95% CI 2.3 to 6.8). A new, fair-quality crossover trial (n=100) not included in the systematic review found capsaicin 0.125 percent gel associated with greater changes from baseline compared to placebo in VAS pain score (0 to 100 scale, mean difference 0.72, 95% CI 0.17 to 1.27), WOMAC pain (0 to 20 scale, mean difference 3.4, 95% CI 2.3 to 4.5), WOMAC stiffness (0 to 8 scale, mean difference 0.82, 955 CI 0.19 to 1.5), and WOMAC function (0 to 68 scale, mean difference 9.0, 95% CI 5.5 to 12).312
Tables
Table 13Incidence of hypertension in the Nurses’ Health Study and Physicians’ Health Study according to use of acetaminophen or NSAIDs
| Study | Acetaminophen use vs. Nonuse: Odds Ratio (95% CI) | NSAID use vs. Nonuse: Odds Ratio (95% CI) |
|---|---|---|
| Nurses’ Health Study I (women 51 to 77 years old)195 | 1.9 (1.3 to 2.9) | 1.8 (1.2 to 2.6) |
| Nurses’ Health Study II (women 34 to 53 years old)195 | 2.0 (1.4 to 2.8) | 1.6 (1.1 to 2.3) |
| Physicians’ Health Study196 | 1.1 (0.87 to 1.3) | 1.0 (0.89 to 1.2) |
CI = confidence interval; NSAID = nonsteroidal anti-inflammatory drug
Table 3Risk of upper gastrointestinal bleeding or perforation with use of an NSAID compared with nonuse of NSAIDs, systematic review of observational studies97
| NSAID | Number of Studies | Pooled Estimate (95% CI) |
|---|---|---|
| Celecoxib | 4 | 1.4 (0.85 to 2.4) |
| Meloxicam | 4 | 4.2 (2.6 to 6.6) |
| Naproxen | 6 | 5.6 (3.8 to 8.3) |
| Ibuprofen | 5 | 2.7 (2.2 to 3.3) |
| Diclofenac | 6 | 4.0 (3.4 to 4.7) |
| Indomethacin | 5 | 5.4 (4.2 to 7.0) |
| Ketoprofen | 5 | 5.6 (3.9 to 7.9) |
| Piroxicam | 5 | 9.9 (6.0 to 16) |
| Ketorolac | 2 | 15 (5.9 to 36) |
CI = confidence interval; NSAID = nonsteroidal anti-inflammatory drug
Table 4Serious gastrointestinal events in observational studies
| Author, Year Study Design Sample Size | Mean age (yrs) Country | Outcome | Main Findings |
|---|---|---|---|
| Garcia Rodriguez, 2007102 Nested case-control Cases: 1561 | NR UK (The Health Improvement Network database) | Upper GI perforation or bleeding | NSAID use vs. nonuse of NSAIDs (CI’s not reported and difficult to estimate from graph) Celecoxib: RR 2.7 Ibuprofen: RR 2.0 Meloxicam: RR 2.7 Diclofenac: RR 3.7 Ketoprofen: RR 5.4 Indomethacin: RR 7.2 Naproxen: RR 8.1 |
| Garcia Rodriguez, 200198 Nested case-control Cases: 2105 | NR UK (GPRD) | Upper GI perforation or bleeding | NSAID use vs. nonuse of NSAIDs Ibuprofen: RR 2.5 (95% CI 1.9 to 3.4) Etodolac: RR 2.2 (95% CI 0.4 to 11) Fenbufen: RR 1.1 (95% CI 0.2 to 5.1) Mefenamic acid: RR 2.7 (95% CI 0.8 to 9.4) Ketoprofen: RR 3.3 (95% CI 1.9 to 5.9) Nabumetone: RR 3.4 (95% CI 1.1 to 11) Tenoxicam: RR 3.4 (95% CI 0.9 to 13) Meloxicam: RR 3.8 (95% CI 0.8 to 17) Naproxen: RR 4.0 (95% CI 2.8 to 5.8) Diclofenac: RR 4.6 (95% CI 3.6 to 5.8) Flurbiprofen: RR 4.6 (95% CI 2.0 to 11) Indomethacin: RR 5.2 (95% CI 3.2 to 8.3) Piroxicam: RR 6.2 (95% CI 3.7 to 10) |
| Hippisley-Cox 2005103 Nested case-control Cases: 9407 | NR; ≥ 25 UK | Complicated GI Event | NSAID use within 90 days vs. no prescription for 3 years Celecoxib: OR 1.2 (95% CI 0.91 to 1.7) Ibuprofen: OR 1.6 (95% CI 1.4 to 1.8) Diclofenac: OR 2.1 (95% CI 1.8 to 2.4) Naproxen: OR 2.0 (95% CI 1.5 to 2.6) Aspirin: OR 1.8 (95% CI 1.6 to 1.9) |
| Lanas, 2006104 Case-control Cases: 2777 | NR Spain | Hospitalization for upper G I bleeding | Celecoxib use vs. nonuse of selective NSAID: RR 1.0 (95% CI 0.4 to 2.1) NSAID use vs. nonuse of nonselective NSAID Ibuprofen: RR 4.1 (95% CI 3.1 to 5.3) Diclofenac: RR 3.1 (95% CI 2.3 to 4.2) Aceclofenac: RR 2.6 (95% CI 1.5 to 4.6) Naproxen: RR 7.3 (95%CI 4.7 to 11) Piroxicam: RR 13 (95% CI 7.8 to −20) Indomethacin: RR 9.0 (95% CI 3.9 to 21) Meloxicam: RR 9.8 (95% CI 4.0 to 24) Ketorolac: RR 14 (95% CI 5.2 to 50) Lornoxicam: RR 7.7 (95% CI 2.4 to 24) Ketoprofen: RR 8.6 (95% CI 2.5 to 29) |
| Laporte 2004105 Case-control Cases=2,813 | NR; ≥ 18 Spain and Italy | Upper GI bleeding | NSAID use vs. nonuse of NSAIDs Aspirin: OR 8.0 (95% CI 6.7 to 9.6) Dexketoprofen: OR 4.9 (95% CI 1.7 to 14) Diclofenac: OR 3.7 (95% CI 2.6 to 5.4) Ibuprofen: OR 3.1 (95% CI 2.0 to 4.9) Indomethacin: OR 10 (95% CI 4.4 to 23) Ketoprofen: OR 10 (95% CI 3.9 to 26) Ketorolac: OR 25 (95% CI 8.0 to 77) Meloxicam: OR 5.7 (95% CI 2.2 to 15) Naproxen: OR 10 (95% CI 5.7 to 18) Nimesulide: OR 3.2 (95% CI 1.9 to 5.6) Piroxicam: OR 16 (95% CI 10 to 24) |
| Mamdani 2002106 Cohort n=143,969 | 75.7 Canada | Upper GI hemorrhage | NSAID use vs. no use of NSAIDs Celecoxib: HR 1.0 (95% CI 0.7 to 1.6) Diclofenac + misoprostol: HR 3.0 (95% CI 1.7 to 5.5) Nonselective NSAIDs: HR 4.0 (95% CI 2.3 to 6.9) NSAID use vs. celecoxib Diclofenac + misoprostol: HR 3.2 (95% CI 1.6 to 6.5) Nonselective NSAIDs: HR 4.4 (95% CI 2.3 to 8.5) |
| Mellemkjaer, 2002107 Cohort n=156,138 NSAID users | NR Denmark | Hospitalization for GI bleeding | NSAID use vs. no use of NSAIDs Diclofenac: RR 4.9 (95% CI 3.5 to 6.6) Ibuprofen: RR 2.4 (95% CI 2.0 to 2.9) Indomethacin: RR 4.3 (95% CI 2.9 to 6.0) Ketoprofen: RR 6.3 (95% CI 4.5 to 8.5) Naproxen: RR 3.0 (95% CI 2.1 to 4.2) Piroxicam: RR 5.0 (95% CI 3.3 to 7.2) |
| Rahme, 2007108 Retrospective cohort N=510,871 | NR; ≥65 Canada | Hospitalization for GI bleeding | NSAID use vs. acetaminophen use Celecoxib: HR 0.82 (95% CI 0.66 to 1.0) Ibuprofen: HR 1.1 (95% CI 0.56 to 2.2) Diclofenac: HR 1.2 (95% CI 0.86 to 1.6) Naproxen: HR 2.8 (95% CI 2.0 to 3.7) |
CI = confidence interval; GI = gastrointestinal; HR = hazard ratio; NR = not reported; NSAID = nonsteroidal anti-inflammatory drug; OR = odds ratio; RR = relative risk; UK GPRD = United Kingdom General Practice Research Database
Table 5Meta-analyses of serious cardiovascular events in trials of celecoxib
| Study, Year Time Period Covered | Number of Studies (Number Randomized to Celecoxib) | Includes Trials of Colorectal Cancer or Alzheimer’s Prevention* | Risk of Cardiovascular Events | Quality |
|---|---|---|---|---|
| White, 2003112 Search dates not reported | 15 (18,942) | No | Antiplatelet Trialists’ Collaboration composite CV events (cardiovascular, hemorrhagic, and unknown deaths; nonfatal MI; or nonfatal stroke) All patients Celecoxib vs. placebo: RR 0.85 (95% CI 0.23 to 3.15) Celecoxib vs. NSAIDs: RR 1.06 (95% CI 0.70 to 1.61) Celecoxib vs. naproxen: RR 0.85 (95% CI 0.29 to 2.46) Aspirin nonusers Celecoxib vs. placebo: RR 0.60 (95% CI 0.11 to 3.29) Celecoxib vs. NSAIDs: RR 0.86 (95% CI 0.48 to 1.56) Celecoxib vs. naproxen: RR 0.82 (95% CI 0.18 to 2.46) | Poor |
| Moore, 200551 Trials completed by December 2003 | 31 (22,192) | No | Myocardial infarction Celecoxib vs. placebo: RR not reported (10 events) Celecoxib 200–400 mg vs. NSAID to maximum daily dose: RR 1.9 (95% CI, 0.87 to 4.1) Celecoxib any dose vs. NSAID to maximum daily dose: RR 1.6 (95% CI 0.93 to 2.6) | Fair |
| Caldwell, 2006111 Searches through April 2005 | 6 (6,859) | Yes | Celecoxib vs. placebo Myocardial infarction: RR 2.3 (95% CI 1.0 to 5.1) Cerebrovascular event: RR 1.0 (95% CI 0.51 to 1.8) Cardiovascular death: RR 1.1 (95% CI 0.38 to 3.0) Composite cardiovascular events: RR 1.38 (95% CI 0.91 to 2.1) Celecoxib vs. placebo, diclofenac, ibuprofen, or paracetamol Myocardial infarction: RR 1.9 (95% CI 1.2 to 3.1) Cerebrovascular event: RR 0.73 (95% CI 0.42 to 1.3) Cardiovascular death: RR 1.0 (95% CI 0.52 to 2.0) Composite cardiovascular events: RR 1.2 (95% CI 0.92 to 1.6) | Fair |
| White, 2007113 Trials completed by October 2004 | 41 (23,030) | No | Celecoxib 200–800 mg vs. placebo Antiplatelet Trialists’ Collaboration composite CV events: RR 1.1 (95% CI 0.47 to 2.7) CV deaths: RR 1.3 (95% CI 0.33 to 4.8) Nonfatal MI: RR 1.6 (95% CI 0.21 to 12) Nonfatal stroke: RR 0.80 (95% CI 0.19 to 3.3) Celecoxib 200–800 mg vs. nonselective NSAIDs Antiplatelet Trialists’ Collaboration composite CV events: RR 0.90 (95% CI 0.60 to 1.3) CV deaths: RR 0.57 (95% CI 0.28 to 1.1) Nonfatal MI: RR 1.8 (95% CI 0.93 to 3.4) Nonfatal stroke: RR 0.51 (95% CI 0.23 to 1.1) | Poor |
| Solomon, 2008114 Search dates not reported | 6 (3664) | Yes | Cardiovascular death, MI, stroke, heart failure, or thromboembolism Celecoxib any dose vs. placebo: HR 1.6 (95% CI 1.1 to 2.3) Celecoxib 400 mg qd vs. placebo: HR 1.1 (95% CI 0.6 to 2.0) Celecoxib 200 mg bid vs. placebo: HR 1.8 (95% CI 1.1 to 3.1) Celecoxib 400 mg bid vs. placebo: HR 3.1 (95% CI 1.6 to 6.1) | Fair |
bid = twice daily; CI = confidence interval; CV = cardiovascular; HR = hazard ratio; MI = myocardial infarction; NSAID = nonsteroidal anti-inflammatory drug; qd = once daily; RR = relative risk
- *
Colon polyp prevention trials: PreSAP, APC; Alzheimer’s prevention: ADAPT
Table 6Rate ratios (95% CI)*: COX-2 inhibitor relative to nonselective NSAID121
| NSAID Group | Vascular Events | Myocardial Infarction | Stroke | Vascular Death |
|---|---|---|---|---|
| Any nonselective NSAID | 1.2 (0.97 to 1.4) | 1.5 (1.2 to 2.0), p=0.0009 | 0.83 (0.62 to 1.1) | 0.97 (0.69 to 1.4) |
| Any nonnaproxen, nonselective NSAID | 0.88 (0.69 to 1.1) | 1.2 (0.85 to 1.7) | 0.62 (0.41 to 0.95), p=0.03 | 0.67 (0.43 to 1.1) |
| Naproxen | 1.6 (1.2 to 2.0) | 2.0 (1.4 to 3.0), p=0.0002 | 1.1 (0.73 to 1.6) | 1.5 (0.90 to 2.4) |
CI = confidence interval; COX = cyclooxygenase; NSAID = nonsteroidal anti-inflammatory drug
- *
Rate ratios below 1 favor COX-2 inhibitors, and rate ratios above 1 favor NSAIDs
Table 7Rate ratios for cardiovascular events (95% CI)*: NSAID use compared with nonuse of NSAIDs128
| NSAID | Number of Studies | Risk of Cardiovascular Events |
|---|---|---|
| Celecoxib | 11 | 1.1 (0.91 to 1.2) |
| Meloxicam | 3 | 1.2 (1.0 to 1.6) |
| Naproxen | 15 | 0.97 (0.87 to 1.1) |
| Diclofenac | 9 | 1.4 (1.2 to 1.7) |
| Ibuprofen | 16 | 1.1 (0.97 to 1.2) |
| Indomethacin | 6 | 1.3 (1.1 to 1.6) |
| Piroxicam | 4 | 1.1 (0.70 to 1.6) |
CI = confidence interval; NSAID = nonsteroidal anti-inflammatory drug
Table 8Cardiovascular events in observational studies
| Author, Year Data Source Sample size | Mean age Country | Rate of Aspirin use | Main Findings |
|---|---|---|---|
| Andersohn 2006132 Nested case-control Cases=3,643 | 69 UK | NR | Acute MI, death from acute MI, or sudden death from CHD Current NSAID use vs. nonuse of NSAIDs: RR (95% CI) Celecoxib: 1.6 (1.2 to 2.0) Diclofenac: 1.4 (1.2 to 1.6) Ibuprofen: 1.0 (0.86 to 1.2) Naproxen: 1.2 (0.84 to 1.6) Other nonselective NSAIDs: 1.1 (0.98 to 1.2) |
| Cunnington, 2008148 Cohort n=71, 026 | <65 years old: 52% USA | NR | MI or ischemic stroke Chronic NSAID use vs. nonchronic or nonuse: HR (95% CI) Celecoxib: 1.0 (0.91 to 1.2) Naproxen: 0.99 (0.64 to 1.5) |
| Fischer, 2005133 Nested case-control Cases=8,688 | NR UK (GPRD) | NR | Acute MI Current NSAID use vs. nonuse of NSAIDs: OR (95% CI) Diclofenac: 1.2 (1.0 to 1.5) Ibuprofen: 1.2 (0.92 to 1.5) Naproxen: 0.96 (0.66 to 1.4) Indomethacin: 1.4 (0.82 to 2.2) Piroxicam: 0.95 (0.53 to 1.7) Ketoprofen: 0.86 (0.44 to 1.7) Fenbufen: 3.1 (1.2 to 8.1) Nabumetone: 0.62 (0.25 to 1.5) Mefenamic acid: 2.3 (0.79 to 6.7) Etodolac: 1.1 (0.40 to 3.2) Tiaprofenic acid: 0.65 (0.17 to 2.5) |
| Fosbol, 2009149 Cohort n=1,028,437 | 43 (median) Denmark | NR | MI or death NSAID use vs. nonuse of NSAIDs: HR (95% CI) Celecoxib: 1.5 (0.99 to 2.2) Diclofenac: 1.6 (1.3 to 1.9) Ibuprofen: 0.88 (0.74 to 1.1) Naproxen: 0.85 (0.49 to 1.5) |
| Garcia Rodriguez, 2004134 Nested case-control Cases: 4,975 | NR UK (GPRD) | NR | MI NSAID use vs. nonuse of NSAIDs: OR (95% CI) Naproxen: 0.89 (0.64 to 1.2) Ibuprofen: 1.1 (0.87 to 1.3.) Diclofenac: 1.2 (0.99 to 1.4) Ketoprofen: 1.1 (0.59 to 2.0) Meloxicam: 0.97 (0.60 to 1.6) Piroxicam: 1.2 (0.69 to 2.2) Indomethacin: 0.86 (0.56 to 1.3) |
| Graham 2005135 Nested case- control Cases=8,143 | NR: 18– 84 USA | Telephone interview subgroup (n=817): 23% | Acute MI requiring admission or sudden cardiac death Current NSAID use vs. remote use: OR (95% CI) Celecoxib: 0.84 (0.67 to 1.0) Ibuprofen: 1.1 (0.96 to 1.2) Naproxen: 1.1 (1.0 to 1.3) Current NSAID use vs. celecoxib use: OR (95% CI) Ibuprofen: 1.3 (1.0 to 1.6) Naproxen: 1.4 (1.1 to 1.8) |
| Helin- Salmivaara, 2006129 Case-control Cases=33,309 | NR Finland | NR | First time MI Current NSAID use vs. nonuse of NSAIDs: OR (95% CI) Indomethacin: 1.6 (1.2 to 2.0) Ibuprofen: 1.4 (1.3 to 1.6) Diclofenac: 1.4 (1.2 to 1.5) Naproxen: 1.2 (1.0 to 1.4) Piroxicam: 1.4 (0.92 to 2.0) Ketoprofen: 1.1 (0.94 to 1.3) Tolfenamic acid: 1.4 (0.90 to 2.2) Nimesulide: 1.7 (1.4 to 2.0) Etodolac: 1.4 (0.44 to 4.2) Nabumetone: 1.3 (0.59 to 2.7) Meloxicam: 1.2 (0.99 to 1.6) Celecoxib: 1.1 (0.83 to 1.3) Recent (within 30 days) NSAID use vs. nonuse of NSAIDs: OR (95% CI) Indomethacin: 1.5 (1.0 to 2.1) Ibuprofen: 1.1 (0.94 to 1.3) Diclofenac: 0.93 (0.77 to 1.1) Naproxen: 1.3 (1.0 to 1.7) Piroxicam: 0.89 (0.49 to 1.6) Ketoprofen: 1.3 (1.0 to 1.7) Tolfenamic acid: 1.3 (0.74 to 2.3) Nimesulide: 1.1 (0.91 to 1.4) Etodolac: 0.95 (0.23 to 4.0) Nabumetone: 3.0 (0.96 to 9.4) Meloxicam: 1.0 (0.77 to 1.4) Celecoxib: 0.95 (0.65 to 1.4) |
| Hippisley-Cox 2005136 Nested case- control Cases: 9,218 | NR; aged 25–100 UK | NR | First ever MI NSAID use within 3 months vs. no prescription for 3 years: OR (95% CI) Celecoxib: 1.2 (0.96 to 1.5) Ibuprofen: 1.2 (1.1 to 1.4) Diclofenac: 1.6 (1.4 to 1.7) Naproxen: 1.3 (1.0 to 1.6) Other nonselective NSAIDs: 1.2 (1.0 to 1.4) |
| Johnsen 2005137 Case-control Cases=10,280 | 70 Denmark | 7% high-dose | Acute MI Current NSAID use vs. nonuse of NSAIDs: RR (95% CI) Celecoxib: 1.2 (0.97 to 1.6) Naproxen: 1.5 (0.99 to 2.3) Other nonaspirin NSAID: 1.7 (1.5 to 1.8) New NSAID use vs. nonuse of NSAIDs: (95% CI) Celecoxib: 2.1 (1.4 to 3.1) Naproxen: 1.6 (0.57 to 4.8) Other nonaspirin NSAID: 2.6 (2.0 to 3.5) |
| Kimmel 2005138 Case-control Cases: 1,718 | NR; aged 40 to 75 USA | 34% | Nonfatal MI NSAID use vs. nonuse of NSAIDs: OR (95% CI) Celecoxib: 0.43 (0.23 to 0.79) Nonselective NSAID: 0.61 (0.52 to 0.71) |
| Levesque 2005139 Nested case- control Cases: 2,844 | NR; ≥ 66 Canada | 22% | Acute MI, fatal or nonfatal NSAID current use vs. nonuse of NSAIDs: RR (95% CI) Celecoxib: 0.99 (0.85 to 1.2) Naproxen: 1.2 (0.75 to 1.8) Meloxicam: 1.1 (0.49 to 2.3) |
| Mamdani 2003140 Cohort n=166,964 | NR; ≥ 66 Canada | 15% | Hospitalization for acute MI NSAID user vs. nonuser control: RR (95% CI) Celecoxib: 0.9 (0.7 to 1.2) Naproxen: 1.0 (0.7 to 1.7) Nonnaproxen nonselective NSAIDs: 1.2 (0.9 to 1.4) |
| Rahme, 2002141 Case-control Cases=4163 | NR (older than 65 years) Canada | NR | Hospitalization for acute MI Exposure to naproxen vs. exposure to other NSAIDs: OR 0.79 (95% CI 0.63 to 0.99) |
| Rahme, 2007130 Retrospective cohort N=283,799 | NR (>65 years) Canada | 24% | Acute myocardial infarction hospitalization Celecoxib vs. diclofenac/ibuprofen: 0.90 (0.76 to 1.1) |
| Rahme, 2007108 Retrospective cohort N=510,871 | NR; ≥ 65 Canada | 22% | Acute myocardial infarction NSAID use vs. acetaminophen use: HR (95% CI) Celecoxib: 0.97 (0.86 to 1.1) Ibuprofen: 1.0 (0.68 to 1.6) Diclofenac: 1.2 (0.96 to 1.4) Naproxen: 1.2 (0.89 to 1.5) |
| Ray 2002147 Cohort n=378,776 | 61.5 USA | NR | Serious CHD (hospital admission for acute MI or death from CHD) Current NSAID use vs. nonuse of NSAIDs: RR (95% CI) Celecoxib: 0.96 (0.76 to 1.2) Naproxen: 0.93 (0.82 to 1.1) Ibuprofen: 0.91 (0.78 to 1.1) New NSAID use vs. nonuse of NSAIDs: RR (95% CI) Celecoxib: 0.88 (0.67 to 1.2) Naproxen: 0.92 (0.73 to 1.2) Ibuprofen: 1.0 (0.77 to 1.3) |
| Schlienger, 2002143 Nested case- control Cases=3,319 | NR UK (GPRD) | NR | First acute MI NSAID use vs. nonuse of NSAIDs: OR (95% CI) Ibuprofen: 1.2 (0.87 to 1.6) Diclofenac: 1.4 (1.1 to 1.8) Piroxicam: 1.6 (0.78 to 3.5) Fenbufen: 2.1 (0.80 to 5.3) Ketoprofen: 1.4 (0.77 to 2.5) Indomethacin: 1.0 (0.58 to 1.8) Flurbiprofen: 2.3 (0.93 to 5.5) Naproxen: 0.68 (0.42 to 1.1) |
| Solomon, 2002144 Case to control Cases=4,425 | NR USA | NR | Hospitalization for MI NSAID use vs. nonuse of NSAIDs: RR (95% CI) Naproxen: 0.84 (0.72 to 0.98) Ibuprofen: 1.0 (0.88 to 1.2) NSAID use vs. ibuprofen use: RR (95% CI) Naproxen: 0.82 (0.67 to 1.0) |
| Solomon 2004145 Case-control Cases=10,895 | NR; > 80 USA | NR | Acute MI Celecoxib use vs. no celecoxib use: OR 0.93 (95% CI 0.84 to 1.0) Celecoxib use vs. naproxen use: OR 0.95 (95% CI 0.74 to 1.2) Celecoxib use vs. ibuprofen use: OR 0.98 (95% CI 0.76 to 1.3) Celecoxib use vs. other NSAID use: OR 0.95 (95% CI 0.82 to 1.1) |
| Solomon, 2008131 Cohort n=175,654 | 80 years USA | NR | MI, stroke, CHF, and out-of-hospital death attributable to cardiovascular disease NSAID use vs. nonuse of NSAIDs: HR (95% CI) Celecoxib: 0.89 (0.83 to 0.94) Diclofenac: 1.2 (1.1 to 1.3) Ibuprofen: 0.96 (0.83 to 1.1) Naproxen: 0.79 (0.67 to 0.93) Other nonselective NSAIDs: 0.87 (0.79 to 0.96) |
| Velentgas 2006146 Cohort n=424,584 | NR (40–64 years) USA | NR | Acute coronary syndrome or MI Current NSAID use vs. current ibuprofen use: RR (95% CI) Celecoxib: 1.0 (0.83 to 1.3) Naproxen: 1.1 (0.93 to 1.4) |
CHD = coronary heart disease; CHF = congestive heart failure; HR = hazard ratio; MI = myocardial infarction; NR = not reported; NSAID = nonsteroidal anti-inflammatory drug; OR = odds ratio; RR = relative risk; UK GPRD = United Kingdom General Practice Research Database
Table 9Systematic review of tolerability of COX-2s compared with NSAIDs
| Review | AE incidence | Withdrawals | ||
|---|---|---|---|---|
| Overall RR (95% CI) | GI-related RR (95% CI) | Any AE RR (95% CI) | GI-related RR (95% CI) | |
| Celecoxib vs. NSAIDs for OA/RA | ||||
| Deeks 200250 | - | - | 0.86 (0.72, 1.04) | 0.54 (0.42, 0.71) |
| Moore 200551 | 0.96 (0.94, 0.98) | 0.84 (0.81, 0.87) | 0.86 (0.81, 0.91) | 0.75 (0.7, 0.8) |
| Chen, 200858 | 0.96 (0.91, 1.0) | 0.75 (0.70, 0.80) | 0.86 (0.73, 1.0) | 0.45 (0.35, 0.56) |
AE = adverse event; CI = confidence interval; COX = cyclooxygenase; GI = gastrointestinal; NSAID = nonsteroidal anti-inflammatory drug; OA = osteoarthritis; RA = rheumatoid arthritis; RR = relative risk
Table 10Toxicity index scores from ARAMIS database studies
| Study | Aspirin | Ibuprofen | Salsalate | Others (Range) |
|---|---|---|---|---|
| Fries 1991177 | 1.19 | 1.94 | 1.28 | 2.17 (naproxen) to 3.99 (indomethacin) |
| Fries 1993176 | 1.33 | 1.89 | NR | 1.90 (naproxen) to 2.86 (tolmetin) |
| Fries 1996175 | 1.77 | 2.68 | 2.00 | 1.63 (sulindac) to 3.09 (ketoprofen) |
| Singh 1997178 | 2.25 | 1.95 | 1.79 | 3.29 (naproxen) to 5.14 (meclofenamate) |
ARAMIS = Arthritis, Rheumatism, and Aging Medical Information System; NR = not reported
Table 11Pain relief in systematic reviews of acetaminophen compared with NSAID
| Systematic Review | Date of Last Search | Number of Head-to-Head Trials Included | Main Results for Outcome of General or Rest Pain |
|---|---|---|---|
| Towheed, 2006180 | Through 7/05 | 12 (4 trials evaluated coxibs) | NSAIDs superior for rest pain (3 trials, SMD 0.20, 95% CI 0.03 to 0.36), overall pain (8 trials, SMD 0.25, 95% CI 0.17 to 0.33), WOMAC pain (2 trials, SMD 0.24, 95% CI 0.09 to 0.38), WOMAC stiffness (2 trials, SMD 0.20, 95% CI 0.05 to 0.34), WOMAC function (2 trials, SMD 0.25, 95% CI 0.11 to 0.40), and global assessment of efficacy (2 trials, RR 1.2, 95% CI 1.1 to 1.4) |
| Zhang, 2004182 | Through 7/03 | 8 (3 trials evaluated coxibs) | NSAIDS superior using WOMAC scale (SMD 0.3, 95% CI 0.17 to 0.44) and clinical response rate (RR 1.24, 95% CI 1.08 to 1.41) |
| Lee, 2004179 | Through 2/03 | 6 (1 trial evaluated a coxib) | NSAIDs superior for rest pain (weighted mean difference 6.33, 95% CI 3.41 to 9.24) |
| Wegman, 2004181 | Through 12/01 | 3 (no trials evaluated coxibs) | NSAIDs superior for general/rest pain (SMD 0.33, 95% CI 0.15 to 0.51) |
CI = confidence interval; NSAID = nonsteroidal anti-inflammatory drug; RR = relative risk; SMD = standardized mean difference; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index
Table 12Adverse events in systematic reviews of acetaminophen compared with NSAID
| Systematic Review | Withdrawal due to Adverse Events | GI Adverse Events |
|---|---|---|
| Towheed, 2006180 | NSAID vs. acetaminophen: RR 0.79 (95% CI 0.59 to 1.0) | Withdrawal due to GI adverse event Nonselective NSAIDs vs. acetaminophen: RR 2.0 (95% CI 1.0 to 3.8) Any GI adverse event Nonselective NSAID vs. acetaminophen: RR 1.5 (95% CI 1.1 to 2.0) COX-2 selective NSAID vs. acetaminophen: RR 0.98 (95% CI 0.80 to 1.2) |
| Zhang, 2004182 | NR | GI discomfort Nonselective NSAID vs. acetaminophen: RR 1.4 (95% CI 1.1 to 1.8) COX-2 selective NSAID vs. acetaminophen: RR 0.65 (95% CI 0.17 to 2.52) |
| Lee, 2004179 | NSAID vs. acetaminophen: OR 1.4, 95% CI 0.93 to 2.3) | NR |
CI = confidence interval; COX = cyclooxygenase; GI = gastrointestinal; NR = not reported; NSAID = nonsteroidal anti-inflammatory drug; OR = odds ratio; RR = relative risk
Table 14Response rates in the Glucosamine/chondroitin Arthritis Intervention Trial (GAIT)224
| Intervention | All Patients | Moderate-Severe Baseline Pain (WOMAC Pain Score 301–400 mm) | Mild Baseline Pain (WOMAC Pain Score 125–300) |
|---|---|---|---|
| Placebo | 60.1% | 54.3% | 61.7% |
| Celecoxib | 70.1% (p=0.008 vs. placebo) | 69.4% (p=0.06 vs. placebo) | 70.3% (p=0.04 vs. placebo) |
| Glucosamine | 64.0% (p=0.30 vs. placebo) | 65.7% (p=0.17 vs. placebo) | 63.6% (p=0.67 vs. placebo) |
| Chondroitin | 65.4% (p=0.17 vs. placebo) | 61.4% (p=0.39 vs. placebo) | 66.5% (p=0.27 vs. placebo) |
| Glucosamine + chondroitin | 66.6% (p=0.09 vs. placebo) | 79.2% (p=0.002 vs. placebo) | 62.9% (p=0.80 vs. placebo) |
GAIT = Glucosamine/chondroitin Arthritis Intervention Trial; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index
Table 15Efficacy, glucosamine and chondroitin trials
| Author, Year Quality | Condition Number Enrolled | Comparison Duration of Study | Main Results |
|---|---|---|---|
| Glucosamine Trials | |||
| Herrero-Beaumont, 2007204 Fair | OA of knee 318 | Glucosamine sulfate 1500 mg powder for oral solution qd Acetaminophen 1 gm po tid Placebo 6 months | Glucosamine sulfate vs. acetaminophen vs. placebo Change from baseline: Lequesne Index (0 to 24): −3.1 vs. −2.7 vs. −1.9; p=0.032 for difference vs. placebo WOMAC total (0 to 100): −12.9 vs. −12.3 vs. −8.2; p=0.039 for difference vs. placebo WOMAC pain (0 to 100): −2.7 vs. −2.4 vs. −1.8; NS WOMAC function (0 to 100): −9.2 vs. −8.7 vs. −5.5; p=0.022 for difference vs. placebo OARSI-A responders: 40% vs. 21.2% for placebo, p= 0.004 |
| Rozendaal, 2008205 Rozendaal, 2009 Good | OA of hip 222 | Glucosamine sulfate 1500 mg po qd or bid Placebo 24 months | Glucosamine sulfate vs. placebo Change from baseline: WOMAC pain (0 to 100): −1.90 ± 1.6 vs. −0.30 ± 1.6, adjusted difference −1.54 (−5.43 to 2.36) WOMAC function (0 to 100): −1.69 ± 1.3 vs. 0.38 ± 1.3, adjusted difference −2.01 (95% CI −5.38 to 1.36) JSN, mm adjusted difference: Minimal: −0.029 (95% CI −0.122 to 0.064) Lateral: −0.017 (95% CI −0.121 to 0.088) Superior: 0.016 (95% CI −0.079 to 0.111) Axial: −0.005 (95% CI −0.118 to 0.108) |
| Wilkens, 2010206 Good | Degenerative lumbar OA 250 | Glucosamine sulfate 1500 mg po qd or tid Placebo 6 months | Glucosamine sulfate vs. placebo Treatment Effect at 1 year (negative values favor glucosamine): RMDQ (0 to 24): −0.8 (95% CI −2.0 to 0.4), p=0.50 NRS LBP (0 to 10): −0.3 (95% CI −0.8 to 0.3). p=.85 Global perceived effect, No. (%):* 34 (30.9%) vs. 32 (29.4%), p=.30 |
| Chondroitin Trials | |||
| Kahan, 2009207 Fair | OA of knee 622 | Chondroitin sulfates 4 & 6 800 mg every evening Placebo 2 years | Chondroitin sulfate vs. placebo At 6 months: WOMAC pain score decrease ≥40%: 41% vs. 34%, p=0.05 No difference in WOMAC total, stiffness, or function At 24 months: minimum JSW loss (mean ± SEM): −0.07 ± 0.03 mm vs. −0.31 ± 0.04 mm Hodges-Lehmann estimator of median effect of treatment: −0.14 (95% CI 0.06 – 0.21 mm, p<0.0001) |
| Mazieres, 2010208 Fair | OA of knee 307 | Chondroitin sulfate 500 mg po bid Placebo 24 weeks | Chondroitin sulfate vs. placebo Change from baseline to week 24, M (SD): Lequesne Index, (0 to 24): −2.4 (3.4) vs. −1.7 (3.3), p=0.109 VAS pain, mm: −26.2 (24.9) mm vs. −19.9 (23.5) mm, p= 0.029 OMERACT-OARSI responders: 68% vs. 56% (p=0.03) |
| Michel, 2005209 Fair | OA of knee 300 | Chondroitin sulfates 4 & 6 800 mg po qd Placebo 2 years | Chondroitin sulfate vs. placebo Changes in WOMAC: Total: −3.9% vs. 2.1% Pain: −11.0% vs. −6.2% Stiffness: −7.8% vs. −4.6% Function: −0.8% vs. 5.9% JSN Minimum difference: 0.12 (95% CI 0.00 to 0.24), p=0.05 JSM Mean difference: 0.14 (95% CI 0.01 to 0.27), p =0.04 |
| Moller, 2010210 Fair | OA of knee (in patients with psoriasis) 129 | Chondroitin sulfate 800 mg po qd Placebo 3 months | Chondroitin sulfate vs. placebo (mean differences at 3 months) Pain intensity (0 to 100 mm VAS): −12 (95% CI −20 to −4) Lequesne Index (0 to 24): −1.7 (95% CI −3.0 to −0.4) SF-36 physical component (0 to 100): 1.7 (95% CI 1.4 to −1.2) SF-36 mental component (0 to 100): −0.3 (95% CI −3.3 to 2.6) |
| Glucosamine/Chondroitin Trials | |||
| Messier, 2007211 Fair | OA of knee 89 | Glucosamine hydrochloride 1500 mg and Chondroitin sulfate 1200 mg qd or tid Placebo 1 year; 6 months alone; 6 months treatment plus exercise | Glucosamine hydrochloride + chondroitin sulfate vs. placebo At 12 months: WOMAC pain (0 to 20): 6.0 (0.5) vs. 5.18 (0.5) WOMAC function (0 to 68): 19.4 (1.2) vs. 20.6 (1.2)b |
| Sawitzke, 2008212 Good | OA of knee 662 | Glucosamine sulfate 500 mg tid Chondroitin sulfate 400 mg tid Combination of Glucosamine and Chondroitin Celecoxib 200 mg qd Placebo 24 months | Glucosamine hydrochloride vs. chondroitin sulfate vs. both vs. placebo Mean loss in JSW over 2 years: 0.013 vs. 0.107 vs. 0.194 vs. 0.111 vs. 1.166 Difference from placebo (negative value = less JSW loss): −0.153 (−0.379, 0.074) vs. −0.059 (−0.287, 0.169) vs. 0.028 (−0.214,0.271) vs. −0.055 (−0.279, 0.170) Disease progression over 2 years, % of patients:18.6 vs. 21.4 vs. 24.4 vs. 20.2 vs. 22.4 |
bid = twice daily; JSM = joint space measurement; JSN = joint space narrowing; JSW = joint space width; NRS LBP = numerical rating scale for low back pain; OA = osteoarthritis; OMERACT-OARSI = Outcomes Measures in Arthritis Clinical Trials-Osteoarthritis Research Society International; po = orally; RMDQ = Roland Morris Disability Questionnaire; qd = once daily; qid = four time daily; tid = three times daily; VAS = visual analogue scale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index
- *
Proportion of patients who had a global perceived effect to the intervention
Table 16Celecoxib in patients with bleeding ulcer history
| Study Sample Size | Treatments | Recurrent Ulcer Bleeding (Difference, 95% CI) | Other Adverse Events | Withdrawals due to Adverse Events |
|---|---|---|---|---|
| Chan 2002233 n=287 | Celecoxib 200 mg bid Diclofenac 75 mg bid plus omeprazole 20 mg qd | 4.9% vs. 6.3% at 6 months (−1.5%, −6.8 to 3.8; NS) | No differences | 13% vs. 12%, NS* |
| Lai 2005234† n=242 | Celecoxib 200 mg qd Naproxen 250 mg tid plus lansoprazole 30 mg qd | 3.7% vs. 6.3% at 6 months (−2.6; −9.1 to 3.7; NS) | No differences for all but dyspepsia: 15% vs. 5.7%, p=0.02 | 10% vs. 7.4%, NS |
| Chan 2007235 n=273 | Celecoxib 200 mg bid plus esomeprazole 20mg bid Celecoxib 200 mg bid | 0% vs. 19% at median 13 months (p=0.03) | No differences | 5.8% vs. 7.4%, NS |
bid = twice daily; CI = confidence interval; NS = not significant; qd = three times daily; tid = twice daily
- *
Includes withdrawals due to lack of efficacy
- †
Open trial
Table 17Observational studies of the cardiovascular risk with concomitant aspirin
| Study | Study Design | Number | Outcome Measure | Referent | Effect (95% CI) | |
|---|---|---|---|---|---|---|
| Any nonselective NSAID | Ko 2002255 | Cohort | 39,043 | Mortality at 1 year | No NSAID, no aspirin | NSAID+ASA OR 0.78 (0.69 to 0.88) ASA alone OR 0.81 (0.77 to 0.86) |
| Kurth 2003*258 | RCT Subgroup analysis | 22,071 | AMI | No aspirin (placebo) | ASA without NSAID OR 0.56 (0.44 to 0.72) 1–59 days NSAID + ASA OR 0.69 (0.46 to 1.0) ≥60 days NSAID + ASA OR 1.57 (0.70 to 6.6) | |
| Kimmel 2004254 | Case-control | 4,393 | First nonfatal MI | Aspirin alone | NSAID+ASA OR 0.92 (0.46 to 1.9) | |
| Fisher 2005131 | Case-control | 2,989 | AMI | No NSAID, no aspirin | NSAID+ASA OR 0.74 (0.57 to 0.97) ASA alone OR 0.87 (0.75 to 1.0) | |
| Ibuprofen | MacDonald 2003256 | Cohort | 7,107 | All-cause and cardiovascular mortality | Aspirin alone | Ibuprofen+ASA All-cause mortality HR 1.9 (1.3 to 2.9) Cardiovascular mortality HR 1.7 (1.0 to 2.8) |
| Patel 2004257 | Cohort | 14,098 | AMI | Aspirin alone | Ibuprofen+ASA OR 0.61 (0.50 to 0.73) | |
| Kimmel 2004254 | Case-control | 4,393 | First nonfatal MI | Aspirin alone | Ibuprofen+ASA OR 2.0 (1.1 to 3.9) | |
| Rahme 2007248 | Cohort | 76,877 | AMI | Acetaminophen alone | Ibuprofen+ASA OR 1.4 (0.8 to 2.4) Ibuprofen only OR 1.0 (0.74 to 1.5) | |
| Fischer 2005131 | Case-control | 2,989 | AMI | No NSAID, no aspirin | Ibuprofen+ASA OR 0.69 (0.42 to 1.2) | |
AMI = acute myocardial infarction; ASA = aspirin; CI = confidence interval; HR = hazard ratio; MI = myocardial infarction; NSAID = nonsteroidal anti-inflammatory drug; OR = odds ratio
- *
Composite outcome = CV mortality, nonfatal MI, and stroke at 1 year.
Table 18Summary of results from placebo-controlled trials of gastroprotective agents262–264
| Treatment | Number of Studies Duration | Prevention of Clinical GI Events* | Prevention of Endoscopic Ulcers | |
|---|---|---|---|---|
| Gastric | Duodenal | |||
| Misoprostol | 8;4–11weeks: 11; ≥ 3 months | OR 0.60, 95% CI 0.36 to 0.98† | 4–11 weeks: RR 0.17, 95% CI 0.09 to 0.31‡ 3 months: RR 0.26; 95% CI 0.17 to 0.39‡ | 4–11 weeks: RR 0.28, 95% CI 0.09 to 0.31‡ 3 months: RR 0.47, 95% CI 0.33 to 0.69‡ |
| Duration NR | RR 0.57, 95% CI 0.36 to 0.91§ | Either: RR 0.33, 95% CI 0.3 to 0.4§ | Reported in gastric ulcers column | |
| H2 blockers | Standard dose¶: 7; ≥3 months Double dose¶: 3; ≥3 months | Not reported | Standard dose: RR 0.73, 95% CI 0.50 to 1.1‡ Double dose: RR 0.44, 95% CI 0.26 to 0.74‡ | Standard dose: RR 0.36, 95% CI 0.18 to 0.74‡ Double dose: RR 0.26, 95% CI 0.11 to 0.65‡ |
| Standard dose Duration NR | RR 0.33, 95% CI 0.01 to 8.14§ | Gastric or duodenal ulcer: RR 0.55, 95% CI 0.4 to 0.7§ | Reported in gastric ulcers column | |
| PPIs | 4, ≥3 months | Not reported | RR 0.40, 95% CI 0.32 to 0.51‡ | RR 0.19, 95% CI 0.09 to 0.37‡ |
| Duration NR | RR 0.46, 95% CI 0.07 to 2.9§ | Gastric or duodenal ulcer: RR 0.37, 95% CI 0.3 to 0.5§ | Reported in gastric ulcers column | |
CI = confidence interval; GI = gastrointestinal; NR = not reported; OR = odds ratio; PPI = proton pump inhibitor; RR = relative risk
- *
Hemorrhage, hemorrhagic erosions, recurrent upper gastrointestinal bleeds, perforation, pyloric obstruction, melena, and death from any of these
- †
Silverstein 1995 (MUCOSA trial)290
- ‡
Rostom 2002264
- §
Hooper 2004262
- ¶
Standard Doses = 150 mg daily, Double Doses = 300 mg daily.
Table 19Head-to-head trials of gastroprotective agents263
| Comparison | Reductions in Ulcer Risk | |
|---|---|---|
| Gastric | Duodenal | |
| Misoprostol vs. ranitidine* (2 trials; n=600) | RR 0.12 95% CI 0.03 to 0.89 | No differences |
| Omeprazole* vs. ranitidine* (1 trial, n=425) | RR 0.32 95% CI 0.17 to 0.62 | RR 0.11 95% CI 0.01 to 0.89 |
| PPI† vs. misoprostol‡ (2 trials; n=838) | No differences | RR 0.29 95% CI 0.15 to 0.56 |
CI = confidence interval; PPI = Proton pump inhibitor; RR = relative risk
- *
Standard dose
- †
Omeprazole or lansoprazole standard doses
- ‡
Secondary prophylaxis trials – misoprostol doses 400 mcg daily in one trial and 800 mcg daily in another trial
Table 20Efficacy, head-to-head trials of topical compared with oral NSAID for osteoarthritis
| Author, Year Quality | Condition Number Enrolled | Comparison | Duration of Study | Main Results |
|---|---|---|---|---|
| Dickson, 1991298 Fair | OA of knee 235 | Piroxicam 0.5% gel tid Ibuprofen 400 mg po tid | 4 weeks | Patient global assessment ‘good’ ‘or excellent’: 64% vs. 60% Pain during day (0–9): 3.0 vs. 3.0, p=0.56 Pain at night (0–9): 2.0 vs. 2.0, p=0.54 |
| Sandelin, 1997300 Fair | OA of knee 208 | Eltenac 1% gel tid Diclofenac 50 mg po bid | 4 weeks | Lequesne Index (0–24): 6.3 vs. 6.9 Pain (0–100 VAS): 28 vs. 30 |
| Zacher, 2001304 Not rated* | OA of fingers 321 | Diclofenac 1% gel Ibuprofen 400 mg po tid | 3 weeks | >=40% improvement in pain on 100 mm VAS: 40% vs. 34%, RR 1.2 (95% CI 0.88–1.6) |
| Tugwell, 2004303 Good | OA of knee 622 | Diclofenac 1.5% in 45.5% DMSO tid Diclofenac 50 mg po tid | 12 weeks | Clinical responder (OMERACT VI criteria38): 66% vs. 70% WOMAC Pain (0–500, mean change): −118 vs. −134, p=0.10 WOMAC Physical Function (0–1700, mean change): −348 vs. −438, p=0.008 WOMAC Stiffness (0–200, mean change): −45 vs. −52, p=0.14 Patient global assessment (0–100, mean change): −27 vs. −32, p=0.08 |
| Rother, 2007299 Good | OA of knee 270 | Ketoprofen 110 mg in 4.8 g transferone every 12 hours Celecoxib 100 mg every 12 hours | 6 weeks | Clinical responder (OMERACT criteria): 69% vs. 64% Patient global assessment of response “good” or “excellent”: 46% vs. 39% WOMAC Pain (0 to 100, mean change): −19 vs. −21 WOMAC Physical Function (0 to 100, mean change): −16 vs. −18 WOMAC Stiffness (0 to 100, mean change): −15 vs. −17 Use of rescue medication (capsules/day): 0.24 vs. 0.16 |
| Underwood, 200849 Fair | OA of knee 282 | Advice to use a topical NSAID, preferably ibuprofen Advice to use an oral NSAID | 1 year | Mean differences in change WOMAC Pain (0 to 100): 1 (95% CI −4 to 6) WOMAC Stiffness (0 to 100): 0 (95% CI −6 to 5) WOMAC Physical Function (0 to 100): 3 (95% CI −2 to 7) WOMAC Global (0 to 100): 2 (95% CI −2 to 6) SF-36: No differences at 1 year in mental or physical component summary scores |
| Simon, 2009301 Good | OA of knee 305 | Diclofenac 1.5% in 45.5% DMSO qid Diclofenac 100 mg po qd | 12 weeks | WOMAC Pain (0 to 20, mean change): −6.0 vs. −6.4, p=0.43 WOMAC Physical Function (0 to 68, mean change): −16 vs. −18, p=0.32 WOMAC Stiffness (0 to 8, mean change): −1.9 vs. −2.1, p=0.60 Patient global assessment (0 to 4, mean change): −1.4 vs. −1.4, p=0.44 |
| Tiso, 2010302 Fair | Chronic knee pain (presumed OA of knee) 20 | Ibuprofen 4% gel qid Ibuprofen 800 mg po tid | 2 weeks | WOMAC Pain (0 to 500, mean change): −83 vs. −84, NS WOMAC Physical Function (0 to 1700, mean change): −312 vs. −323, NS WOMAC, Stiffness (0 to 200, mean change): −48 vs. −26, NS SF-36: No differences on mental component score, physical component score, or subscales |
CI = confidence interval; NSAID = nonsteroidal anti-inflammatory drug; OA = osteoarthritis; OMERACT = Outcomes Measures in Arthritis Clinical Trials; po = orally; tid = three times daily; qd = once daily; qid = four times daily; VAS = visual analogue scale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index
- *
Non-English language study, data as reported in Mason et al305.
Table 21Adverse events: Head-to-head trials of topical compared with oral NSAID for osteoarthritis
| Author, Year Quality | Condition Number Enrolled Comparison | Withdrawal due to Adverse Events Any Adverse Events | Gastrointestinal Adverse Events | Skin Adverse Events | Other Adverse Events |
|---|---|---|---|---|---|
| Dickson, 1991298 Fair | OA of knee 235 Topical piroxicam 0.5% vs. ibuprofen 400 mg po tid | Withdrawal due to adverse events: 7.7% (9/117) vs. 5.9% (7/118) Any adverse event: 26% (31/117) vs. 23% (27/118) | Upper GI: 10% vs. 8.5% Other GI: 2.6% vs. 0.8% | Rash: 0.8% vs. 0.8% | CNS: 6.0% vs. 6.8% |
| Sandelin, 1997300 Fair | OA of knee 208 Eltenac 1% gel vs. diclofenac 50 mg po bid | Withdrawal due to adverse events: 3.2% (4/126) vs. 1.2% (1/82) Any adverse event: 27% (34/126) vs. 24% (20/82) | GI: 4.8% vs. 13% | Local skin reaction: 13% vs. 1.2% | CNS: 9.5% vs. 7.3% |
| Zacher, 2001304 Not rated* | OA of fingers 321 Diclofenac 1% gel vs. ibuprofen 400 mg po tid | Withdrawal due to adverse events: 3.0% (5/165) vs. 10% (16/156) | Data not available | Data not available | Data not available |
| Tugwell, 2004303 Good | OA of knee 622 Diclofenac 1.5% in 45.5% DMSO vs. diclofenac 50 mg po tid | Withdrawal due to adverse events: 21% (64/311) vs. 25% (79/311) | Any GI adverse event: 35% vs. 48% (p=0.0006); severe 7.4% vs. 21% (p=0.002) Abdominal pain: 12% vs. 22% (p=0.0008); severe 5.6% vs. 19% Diarrhea: 9% vs. 17% (p=0.001), severe 3.7% vs. 17% Dyspepsia: 15% vs. 26% (p=0.001), severe 4.2% vs. 14% Melena: 1% vs. 2% (p=0.36) Nausea: 8% vs. 13% (p=0.36) Vomiting: 2% vs. 2% (p=0.56) AST normal to abnormal: 2% vs. 10% (p=0.0001) Hemoglobin (g/l): normal to abnormal 2% vs. 10% (p<0.0001), mean change from baseline 0.9 vs. −2.2 (p<0.0001) | Dry skin: 27% vs. 1% (p<0.0001 Rash: 12% vs. 2% (p<0.0001 Pruritus: 6% vs. 0.6% (p<0.0001) Vesiculobullous rash: 5% vs. 0% (p<0.0001) | Asthma: 0.6% vs. 3% (p=0.002) Dizziness: 0.6% vs. 4% (p=0.002) Dyspnea; 0% vs. 2% (p=0.01) Mean blood pressure increased 5 mm Hg or greater: 24% vs. 28% (p=0.30) Creatinine: normal to abnormal 1% vs. 3% (p=0.08), mean change from baseline 0.3 vs. 3.3 (p=0.003) |
| Rother, 2007299 Good | OA of knee 270 Ketoprofen 110 mg in 4.8 g transferone q 12 h vs. celecoxib 100 mg every 12 hours | Withdrawal due to adverse events: 17% (23/138) vs. 14% (18/132), p=0.15 Any adverse event: 54% vs. 50% | GI adverse event: 9.4% vs. 14% Abdominal pain, upper: 1.4% vs. 3.0% Diarrhea: 0.7% vs. 1.5% Dyspepsia: 0.7% vs. 3.0% Gastritis: 2.2% vs. 0% Nausea: 1.4% vs. 2.3% | Any skin: 28% vs. 20% Dermatitis allergic: 1.4% vs. 0.8% Erythema: 21% vs. 14% Exanthema: 2.2% vs. 1.5% Pruritus: 0% vs. 3.8% Skin irritation: 1.4% vs. 0% Urticaria: 1.4% vs. 0.8% | Any respiratory, thoracic, and mediastinal disorders: 12% vs. 11% |
| Underwood, 200849 Fair | OA of knee 282 Advice to use topical NSAID (preferably ibuprofen) vs. advice to use an oral NSAID (preferably ibuprofen) | Any defined minor adverse event: 53% vs. 57% (mean difference 0%, 95% CI − 11% to 12%) | GI minor event: 42% vs. 40% (mean difference 2%, 95% CI −9% to 14%) Liver enzymes >upper limit of normal: 2.7% vs. 2.2 (mean difference 0.4%, 95% CI −3.4% to 4.3%) Change in hemoglobin (g/l): 0.2 vs. 0.7, difference 0.5 (95% CI −1.3 to 2.3) | Not reported | Unplanned hospitalization through 1 year (rate per 100 per year): 4.5 vs. 1.4 (mean difference 3.1, 95% CI −1.0 to 7.2) Renovascular minor adverse event: 16% vs. 15% (mean difference 1%, 95% CI −8% to 9%) Respiratory: 7% vs. 17% (mean difference −9%, 95% CI −17% to −2%) Change in systolic blood pressure (mm Hg): 2.5 vs. 4.4, difference 1.9 (95% CI −1.7 to 5.5) Change in serum creatinine (micromol/l): 2.4 vs. − 1.3, difference −3.7 (−6.5 to −0.9) |
| Simon, 2009301 Good | OA of knee 305 Diclofenac 1.5% in 45.5% DMSO qid vs. diclofenac 100 mg po qd | Withdrawal due to adverse events: 10% (16/154) vs. 13% (19/151) Any adverse event: 62% vs. 62% Serious adverse event: 0% vs. 0.7% | Any GI adverse event: 6.5% vs. 24% Abdominal pain: 3.2% vs. 7.3% Dyspepsia: 2.6% vs. 4.0% Diarrhea; 1.3% vs. 4.6% Liver function tests abnormal: 1.9% vs. 7.9%; AST normal to abnormal 6.9% vs. 20% Rectal hemorrhage: 0.6% vs. 0% Nausea: 0% vs. 2.0% Hemoglobin normal to abnormal: 2.1% vs. 5.8%; mean change (g/l): −1.0 vs. −3.8 | Any skin/appendages event: 27% vs. 7.3% Dry skin: 18% vs. 2.6% Contact dermatitis: 2.6% vs. 0.7% Rash: 2.6% vs. 0% Contact dermatitis with vesicles: 1.9% vs. 0.7% | Respiratory disorder: 3.2% vs. 5.3% Creatinine normal to abnormal: 2.8% vs. 7.2%; mean change (micromol/l): −0.4 vs. 3.1 |
| Tiso, 2010302 Fair | Chronic knee pain (presumed OA of knee) 20 Ibuprofen 4% gel qid vs. ibuprofen 800 mg po tid | Not reported | Stomachache: 0% vs. 10% Constipation: 0% vs. 10% Diarrhea: 0% vs. 10% | Rash: 11% vs. 0% | Dizziness: 11% vs. 20% Headache: 0% vs. 20% |
AST = alanine aminotransferase; CI = confidence interval; CNS = central nervous system; DMSO = Dimethyl sulphoxide; GI = gastrointestinal; NSAID = nonsteroidal anti-inflammatory drug; OA = osteoarthritis; po = orally; qid = four times daily; tid = three times daily
- *
Non-English language study, data as reported in Mason et al305.