NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Forman-Hoffman V, Middleton JC, Feltner C, et al. Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder: A Systematic Review Update [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 May. (Comparative Effectiveness Review, No. 207.)
This section contains findings organized by Key Question (KQ) and grouped by interventions (i.e., by type of psychological treatment or by drug class). For each KQ, we first give the key points and then proceed with a more detailed synthesis of the literature.
KQ 1 addresses the efficacy of psychological treatments and their comparative effectiveness with each other. For each type of psychotherapy, we first address efficacy by evaluating studies with inactive comparison groups (e.g., wait-list or treatment as usual/usual care). We group treatment-as-usual and usual-care comparators together when we synthesize findings and label the combined group “usual care” throughout the report. By the term inactive, we mean comparators that do not involve a specific therapeutic intervention. We then proceed to address comparative effectiveness of a given psychotherapy by evaluating studies with active comparison groups (i.e., head-to-head studies involving other specific psychological interventions).
KQ 2 addresses efficacy of pharmacological treatments and their comparative effectiveness with each other. As with KQ 1, we first address efficacy for each type of pharmacotherapy by evaluating studies with inactive comparators (e.g., placebo). We then proceed to address comparative effectiveness by evaluating head-to-head pharmacological studies (i.e., drug vs. drug).
KQ 3 addresses the direct (head-to-head) evidence on comparative effectiveness of a psychological versus a pharmacological treatment.
KQ 4 synthesizes the evidence on adverse effects (AEs) associated with treatments of interest for adults with posttraumatic stress disorder (PTSD).
In addition, we examine whether either the efficacy or the comparative effectiveness of any studies included in KQ 1, 2, or 3 differs by patient characteristics or type of trauma exposure (KQs 1a, 2a, and 3a, respectively). We required studies described in these sections to compare efficacy or comparative effectiveness between one or more of the subgroups of interest, ideally via interaction analyses. Because of the substantial heterogeneity of the samples, interventions, and comparators of each included study, we did not conduct meta-analysis to obtain pooled estimates of subgroup differences across studies.
To answer this question, we present findings from included studies that reported outcomes for subgroups of interest (defined by patient or trauma factors) and compare the efficacy or comparative effectiveness across subgroups.
Findings discussed in this chapter come from studies rated as having low or medium risk of bias. Evidence tables in Appendix F provide additional information about the characteristics and findings from each study rated as having low or medium risk of bias. We excluded high risk of bias studies and this chapter does not include findings from these studies. Appendix G contains information about these high risk of bias studies that otherwise met all review inclusion criteria.
At the conclusion of this chapter, we include discussion of the evidence found in support of our two contextual questions.
Results of Literature Searches
Results of our searches appear in Figure 2. We included published articles reporting on 193 studies (207 articles). Of the included studies, all were randomized controlled trials (RCTs). We assessed the majority of included studies as medium risk of bias. We assessed eleven studies as low risk of bias. Additional details describing the included studies are provided in the relevant sections of this results chapter.
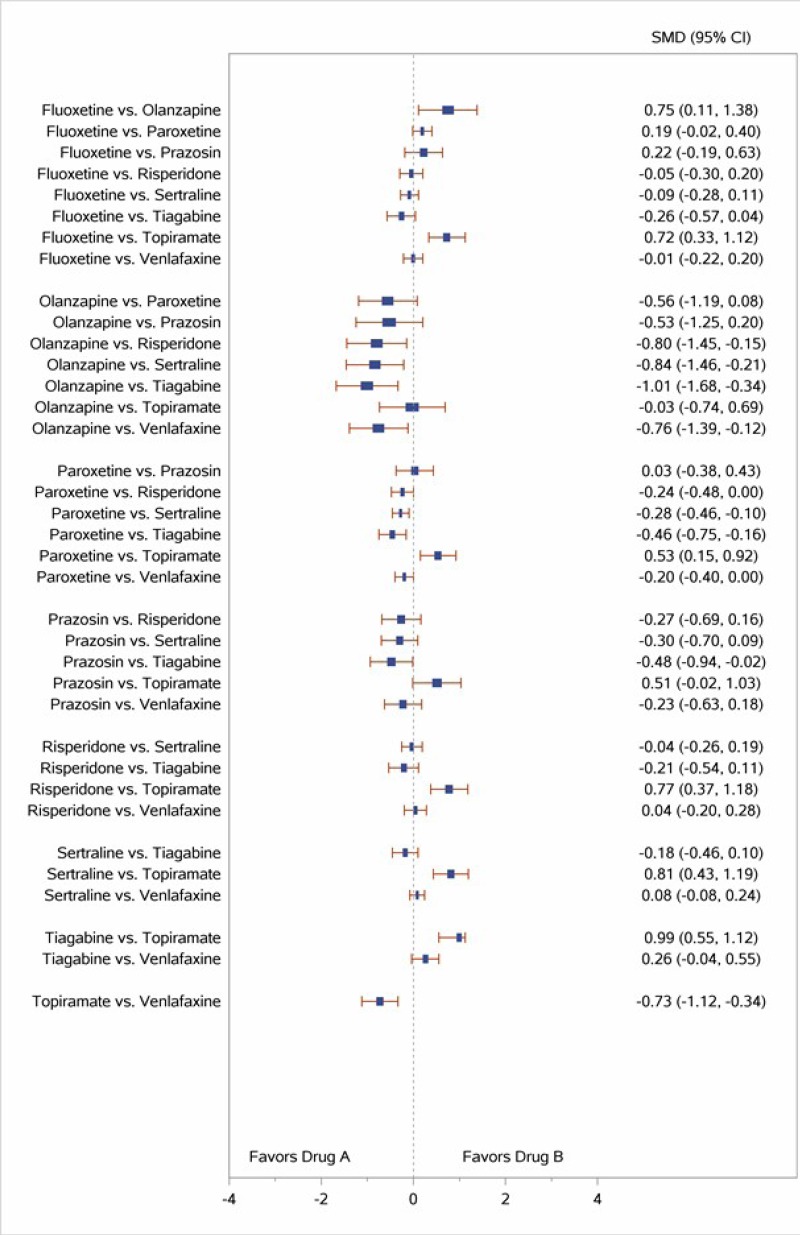
Table 4 describes common outcome measures used in this literature. For further details about these instruments and scales, see Appendix B. Definitive thresholds for clinically significant changes are not well established for many of these measures, although some general guidelines exist (as noted in the table). For continuous outcomes for which a research team combined data from different scales measuring the same construct, a standardized mean difference (SMD) effect size of ~0.5 (a “medium” effect size)118 or higher has been considered a threshold for clinically significant benefit.
KQ 1. Efficacy and Comparative Effectiveness of Different Psychological Treatments
We organized this section by type of psychological treatment and present the information in the following order: (1) cognitive behavioral therapy (CBT)-cognitive interventions; (2) CBT-coping skills; (3) CBT-exposure; (4) CBT-mixed therapies; (5) eye movement desensitization and reprocessing (EMDR); and (6) other psychotherapies, which include Seeking Safety (SS), imagery rehearsal therapy (IRT), interpersonal therapy (IPT), mindfulness-based stress reduction (MBSR), narrative exposure therapy (NET), brief eclectic psychotherapy (BEP), trauma affect regulation (TAR), memory specificity training (MEST), and emotional freedom techniques (EFT). Table 5 presents the organization used to categorize the classes of psychological treatments identified by studies included in this review.
The primary outcomes of interest that investigators used to determine the effectiveness of treatments for adults with PTSD include PTSD symptoms, loss of PTSD diagnosis, and symptom remission, as defined by study authors based on loss of symptoms below a predefined threshold level). We also comment on other outcomes of interest, such as prevention or reduction of coexisting medical or psychiatric conditions (especially depression, anxiety, and substance use problems), quality of life, and disability or functional impairment (to include returning to work or active duty status). The key points are based primarily on meta-analyses. When the lack of available evidence prevented pooling findings via meta-analysis, we relied on qualitative synthesis of findings. Table 6 presents the summary of efficacy and strength of evidence (SOE) of PTSD psychological treatment studies included in this review.
Within each section, we focus first on studies with inactive comparison groups (e.g., wait-list or usual care) to determine whether evidence supports the efficacy of each type of intervention. We then address studies with active comparison groups (i.e., head-to-head comparative evidence), or we provide cross-references for where those studies are addressed. In some cases, the active comparator was not an intervention for which we intended to assess the comparative effectiveness with an included treatment type (e.g., present-centered therapy [PCT] or patient education).
Tables describing characteristics of included studies are organized similarly. For most sections, we first provide details on studies that use any inactive comparators (in alphabetical order by last name of the first author) (i.e., those about efficacy) and then the details on any additional studies that included only active comparators.
In the bulleted text below, we summarize the main overall key points and then the key points for each type of psychotherapy. We also report grades for the SOE, where appropriate, which we determined after considering the evidence base of studies we had assessed as either low or medium risk of bias. For continuous outcomes such as PTSD, depression, and anxiety symptoms or ratings of quality of life or functioning, we present the between-group mean difference for single studies or the SMD when describing more than one study to indicate the between-group difference in pre- to posttreatment or pre- to followup assessments. For dichotomous outcomes like remission and loss of PTSD diagnosis, we report the risk difference (RD) between groups.
For outcomes with evidence from three or more studies with low heterogeneity across studies or five or more studies testing the same intervention types, we present the pooled estimate from meta-analysis and the 95 percent confidence interval (CI). When we determined that three or four studies had substantial heterogeneity in sample, intervention, or study characteristics or two or fewer studies testing the same intervention presented data for an outcome, we qualitatively synthesized the findings and present findings from the individual studies.
All included studies are cited in the detailed synthesis section and related tables and figures presented for each treatment. Section headings within each detailed synthesis section include each outcome reported by at least one included study of that treatment type. If an outcome does not appear in the section, no included study testing the intervention of interest reported data on it.
Appendices contain additional information about the risk of bias assessments (Appendix E), individual study characteristics and findings for each outcome presented in evidence tables (Appendix F), characteristics and consistency of findings of high risk of bias studies not synthesized in the text (Appendix G), forest plots depicting individual and pooled study findings (Appendix H), and detailed information about each component contributing to the SOE grade (Appendix I).
Key Points: Overall—Efficacy of Psychological Treatments
- For PTSD symptoms reduction, CBT-exposure and CBT-mixed therapies provide high SOE of efficacy. Cognitive processing therapy (CPT), cognitive therapy (CT), EMDR, and NET provide moderate SOE of efficacy; TAR and IRT provide low evidence of efficacy.
- Low SOE supports no difference in efficacy of SS.
- For loss of PTSD diagnosis, CBT-exposure and CBT-mixed therapies provide high SOE of efficacy; CPT, CT, and EMDR provide moderate SOE of efficacy; NET and BEP provide low evidence of efficacy.
- Studies provide insufficient evidence of differences in efficacy by subgroups of interest defined by patient characteristics or type of trauma.
Key Points: Overall—Comparative Effectiveness of Psychological Treatments
- Few studies have tested comparative effectiveness of psychological interventions, precluding the use of meta-analysis to pool estimates.
- Moderate SOE favors CBT-exposure over relaxation for reduction in PTSD symptoms, loss of PTSD diagnosis, and reduction in depression symptoms.
- Low SOE favors greater reduction in PTSD symptoms for CBT mixed (CBT-M) over relaxation therapy.
- Low SOE for no difference in effectiveness for reduction in PTSD symptoms for CBT-exposure versus EMDR and for reduction in depression symptoms for CBT-exposure versus CBT-exposure+cognitive restructuring (CR)
Key Points: CBT—Cognitive Interventions
- Moderate SOE supports the efficacy of CPT and CT for reduction in PTSD symptoms, loss of PTSD diagnosis, and reduction in depression symptoms
- Moderate SOE supports the efficacy of CT for reduction in anxiety symptoms and reduction in disability.
Key Points: CBT—Coping Skills
- Insufficient evidence exists to determine the efficacy of relaxation, stress inoculation training (SIT), and structured approach therapy (SAT), with single studies testing each intervention.
- Moderate SOE supports the effectiveness of CBT-exposure compared with relaxation for reduction in PTSD symptoms, loss of PTSD diagnosis, and reduction in depression symptoms
- Low SOE supports the effectiveness of CBT-mixed compared with relaxation for reduction in PTSD symptoms.
Key Points: CBT—Exposure
- High SOE supports the efficacy of CBT-exposur etherapy for reduction in PTSD symptoms, loss of PTSD diagnosis, and reduction in depression symptoms, and low SOE for anxiety symptoms.
- Moderate SOE provides comparative effectiveness of CBT-exposure compared with relaxation for reduction in PTSD symptoms and loss of PTSD diagnosis, and low SOE favors CBT-exposure over relaxation for reduction in depression symptoms.
- Low SOE shows no difference in effectiveness between CBT-exposure and EMDR for reduction in PTSD symptoms and between CBT-exposure and CBT-exposure+CR for reduction in depression symptoms.
Key Points: CBT—Mixed
- High SOE supports the efficacy of CBT-mixed for reduction in PTSD symptoms, loss of PTSD diagnosis, and reduction in depression symptoms.
- Moderate SOE supports the efficacy of CBT-mixed for reduction in anxiety symptoms and reduction in substance use issues.
- Low SOE supports the efficacy of CBT-mixed for reduction in disability/functional impairment.
- Low SOE supports the comparative effectiveness of CBT-mixed over relaxation for reduction in PTSD symptoms.
Key Points: EMDR
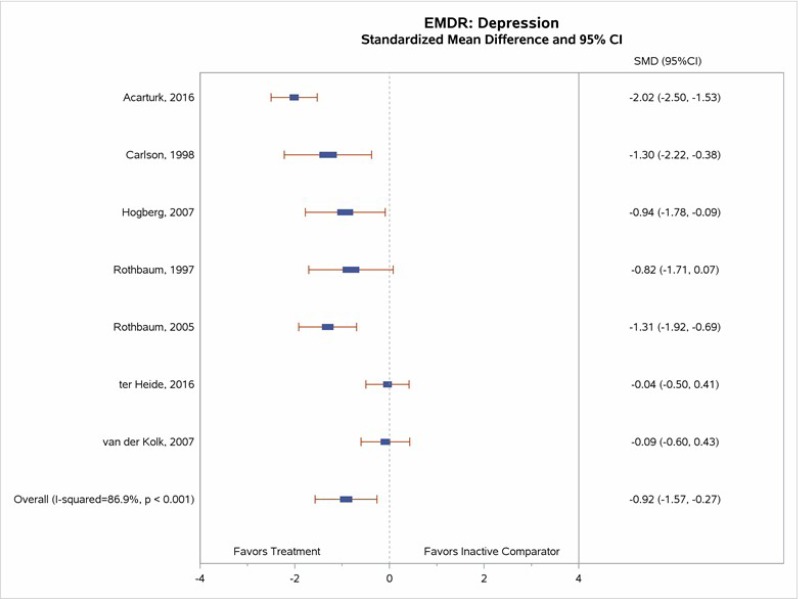
- Moderate SOE supports the efficacy of EMDR for reduction in PTSD symptoms, loss of diagnosis, and reduction in depressive symptoms.
- Low SOE shows no difference in effectiveness for reduction in PTSD symptoms between EMDR and CBT-exposure.
Key Points: Other Psychological Interventions
- Low SOE supports the efficacy of TAR for reduction in PTSD symptoms.
- Low SOE supports the efficacy of BEP for loss of PTSD diagnosis, reduction in depression symptoms, and reduction in anxiety symptoms.
- Low SOE supports efficacy of IRT for reduction in PTSD symptoms.
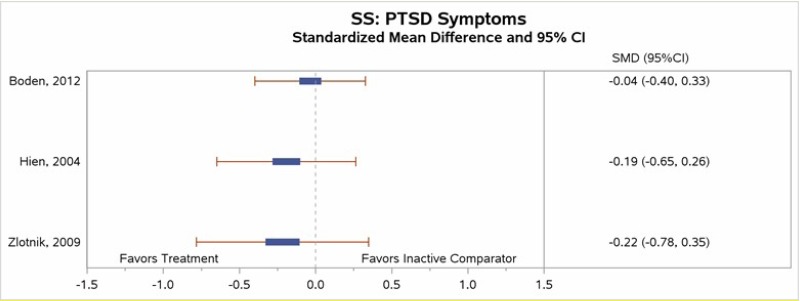
- Moderate SOE supports the efficacy of NET for reduction in PTSD symptoms and low SOE supports its efficacy for loss of PTSD diagnosis. Low SOE supports no difference of SS versus inactive comparator on reduction in PTSD symptoms.
Detailed Synthesis: CBT—Cognitive Interventions
Characteristics of Studies
Table 7 summarizes the characteristics of the 14 cognitive intervention studies that met our inclusion criteria. We divided the 14 cognitive interventions further into CPT (7 studies), CR (1 study), CT (5 studies), and meta cognitive therapy (MCT) (1 study).
Of the seven CPT interventions, four had wait-list comparators,1–3, 6 one had a usual-care comparator,4 and three had active intervention comparators (prolonged exposure [PE],3 MEST,124 and group PCT).127 The single CR study had three active comparators: relaxation, PE, and a combined CR and PE.122 Five studies tested CT: three had wait-list comparators,5, 8, 9 one had a usual care comparator,7 and two had active comparators9 (self-help booklet5 and imaginal exposure129). The single MCT study had one active comparator, PE, and one inactive comparator, wait-list.19 Further details describing the included studies are provided in Appendix F.
Of the seven CPT studies, sample sizes ranged from 16 to 171. Duration of treatment ranged from 6 to 17 weeks. Five studies included at least one posttreatment followup assessment after 1 to 12 months,1–4, 124 one study reported followup data but had a cross-over design affecting time period comparisons across groups,6 and one study reported outcomes at 5 to 10 years followup.126 Three studies enrolled all or a majority of females with sexual abuse or assault trauma types,2, 3, 6 and three studies enrolled all or a majority of males with combat-related trauma types.1, 4, 127 The mean age of participants in the CPT studies ranged from 32 to 54 years. The primary outcome measures for these studies were the Clinician-Administered PTSD Scale (CAPS), PTSD Checklist (PCL), Modified PTSD Symptom Scale (MPSS), and PTSD Symptom Scale (PSS).
The single CR study that contained three active comparators included males and females with exposure to mixed trauma types.122 Participants completed 10 sessions over a mean of 16 weeks and had followup assessments at 1, 3, and 5 months posttreatment. The primary outcome measure was the Impact of Event Scale (IES).
Of the five CT studies, sample sizes ranged from 28 to 121. Duration of treatment ranged from about 3 to 5 months. Although one study did not include a followup assessment after posttreatment,7 the other four studies included at least one followup period 6 to 12 months posttreatment. All four studies with inactive comparators enrolled a majority of female participants;5, 7–9 two of these also had active comparator arms.5, 9 The single study that compared CT with imaginal exposure (IE)129 had similar proportions of male and female participants. One study included those with motor vehicle accident (MVA) trauma types;5 the other study participants had mixed types of trauma exposures. The mean age of participants in CT studies ranged from 37 to 44. All studies used the CAPS as the primary outcome of interest.
The single MCT study that had one active comparator (PE) and one inactive comparator (wait-list) included males and females with exposure to mixed trauma types. The single CR study that contained three active comparators included males and females with exposure to mixed trauma types.122 Participants completed eight sessions and did not include any followup assessments after the end of treatment. The primary outcome measure was the Posttraumatic Diagnostic Scale (PDS).
Results for Cognitive Interventions Compared With Inactive Comparators
Under each outcome heading below, we first present our data synthesis for studies testing CPT against an inactive comparator. Then we present results for the CR study, CT studies, and MCT study with inactive comparator groups.
PTSD Symptoms
All included studies reported measures of PTSD symptoms. All five studies comparing CPT with inactive comparators found that subjects in the CPT arm had a greater reduction in CAPS-assessed symptoms of PTSD than those in the inactive comparator arm.1–4, 6 The meta-analysis that pooled CAPS scores (Figure 3) found a much greater decrease in PTSD symptoms for subjects treated with CPT therapy than for those in inactive comparator groups (SMD, −1.35; 95% CI, −1.77 to −0.94, I2=71.1%, 5 studies, N=399, moderate SOE). The meta-analysis had considerable statistical heterogeneity, but the direction of effects was consistent. The differences were only in the exact magnitude of benefit; all studies found moderate or large magnitudes of benefit. In addition, two of three studies that compared CPT with a wait-list group found that changes were maintained at 3 to 6 months (posttreatment) followup.2, 3
All four studies that compared CT with inactive control groups reported significantly greater decreases in PTSD symptoms for those treated with CT than those in inactive comparator groups (meta-analysis not performed because of heterogeneity in sample and study characteristics, SMD of individual studies ranged from −2.0 to −0.3; 4 studies; N=236; moderate SOE).5, 7–9 The single study that compared MCT with an inactive comparator reported significantly greater decreases in PDS-measured PTSD symptoms, favoring MCT, as measured by the PDS (between-group mean difference=−27.7, 1 study, N=21; insufficient SOE).19
Loss of PTSD Diagnosis
Several cognitive intervention studies reported data on posttreatment diagnostic status. The four CPT studies that reported loss of diagnosis outcomes favored CPT over inactive comparator (risk difference [RD], 0.44; 95% confidence interval [CI], 0.26 to 0.62; I2, 77.9%; 4 studies, N=299; moderate SOE)1–4 (Figure 4). All four studies comparing CPT with wait-list reported followup assessments showing that, over time, the greater changes seen in loss of PTSD diagnosis among CPT participants than inactive comparator participants persisted at 1 month,1 12 months,2, 3 and 5 to 10 years after the end of treatment.126
Three5, 8, 9 of the four studies5, 7–9 that compared loss of PTSD diagnosis between CT and inactive comparators found significantly higher rates of loss of PTSD diagnosis at posttreatment among those who received the CT intervention as compared with those who received an inactive comparator (RD, 0.55; 95% CI, 0.28 to 0.82; I2, 88.5%; 4 studies, N=314; moderate SOE) (Figure 5).
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
All but one127 cognitive intervention study assessed coexisting depressive symptoms using the Beck Depression Inventory (BDI) or BDI-II, and some also assessed anxiety symptoms.1–6 Our meta-analysis of the five CPT studies reporting BDI or BDI-II scores (Figure 6) found greater improvement in depression symptoms for subjects treated with CPT than for those in inactive comparison groups (SMD, −1.09; 95% CI, −1.52 to −0.65, I2=75.0, 5 studies, N=399, moderate SOE).1–4, 6
In three studies that included followup assessments of depressive symptoms, subjects maintained significant decreases in symptoms at 3 months3–5 and 9 months3 after the end of treatment assessment. In another study, authors found a pre- to posttreatment depressive symptom effect size of 1.16 for CPT versus inactive comparator group, which declined to 0.49 at the 1-month followup.1 The authors attributed the attenuation of between-group effect size differences to decreases in depressive scores in the wait-list group at followup, not to increases in depressive symptom scores in the CPT group. From the above findings and our meta-analysis, we concluded that evidence supports the efficacy of CPT for reducing depression symptoms (moderate SOE).
Two studies of CPT assessed anxiety symptoms as an outcome using the State-Trait Anxiety Inventory (STAI).1, 4 One found CPT to be no more effective in reducing symptoms of anxiety than wait-list;1 the other found greater improvement in anxiety for subjects treated with CPT than those receiving usual treatment from intake to posttreatment (p=0.018).4, 5 We concluded insufficient evidence to determine the efficacy of CPT for reducing anxiety symptoms based on lack of consistency and imprecise findings of two studies.
All four studies that compared CT with inactive control groups assessed depressive and anxiety symptoms;5, 7–9 four of four studies found significant between-group differences in depression symptoms and three of four studies found significant between-group differences at posttreatment favoring the CT group (moderate SOE). In two of the studies, between-group differences favoring the CT group persisted at the 3- and 9-month followup assessments (p<0.001 for all comparisons).5, 7, 9
The single MCT study reported significantly greater decreases in both depressive symptoms assessed with the BDI and anxiety symptoms assessed with the Beck Anxiety Inventory (BAI) among MCT group participants than inactive comparator participants (insufficient SOE).19
Quality of Life
Two studies compared quality-of-life outcomes between CPT and inactive comparator groups.4, 6 One study4 reported significant time by condition interactions for social quality-of-life measures but not for physical quality-of-life measures.4 The other study found that CPT participants had significantly greater changes in quality-of-life ratings at posttreatment than those in the inactive comparator group (insufficient SOE).6
Two studies compared CT and inactive comparator groups on quality-of-life outcomes. One study of adults with PTSD and serious mental illness found that the CT group subjects had7 better quality-of-life outcomes than the usual-care group for the physical quality-of-life measures (p=0.002) but not for mental quality-of-life measures (p=0.13). The other CT study with quality-of-life outcomes reported significant improvements among those enrolled in the CT groups as compared with the inactive comparator groups (insufficient SOE).9
Disability or Functional Impairment
Three studies that compared CT to an inactive comparator assessed disability using the Sheehan Disability Scale.5, 8, 9 All studies reported significant improvements in disability among CT group participants as compared with inactive control participants at posttreatment and followup assessments (moderate SOE).
Results for Cognitive Interventions Compared With Active Comparators
Two studies tested cognitive interventions with a comparator for which we did not aim to assess comparative effectiveness (CPT versus PCT9, 127 and CT versus self-help booklet5).
Two studies compared CPT with exposure therapy,3, 122 one study compared CT with IE,129, 130 and one study compared MCT with PE.19 Assessment of these comparative effectiveness studies appears in the CBT-Exposure section below.
One study compared CPT with MEST.124 Comparative effectiveness findings appear in the Other Psychological Interventions section below.
One study compared CR with a relaxation group and a combination of PE and CR.122 The CR versus relaxation comparisons appear in the CBT-Coping Skills section (below). The authors did not report data on the comparative effectiveness of CR and the combination of PE and CR.
Detailed Synthesis: CBT—Coping Skills
Characteristics of Studies
Table 8 summarizes the characteristics of the six studies meeting our inclusion criteria.14, 46, 122, 131–133 Further details describing the included studies are provided in Appendix F.
The studies in this section had a “coping skills” arm(s)—either relaxation training, SIT, or SAT. SIT is a cognitive behavioral intervention for PTSD in which the basic goal is to help subjects gain confidence in their ability to cope with anxiety and fear stemming from trauma-related reminders. In SIT, the therapist helps patients increase their awareness of trauma-related cues for fear and anxiety. In addition, clients learn a variety of coping skills that are useful in managing anxiety, such as muscle relaxing and deep breathing. SAT contains components of SIT to include psychoeducation, skills training, and an application phase to practice new coping skills.
Two of the six studies compared coping skills interventions with inactive comparators.14, 46, 122, 131–133 One compared PE, SIT, combined PE+SIT, and a wait-list group,14 and the other compared relaxation, EMDR, and usual care.46 One enrolled women who were victims of sexual or nonsexual assault,14 and the other study enrolled combat veterans with mixed trauma types.46 Duration of treatment ranged from 6 to 9 weeks, and both had multiple followup assessments up to 9 months after the end of treatment. The primary outcome measure for one study was the PSSI;14 and the other used the CAPS as the primary outcome measure.46
All six studies made comparisons with active psychotherapy interventions. Sample sizes ranged from 35 to 110. Duration of treatment ranged from 6 to 16 weeks. All but one study132 included at least one followup assessment. Two studies enrolled combat veterans;46, 131, 136 one enrolled victims of sexual and nonsexual assault;14 and the other three studies enrolled heterogeneous groups of subjects with a variety of index trauma types (e.g., physical assault, road accidents, nonroad accident, witnessing a trauma or homicide, sexual assault, being held hostage, bombing, combat). Mean age for subjects in the studies ranged from 35 to 48.5. Whereas three studies had over 75 percent female participants,14, 132, 133 two studies had samples comprising at least 75 percent males.46, 131 The primary outcome for nearly all studies was the CAPS; one study used the PSS-I.14
Results for Coping Skills Compared With Inactive Comparators
PTSD Symptoms
Both studies that compared a coping skills intervention with inactive comparators reported measures of PTSD symptoms (Table 9).14, 46 One small study found significantly greater decreases in PTSD symptoms at posttreatment among participants in the SIT versus wait-list group.14 The other study found a greater, but nonstatistically significant reduction in PTSD symptoms in the relaxation arm as compared with the treatment-as-usual arm (insufficient SOE).46
Loss of PTSD Diagnosis
One small study reported loss of diagnosis data across treatment groups; comparisons favored SIT (RD, 0.42, p<0.001; insufficient SOE).14
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
Two studies reported on coexisting comorbid depression symptoms and comorbid anxiety symptoms (Table 10).14, 46 The study that included SIT and wait-list arms found that subjects treated with SIT had greater decreases in depression symptoms than those in the wait-list group; between-group differences in pre- to posttreatment changes in anxiety symptoms did not reach statistical significance (insufficient SOE).14
The study comparing relaxation and usual care found decreases in both depression and anxiety symptoms in the relaxation group; however, the authors reported no statistically significant between-group difference on measures of anxiety and did not provide data on between-group differences for depression (insufficient SOE).46
Results for Coping Skills Compared With Active Comparators
Of the six studies comparing a coping skills therapy with an active comparator, four included comparisons with exposure-based interventions,14, 122, 132, 133 two included comparisons with EMDR,46, 133 two included comparisons with CBT-mixed therapies,14, 122 one included a comparison with CR,122 one included a comparison with IPT,132 and one compared to an active control condition for which we did not aim to assess comparative effectiveness (PTSD family education [PFE]).131 For assessment of the comparisons with exposure-based therapies, see the CBT—Exposure section (below). For assessment of the comparisons with CBT-mixed therapies, see the CBT—Mixed section (below). For assessment of the comparisons with EMDR, see the EMDR section (below). For assessment of the comparisons with IPT, see the Other Psychological Interventions section (below).
Results for Coping Skills Compared With Active Comparators: Relaxation Training Versus Cognitive Restructuring
One study assessed the comparative effectiveness of four PTSD treatments; subjects in one arm received relaxation training and subjects in another arm received CR (the other two treatment arms tested CBT-exposure interventions; the CBT-exposure section details the findings from these comparisons).122
PTSD Symptoms
The study that enabled comparisons between relaxation training and CR found no significant between-group differences in the percentage of subjects who experienced a 50 percent pre- to posttreatment decrease in PTSD symptoms as assessed by the PSS (insufficient SOE).122
Loss of PTSD Diagnosis
More subjects in the CR group experienced a loss of PTSD diagnosis than those in the relaxation training group; the difference was not statistically significant (RD=−0.05 favoring CR, p=ns; insufficient SOE).122
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
CR group subjects had greater depressive symptoms decreases than relaxation training group subjects, but not significantly so (RD=0.10, p=ns; insufficient SOE).122
Detailed Synthesis: CBT—Exposure
Characteristics of Studies
Table 11 summarizes the characteristics of the 25 studies meeting our inclusion criteria. Types of exposure therapy tested included IE, in vivo exposure, PE (which includes both components of IE and in vivo exposure, or modified PE, mPE), virtual reality exposure (VRE), written exposure therapy (WRE), and Concurrent Treatment of PTSD and Substance Use Disorders using Prolonged Exposure (COPE). Further details are provided in Appendix F. Of the 25 included studies, 14 studies (15 articles because one study reported similar outcomes across two separate publications) compared exposure therapy with an inactive comparator: wait-list,3, 11–14, 16–19, 21, 122, 137 or usual care,10, 15, 20, 41, 138, 139 and 17 included one or more active comparators.3, 12–14, 16, 19, 41, 42, 122, 129, 132, 133, 138–140
These studies generally enrolled subjects with severe or extreme PTSD symptoms.10, 15, 19, 20, 41, 122, 133 Sample sizes ranged from 24 to 284. Four studies (one of which presented outcomes in 2 articles) assessed followup at the end of active treatment;19–21, 132, 137 the remainder included posttreatment followups after 3, 4½, 6, 9, or 12 months. Fourteen of the studies enrolled a heterogeneous group of subjects with a variety of index trauma types (e.g., accident, disaster, physical assault, sexual assault, witnessing death or serious injury), 4 studies enrolled a majority of subjects with sexual assault-related PTSD,3, 12–14 4 enrolled subjects with combat-related PTSD,15, 18, 139, 141 1 enrolled subjects with combat- or terror-related PTSD,15 1 enrolled natural disaster victims,11 and one enrolled patients involved in an MVA.17 One involved patients with both a psychotic disorder and PTSD.16 Mean age ranged from 27 to 63. Ten studies enrolled two-thirds or more female subjects. The primary outcome for the majority of studies was some version of the CAPS (CAPS, CAPS-2, or CAPS-Sx); 4 studies identified the PSS-I as the primary outcome measure;12, 14, 15, 142 and 1 study used the PDS.19
Results for Exposure Therapy Compared With Inactive Comparators
PTSD Symptoms
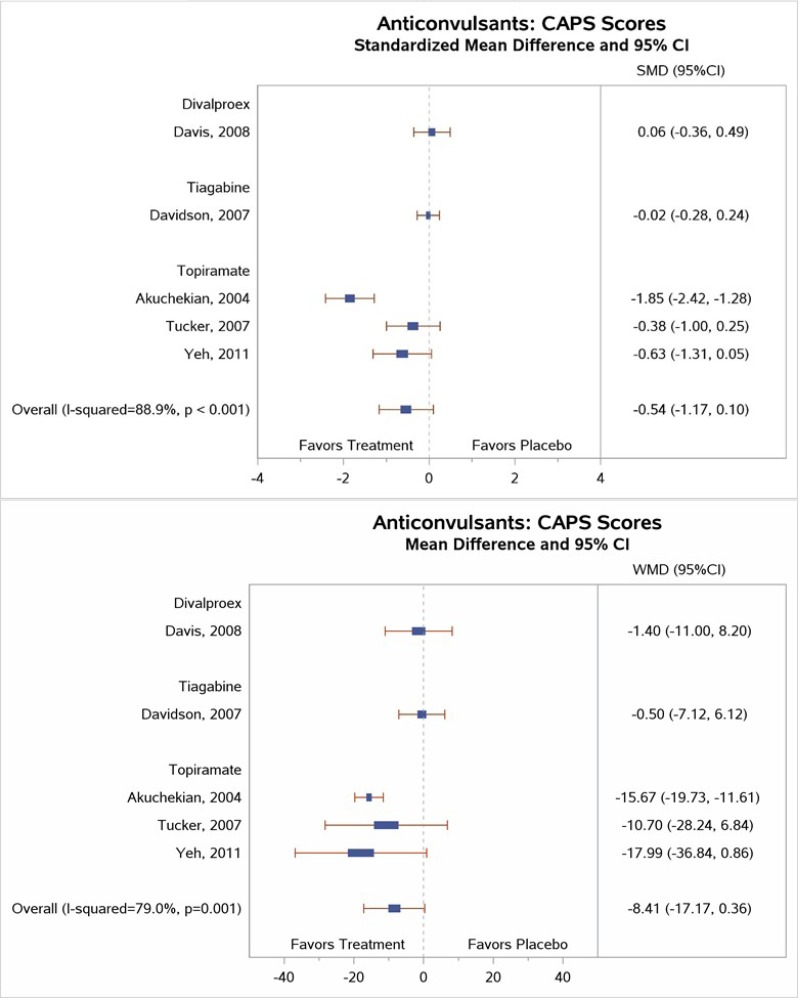
Thirteen of the 14 studies (data for 1 study reported in 2 articles) comparing various exposure therapies with an inactive comparator reported measures of PTSD symptom change; one study compared each of two exposure therapies (VRE and PE) to placebo,18 allowing 14 comparisons. All 13 studies reported outcomes in 14 publications reported greater decreases in PTSD symptoms (outcome measures included CAPS, PSS-I, and PDS) in the exposure group than in the control group.3, 10–21, 137 Our meta-analysis of pooled data from these studies (Figure 7) found a greater decrease in PTSD symptoms for subjects treated with exposure than for those in control groups; the effect size was very large (SMD, −1.23; 95% CI, −1.50 to −0.97, 13 studies [14 comparisons]; N=885; I-squared, 67.5%; high SOE).
Our meta-analysis of the studies reporting CAPS scores found a greater decrease in PTSD symptoms among subjects treated with exposure than those in an inactive comparator group (SMD, −1.12; 95% CI, −1.42 to −0.82; 8 studies [9 comparisons], N=689; I-squared=68.66%; high SOE) (Figure 8).
Among those studies that assessed followup measures longer-term, the effects for decreases in PTSD symptoms were maintained at 3, 6, 9, or 12 months.
Loss of PTSD Diagnosis
Six of the studies comparing subjects who received exposure therapy with those in inactive comparator groups reported loss of PTSD diagnosis between groups. Participants treated with exposure therapy had greater rates of loss of PTSD diagnosis as compared with participants in the inactive comparator groups (RD, 0.56; 95% CI, 0.35 to 0.78; 6 studies, N=409; high SOE) (Figure 9).
Prevention or Reduction of Comorbid Conditions
Ten studies (from 11 publications and involving 11 comparisons) with inactive comparators reported on changes in depression symptoms as measured by the BDI.3, 11–15, 18–21, 137 All but 120 reported a significantly greater decrease in depression symptoms for exposure intervention patients than for inactive comparators. Results of our meta-analysis indicated a greater reduction in BDI depressive symptom scores for subjects treated with exposure than for those in wait-list or usual-care inactive comparator groups (SMD, −0.76; 95% CI, −0.91 to −0.60; I2=19.4%, 10 studies [11 comparisons], N=7,152, Figure 10; high SOE).
Three studies reported on anxiety symptoms, two using the Beck Anxiety Inventory18, 19 and one using the State Trait Anxiety Inventory.20 The two using the Beck Anxiety Inventory indicated significant benefit for exposure therapy, while the study using the State Trait Anxiety Inventory (which involved patients with comorbid substance dependence) did not show significant benefit (low SOE).
Disability or Functional Impairment
Three studies compared functional impairment across groups using different scales (insufficient SOE). One study that compared in vivo exposure with wait-list included a measure of work and social adjustment and found significantly greater decreases in functional impairment in the in vivo group than the inactive comparator group.11 Another study examined differences in four subscales of the World Health Organization Quality of Life brief version (WHOQOLBREF) and found significantly greater increases in the physical health and psychological health (but not social relationships or environmental) subscales among those in the PE group than those in the wait-list control group.21 A third study that compared PE, PE+CR, and wait-list that included the Social Adjustment Scale found greater, but not statistically significant, increases among the PE group subjects than the inactive comparator subjects (see Appendix F for details).12
Results for Exposure Therapy Compared With Active Comparators
The 17 studies that compared exposure therapy with an active comparator included comparisons with EMDR,13, 16, 133 a coping skills intervention (relaxation training 122, 132, 133 or SIT14), a cognitive intervention (CPT, CR, CT, or MCT),3, 19, 122, 129 IPT,132 PE+CR,12, 42, 122 IE+CR,41 PE plus SIT,14 IRT142 or another type of active control (PCT,138, 139 relapse prevention,143 active monitoring comparison group [AMCG],143 supportive counseling [SC],41 or a health information–based active control).140
In this section, we address the 17 studies comparing CBT-exposure therapy with an active comparator. We do not report on comparisons between exposure therapy arms and interventions for which we did not aim to assess comparative effectiveness (i.e., comparisons between different types of exposure interventions39, 40, 144–148 or between exposure interventions and PCT, SC, relapse prevention, AMCG, or health information comparators).3, 12–14, 16, 18, 19, 41, 42, 122, 129, 132, 133, 140, 143
Results for Exposure Therapy Compared With Active Comparators: Exposure Therapy Versus Cognitive Interventions
Four studies compared exposure therapy and either CPT, CR, CT, or MCT.3, 19, 122, 129 Of these, one compared PE with CR,122 one compared IE with CT,129 one compared PE with CPT,3 and one compared PE with MCT.19 We did not perform quantitative meta-analysis to pool the findings because each intervention and comparator were different across each of the 4 studies.
PTSD Symptoms
The results from each different cognitive intervention-exposure intervention comparison found no significant differences in pre- to posttreatment PTSD symptom changes between groups;3, 122, 129 results from one smaller study (11 patients per group) suggested greater benefit from MCT than PE (p=0.05; insufficient SOE).19
Loss of PTSD Diagnosis
Three studies testing different exposure interventions reported data on loss of PTSD diagnosis between exposure and cognitive intervention groups.3, 122, 129 Two studies favored exposure (RD range 0.08 to 0.16), but differences were not significant;122, 129 one found a zero RD between groups (insufficient SOE).3
Prevention or Reduction of Comorbid Conditions
All four studies used the BDI to measure change in depression symptom scores.3, 19, 122, 129 Although point estimates favored CT and CPT over the exposure arms, no study found a statistically significant difference between the interventions (insufficient SOE).
Two studies compared anxiety symptoms between groups (insufficient SOE). One study found no significant differences between IE and CT groups at the end of treatment or 12-month followup assessments on BAI-assessed anxiety symptoms.129 A second study also used the BAI to compare PE with MCT19 and also found no statistically significant between-group differences (insufficient SOE).
Return to Work or Active Duty
One study of CT and IE reported the impact of interventions on return-to-work outcomes.129 At 6 months followup, the difference in percentage working between treatment groups did not reach statistical significance (RD, 0.07; insufficient SOE).
Results for Exposure Therapy Compared With Active Comparators: Exposure Therapy Versus Coping Skills Therapies
Four studies compared exposure therapy with a coping skills therapy.14, 122, 132, 133 One compared PE with SIT,14 and the others compared PE with relaxation therapy.
PTSD Symptoms
All four studies compared PTSD symptom changes from pre- to posttreatment between exposure therapy and coping skills intervention groups.14, 122, 132, 133 The results of our meta-analyses indicated that PE had greater decreases in PTSD symptoms than relaxation (SMD, -0.45; 95% CI, -0.78 to -0.13; 3 studies, N=155, moderate SOE). The study comparing PE with SIT found no significant between-group differences in decreases in PTSD symptoms at the end of treatment assessment (between-group mean difference, -1.8 favoring PE, insufficient SOE).14
Loss of PTSD Diagnosis
Two studies compared loss of PTSD diagnosis between CBT-exposure and relaxation group participants.14, 122, 133 In each study, a greater proportion of subjects treated with CBT-exposure had loss of PTSD diagnosis at the end of treatment than subjects receiving each of the coping skills interventions (RD range 0.20 to 0.47 favoring CBT-exposure; moderate SOE).
The single study that compared loss of PTSD diagnosis between PE and SIT group subjects found no statistically significant difference between the two intervention groups (RD, 0.18; p=ns; insufficient SOE).14
Prevention or Reduction of Comorbid Conditions
Four studies compared CBT-exposure and CBT-coping interventions (relaxation or SIT) using BDI-related measures14, 122, 133 or the Hamilton Depression Rating Scale (HAM-D) to assess depression symptoms.132 The single study comparing exposure with SIT found no difference in depression symptom changes between treatments at the end of treatment (insufficient SOE).14
The meta-analysis of the three studies that compared CBT-exposure (PE) with relaxation therapy,122, 132, 133 found that PE had greater decreases in depressive symptoms than relaxation (SMD, -0.39; 95% CI, -0.71 to -0.07; 3 studies, N=155, moderate SOE).
Results for Exposure Therapy Compared With Active Comparators: Exposure Therapy Compared With Eye Movement Desensitization and Reprocessing
PTSD Symptoms
All three studies13, 16, 133 that compared PE with EMDR found no statistically significant difference in pre- to posttreatment PTSD symptom changes between EMDR and PE intervention groups (meta-analysis not performed for any of the PE versus EMDR pooled findings because of substantial heterogeneity in intervention characteristics and sample characteristics, low SOE for no difference).
Loss of PTSD Diagnosis
Three studies compared the effectiveness of PE versus EMDR on loss of PTSD diagnosis with different results (insufficient SOE). In two studies, more participants in the PE group than in the EMDR group lost their PTSD diagnosis at posttreatment, but differences were not statistically significant (RD range 0.20 to 0.28 across 2 studies, p=ns13, 133). In contrast, in another study, slightly fewer participants in the PE group lost their PTSD diagnosis than in the EMDR group (RD=0.03, p=ns).16
Results for Exposure Therapy Compared With Active Comparators: Exposure Therapy Versus Exposure Plus Cognitive Restructuring
Four studies compared exposure therapy with exposure+CR.12, 41, 42, 122 Three tested PE against PE+CR,12, 42, 122 whereas the other tested IE against IE+CR.41
PTSD Symptoms
Two studies found no difference between subjects treated with exposure and those treated with PE+CR on measures of PTSD symptoms (insufficient SOE).12, 122 Another study found no significant difference at the end of treatment but an advantage for IE plus CR at posttreatment followup (insufficient SOE).41 Finally, one study found that exposure plus CR led to significantly greater decreases in PTSD symptoms at the end of treatment as compared with exposure alone (insufficient SOE).42
Loss of PTSD Diagnosis
Three of these four studies reported data on loss of PTSD diagnosis between groups at posttreatment.41, 42, 122 One study favored the PE group,122 and the other two favored the combined PE+CR group (meta-analysis not performed for exposure versus exposure plus CR findings because of heterogeneity in intervention and sample characteristics, insufficient SOE).41, 42
Prevention or Reduction of Comorbid Conditions
All four studies used the BDI to assess depression symptoms. Each found no statistically significant difference between interventions from baseline to the end of treatment (low SOE for no difference).
Results for Exposure Therapy Compared With Active Comparators: Prolonged Exposure Versus Interpersonal Psychotherapy
As noted above, one study compared PE (N=38), IPT (N=40), and relaxation (N=32).132 We previously compared exposure and relaxation; here, we report the PE versus IPT comparison (insufficient SOE for each outcome). Both types of interventions led to substantial decreases in PTSD symptoms at posttreatment, but the authors found no significant between-group differences. In addition, the proportions of subjects who entered remission did not differ (RD=0.03); the groups also did not differ with respect to decreases in depressive symptoms as measured by the HAM-D or changes in quality-of-life ratings as measured by the Quality of Life Enjoyment and Satisfaction scale.132 We concluded that evidence is insufficient to determine the comparative effectiveness of PE versus IPT for PTSD symptoms, remission, depressive symptoms, and quality of life based on this single study.
Results for Exposure Therapy Compared With Active Comparators: Prolonged Exposure Versus Imagery Rehearsal Therapy
One study compared PE and IRT.142 Significant differences were not found for decreases in PTSD symptoms, percentage recovering or with symptom improvement, or decreases in depression symptoms or psychological symptom severity between treatment groups. The treatment* time interaction for the psychological subscale of the quality of life assessment scale approached significance (p=0.05), with the PE group participants having slightly greater improvements at posttreatment that evened out at the 3-month followup assessment. We concluded that evidence is insufficient to determine the comparative effectiveness of PE versus IRT for PTSD symptoms, remission, depressive symptoms, and quality of life based on this single study.
Detailed Synthesis: CBT—Mixed Interventions
Characteristics of Studies
Table 12 summarizes the characteristics of the 31 studies meeting our inclusion criteria. Further details about these studies appear in Appendix D. The studies in this section are somewhat heterogeneous in several ways: how authors define and describe “cognitive behavioral therapy,” duration of the intervention, and mode of delivery. Elements of the CBT arm of the studies considered here include psychoeducation, self-monitoring, stress management, relaxation training, skills training, exposure (imaginal, in vivo, or both), cognitive restructuring, guided imagery, mindfulness training, breathing retraining, crisis/safety planning, and relapse prevention. The studies varied as to how many sessions (if any) were dedicated to these elements and whether homework was assigned as part of the intervention.
Twenty-three of these 31 studies included an inactive comparator, such as a wait-list (16 studies), usual care (4 studies), or SC (4 studies).12, 14, 22–41, 149 Thirteen of the 31 studies made comparisons with active interventions (i.e., other psychotherapies).12, 14, 39–42, 122, 144–146, 148, 151, 152 Of these 13 studies, 5 included an exposure-based intervention as the comparison;12, 14, 41, 42, 122 1 used structured writing therapy (SWT);40 1 used PCT;39 2 used relaxation;122, 152 2 used another CBT-mixed intervention;148, 151 1 used a CBT for Alcohol Use Disorder (AUD) plus SC;146 1 used a group integrated cognitive behavioral therapy (ICBT) for depression and substance use disorder (SUD) followed by individual ICBT for depression and SUD;145 and 1 study that tested a dialectical behavior therapy-(DBT)-PE combination therapy used DBT alone as the comparison group.144
Of the 24 studies with inactive comparators, sample sizes ranged from 23 to 190. Duration of treatment ranged from 4 to 24 weeks. Although 3 studies did not include a followup assessment22, 24, 25 and the followup interval for 1 was unclear,40 the remainder of the studies with inactive comparators included at least one posttreatment followup assessments after 1 to 12 months.40 The majority of studies enrolled a heterogeneous group of subjects with a variety of index trauma types and comorbid mental health problems (e.g., depression, personality disorders, SUD/AUD). Mean age ranged from 30 to 61 years. Most studies enrolled a large majority of female subjects. The primary outcome measure for 13 of these studies was some version of the CAPS (CAPS, CAPS-2, or CAPS-Sx);22–24, 27–29, 34–39, 41 4 studies used a form of the PSS (PSS-I or PSS-SR);12, 14, 32, 33 3 studies used the PDS;23, 24 5 studies used the PCL;25, 26, 30, 31, 149, 153 and 2 the IES.24, 40
Of the 13 studies with active comparators,40–42, 122 sample sizes ranged from 24 to 190. Duration of treatment ranged from 8 to 16 weeks, with the exception of the study testing DBTPE vs. DBT,144 where treatment lasted for 1 year. All studies also included posttreatment followup assessments ranging from 1 to 12 months. The majority of studies enrolled a heterogeneous group of subjects with a variety of index trauma types and comorbid mental health conditions (e.g., depression, SUD/AUD, personality disorders, intentional self-injury). Mean age ranged from 33 to 50. Most studies enrolled a large majority of female subjects. The primary outcome for 6 studies was some version of the CAPS (CAPS, CAPS-2, or CAPS-Sx); 3 used the PSS-I,12, 14, 146 2 used the PCL,144, 152 1 used the PDS,146 and 1 used the IES.40
Results for CBT-Mixed Interventions Compared With Inactive Comparators
PTSD Symptoms
Of the 23 studies with inactive comparators, 21 compared PTSD symptom changes pre- to posttreatment between CBT-M and inactive comparator groups, half (n=11) of which used the CAPS to report outcomes. Among the 11 studies that used the CAPS, 8 reported decreases in PTSD symptoms as assessed by the CAPS that were statistically significant. Full evidence tables from each of these studies can be found in Appendix F.
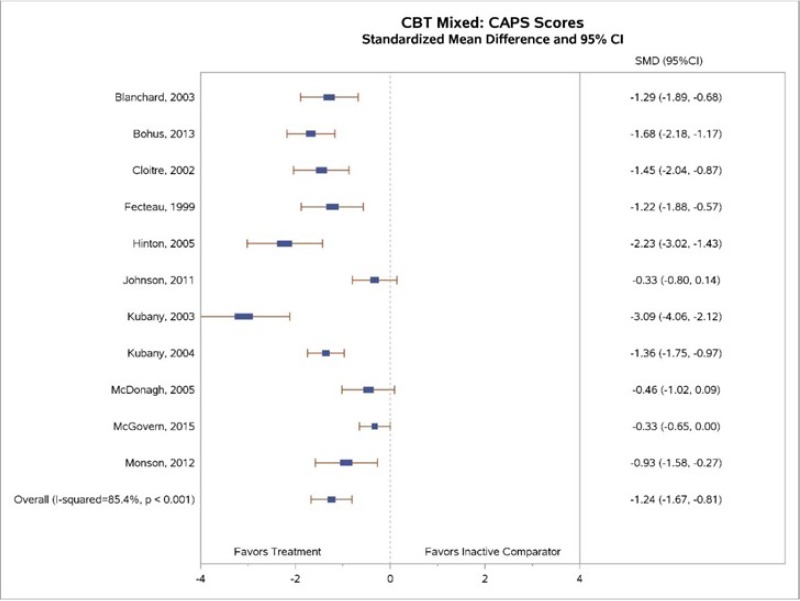
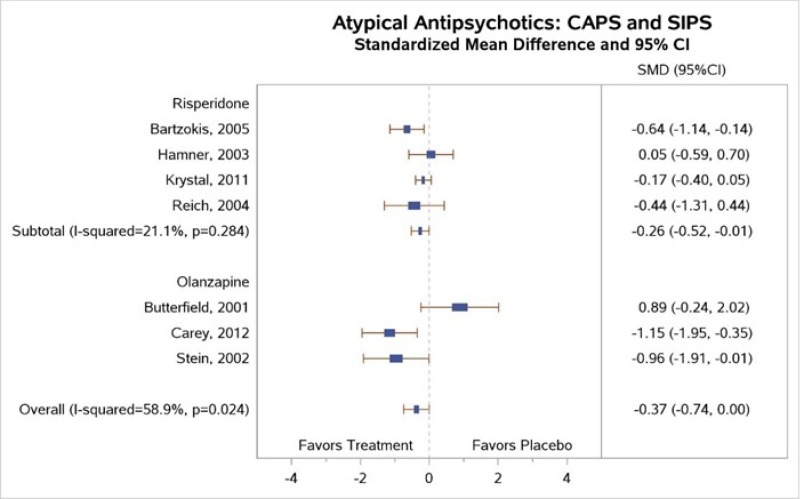
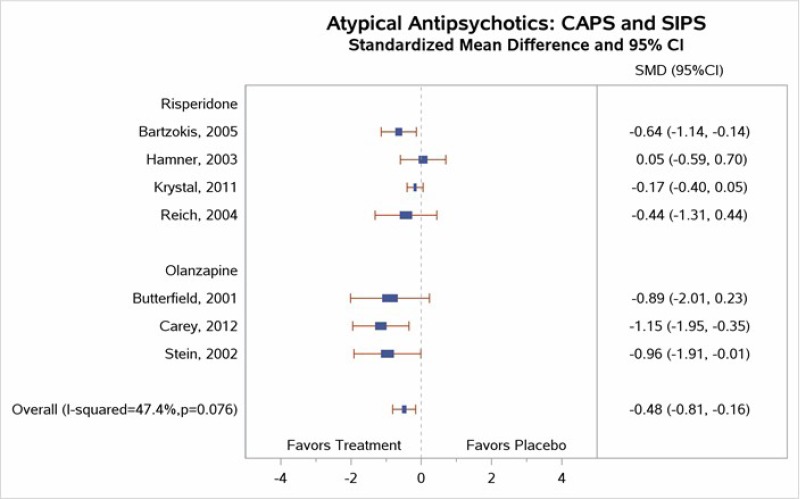
Our meta-analysis (Figure 11) found greater decreases in CAPS-rated PTSD symptoms for CBT-M interventions than for inactive controls (SMD, −1.24; 95% CI, −1.67 to −0.81; 11 studies, N=709; high SOE). Statistical heterogeneity was substantial (I2=85.4%). Much of the heterogeneity may be explained by the diversity of both interventions (as explained above, these interventions used various CBT components). Six studies found a similarly large decrease in PTSD scores assessed by CAPS for CBT-M intervention groups compared with wait-list or usual-care controls—about a 30-point greater reduction.23, 28, 34, 36–38 One study with a wait-list control found even greater decreases (about a 68-point decrease).35 Three of the 10 studies found little to no decrease.27, 29, 39 One of these compared CBT-M interventions with usual care (in which the control patients were often receiving some form of treatment)29 and another with standard care27 rather than with wait-list; this likely biased results toward the null.
We conducted additional meta-analyses to pool pre- to posttreatment differences in PTSD symptoms across groups using additional outcome measures reported across all studies with inactive comparators (CAPS, PSS-I, IES, PCL, PDS). Our meta-analysis found greater decreases in PTSD symptoms for CBT-mixed interventions compared with inactive controls (SMD, −1.01; 95% CI, −1.28 to −0.74; 21 studies, N=1,349, Appendix H; high SOE). Similar to the CAPS meta-analysis, statistical heterogeneity was substantial (I2=81.1%). However, also like the synthesis of CAPS data, the differences in findings were in the magnitude (not the direction) of the effect; all point estimates favored CBT-mixed interventions, and the vast majority of individual studies reached statistical significance.
Three of the 10 studies reported data on PTSD symptoms assessed by CAPS at 3- to 6-month followups (Appendix H).23, 29, 36 Of these, 2 found significant between-group differences favoring the CBT-mixed intervention over inactive comparators,23, 36 and the other study found no significant differences between groups.29 These findings mirrored those found at the end of treatment assessments.
Adding three additional studies that used PTSD symptom measures other than the CAPS to the analysis permitted pooled analysis via meta-analysis. Of the six studies, four found statistically significant between-group differences from pretreatment to followup assessment, favoring CBT-mixed interventions over wait-list23, 32, 36 and SC groups;33 two studies found no significant pretreatment to followup assessment between a CBT-mixed intervention and wait-list26 or usual care (Appendix H).29 Meta-analysis of the six studies found that between-group differences in PTSD symptom changes persisted after the end of treatment but with a somewhat smaller effect size than found at the posttreatment assessment (SMD, −0.8; 95% CI, −1.3 to −0.2; Appendix H; high SOE). Determining with confidence how much of the between-group differences in PTSD symptom decreases persist at longer-term followup is difficult, partly because of the potential for reporting bias (i.e., studies not reporting followup data because the significant differences did not persist).
Remission
One small CBT-mixed study reported that a greater percentage of subjects in the CBT-mixed group achieved remission compared with inactive comparator subjects (RD=0.40 using the PCL,30 insufficient SOE).
Loss of PTSD Diagnosis
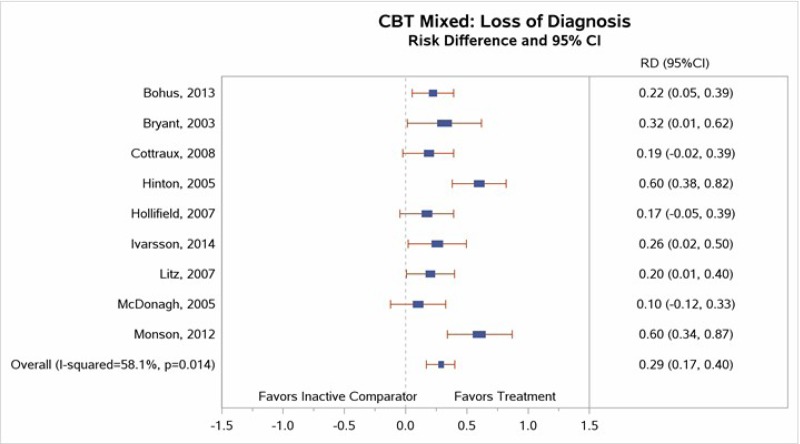
Nine studies reported sufficient data on loss of PTSD diagnosis to permit meta-analysis.22–24, 31–34, 39, 41 Our meta-analysis (Figure 12) found a large effect size (RD, 0.29; 95% CI, 0.17 to 0.40; I2=58.1%, 9 studies, N=474) for loss of PTSD diagnosis between CBT-mixed and inactive comparator subjects (high SOE).
Two of the studies also reported 3- to 6-month loss of PTSD diagnosis followup data.33, 41 Significant findings from both studies suggested that the between-group differences in loss of PTSD diagnosis favoring the CBT-mixed interventions over inactive comparators persisted over time.
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
Fifteen of the 24 studies that compared CBT-mixed interventions with an inactive control reported data on depression symptoms using the BDI. All but one of these reported point estimates favoring subjects treated with CBT-mixed interventions; the vast majority reported these findings to be statistically significant. Meta-analysis of these studies found greater improvement in depression symptoms for subjects treated with CBT-mixed interventions than for those in inactive comparator (SMD, −0.87; 95% CI, −1.14 to −0.61; I2=72.0%, 15 studies, N=929; Appendix H; high SOE).
Five of the studies reported sufficient 3- to 6-month postintervention followup data for between-group changes in depressive symptoms. Meta-analysis of the five studies found that improvements were maintained but with a slightly smaller effect size (SMD, −0.55; 95% CI, −0.78 to −0.31; 5 studies, N=286, moderate SOE, Appendix H).
A number of studies also reported reduction in anxiety symptoms; a variety of different measures were used. The most commonly reported measure was the STAI, reported by five of the studies that compared CBT-mixed interventions with an inactive comparator. Most found greater decreases in anxiety symptoms for subjects treated with CBT-mixed interventions than for those in inactive comparator groups. Meta-analysis of these studies found greater between-group decreases in anxiety symptoms among subjects treated with CBT-mixed interventions than those in inactive comparator groups from pre- to posttreatment (SMD, −0.79; 95% CI, −1.31 to −0.27; 5 studies, N=257; I squared=82.9; Appendix H; moderate SOE).
A few studies testing interventions targeting individuals with comorbid PTSD and substance use problems reported on various substance use outcome measures using a wide variety of measures. One study of veterans with comorbid PTSD and SUD found the CBT-M group had a lower mean percentage of heavy drinking days at posttreatment than controls;149 another found significant decreases in positive toxicology tests and self-reported amount and frequency of substance use among CBT-M group participants as compared with controls (moderate SOE).27
Quality of Life
Five studies reported data on quality of life.24, 26, 31, 39, 149 The use of four different quality-of-life measures149 across the five studies (one of which included only subscale data149), however, precluded the use of meta-analysis to pool findings.149
The five studies had mixed findings (insufficient SOE). Three studies found no differences between groups,26, 39, 149 and two studies reported greater improvements among CBT-mixed participants than inactive control participants.24, 31 Taken together, this evidence is insufficient to determine the efficacy of CBT-mixed interventions for improving quality of life.
Disability or Functional Impairment
Six studies reported data on disability or functional impairment23, 30–32, 36, 37 using a variety of measures (Table 13). We did not use meta-analysis to pool findings because of the diversity of measures that did not assess the same types of disability and functional impairment. Four of the six studies found significantly greater improvements in disability or functional outcomes for those who received CBT-mixed interventions than those who received an inactive control (low SOE).
Results for CBT-Mixed Interventions Compared With Active Comparators
Of the 12 studies comparing a CBT-mixed intervention with an active comparator, 5 compared a CBT-mixed intervention against an exposure-based intervention.12, 14, 41, 42, 122 Assessment of head-to-head comparisons with exposure-based interventions is covered in the CBT—Exposure section (above). Several of the other studies made comparisons with interventions for which we did not aim to assess comparative effectiveness39, 40, 144–146, 148 (e.g., comparisons with other CBT-mixed interventions,144–146, 148 SWT,40 or an active control [PCT].39 In this section, we address the 2 studies comparing CBT-mixed interventions and relaxation interventions.122, 152
Results for CBT-Mixed Interventions Compared With Active Comparators: CBT-Mixed Versus Relaxation
PTSD Symptoms
Both studies that evaluated CBT-M versus relaxation reported significantly greater decreases in PTSD symptoms among CBT-mixed intervention participants than relaxation participants (low SOE).122, 152 One reported large between-group differences in PTSD symptoms assessed by the CAPS (between-group mean difference, -24), and the other reported a large between-group effect size (between-group mean difference, −21.2) in PCL-measured PTSD symptoms between groups that persisted122 at followup (p<0.05).
Disability or Functional Impairment
One study reported data on disability or functional impairment using the General Health Questionnaire (GHQ) Global Improvement measure (insufficient SOE).122 A greater (but not statistically different) percentage of subjects in the CBT arm than in the relaxation arm showed improvements in functioning (RD, 0.15; p=NS).
Detailed Synthesis: Eye Movement Desensitization and Reprocessing
Characteristics of Studies
Table 14 summarizes the characteristics of the 10 studies meeting our inclusion criteria. Further details describing the included studies are provided in Appendix F. Eight studies had an inactive comparator, such as wait-list,13, 16, 44–46, 48 “stabilization as usual,”43 or placebo.47 Five had active comparisons with either PE,13, 16, 133 BEP,154 or relaxation training.46, 133
Sample sizes ranged from 21 to 155. Duration of treatment ranged from 4 to 8 weeks. Although one study did not include a followup assessment48 and another included only a followup at 5 weeks posttreatment, the rest of the EMDR studies included posttreatment followups after 3, 6, or 9 months. Two of the studies enrolled a heterogeneous group of subjects with a variety of index trauma types (e.g., sexual assault, physical assault, witnessing traumatic events, accidents, and combat), one study enrolled a majority of subjects with combat-related PTSD,46 one enrolled Swedish public transportation workers who witnessed train accidents or were physically assaulted,48 two enrolled female victims of sexual assault,13, 45 two enrolled refugees,43, 44 and one enrolled participants with comorbid psychotic disorders with mixed trauma types.16 Mean age was roughly similar across studies, ranging from 34 to 49 years. Four studies enrolled 70 percent or more female subjects.13, 44, 45, 47 The primary outcome for the majority of studies was some version of the CAPS (CAPS, CAPS-2, or CAPS-Sx); two studies identified other primary outcomes, including the PSS-I45 or IES.44, 48
Among the studies with inactive comparators described above, two also included an active comparator arm of either PE13 or relaxation.46 Another study compared EMDR with either PE or relaxation therapy in a sample of individuals with PTSD with mixed trauma exposure types.133 A fourth study included an active comparator (PE) and an inactive comparator (wait-list).16
Results for EMDR Compared With Inactive Comparators
PTSD Symptoms
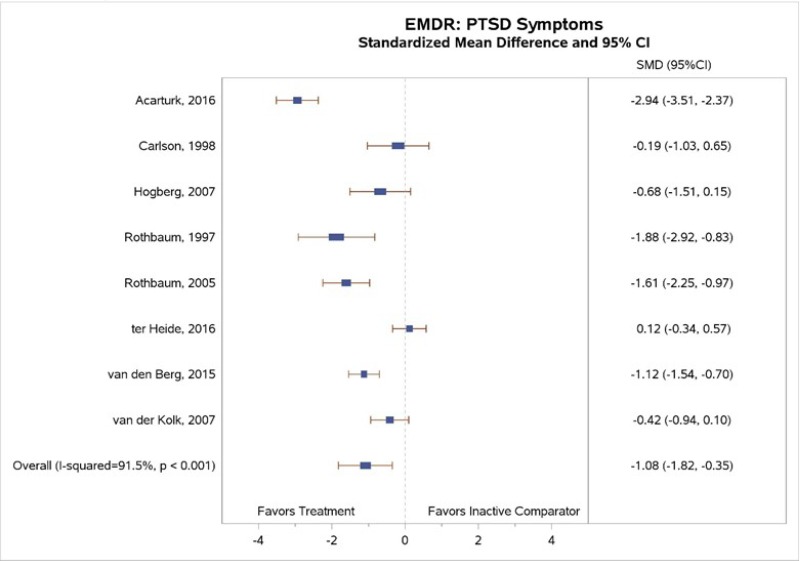
All eight EMDR studies with inactive comparators measured PTSD symptom change. Our meta-analysis (Figure 13) found greater decreases in PTSD symptoms for EMDR than for inactive comparator subjects (SMD, −1.08; 95% CI, −1.82 to −0.35; I squared=91.5%, 8 studies; N=449).13, 16, 43–48 Differences between EMDR and comparator groups reached statistical significance in four of eight studies;13, 16, 44, 45 point estimates varied widely across studies (moderate SOE). The two studies that found a significant pre- to posttreatment benefit of EMDR and included followup assessments reported the maintenance of benefit at the 1-month44 and 9-month16 followup assessments.
Loss of PTSD Diagnosis
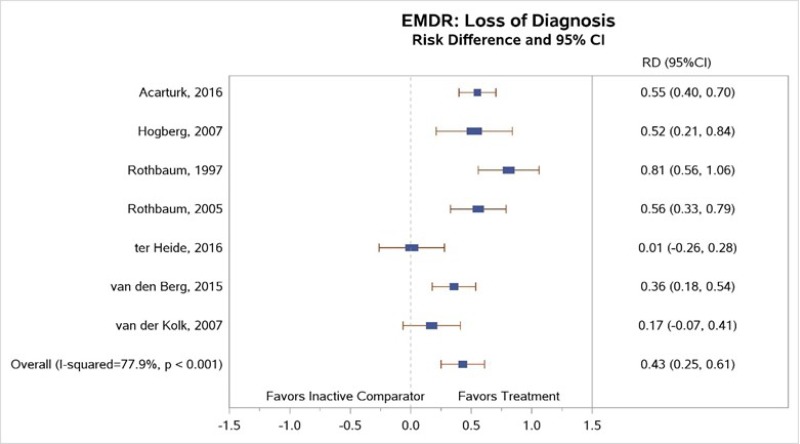
Of the studies that compared EMDR with inactive comparators, seven of the eight studies reported sufficient data to permit meta-analysis. Although one study that compared EMDR to stabilization as usual43 did not find significant differences between groups, the other seven studies found a greater loss of diagnosis among EMDR subjects than inactive comparator subjects at posttreatment and at followup assessments.13, 16, 44, 45, 47, 48 Our meta-analysis (Figure 14) found large between-group differences (RD, 0.43; 95% CI, 0.25 to 0.61) in loss of diagnosis at posttreatment assessment (moderate SOE).47
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
Seven studies comparing EMDR with inactive comparators included a measure of depression symptoms (BDI, HAM-D, or Hopkins Symptom Check List [HSCL]-depression). All demonstrated better improvements in depression symptoms among EMDR group subjects, with four of the seven study comparisons reaching statistical significance.13, 43, 44, 46, 48 Our meta-analysis (Figure 15) indicated a significant effect size (SMD, −0.91; 95% CI, −1.58 to −0.24; 7 studies; I squared=87.5%, N=347; moderate SOE).
Three studies used STAI,13, 45, 46 and one used the anxiety subscale of the HSCL43 to assess anxiety symptoms. Although all studies found that EMDR improved anxiety symptoms more than inactive controls, results did not reach statistical significance in three of the four studies (meta-analysis not conducted because of heterogeneity in sample characteristics, insufficient SOE).13, 43, 45, 46
Quality of Life
One study assessed quality-of-life outcomes.43 Differences in quality-of-life changes from pre- to posttreatment did not significantly differ between the EMDR and stabilization-as-usual groups (insufficient SOE).
Results for EMDR Compared With Active Comparators: Relaxation
Of the studies comparing EMDR with an active comparator, three compared EMDR and exposure therapy;13, 16, 133 as assessed in the CBT—Exposure section (above); one study compared EMDR with BEP154 as assessed in the Other Psychological Intervention section (below). Two studies compared EMDR and relaxation.46, 133
PTSD Symptoms
One study found no statistically significant pre- to posttreatment difference in CAPS-assessed PTSD symptoms between subjects treated with EMDR and those treated with relaxation;133 one found that EMDR led to greater PTSD symptom decreases than relaxation (N=13) on the Mississippi Scale for Combat Related PTSD but not on the IES (insufficient SOE).46
Loss of PTSD Diagnosis
Two studies that compared EMDR with relaxation both reported loss of PTSD diagnosis at some assessments.46, 133 One reported loss of diagnosis at the end of treatment favoring EMDR over relaxation (RD, 0.20, p=ns), but differences did not reach statistical significance (insufficient SOE).133
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
Both studies used the BDI to measure depression symptoms; one also reported on anxiety symptoms using the STAI.46 Neither study found a statistically significant difference between groups for reducing depression symptoms (insufficient SOE). One study reported a large between-group effect size (>0.90 using BDI) that was not statistically significant.46, 133 The study that reported between-group differences of anxiety symptoms found that relaxation was less effective than EMDR (p<0.01) for reducing symptoms of anxiety at the end of treatment (insufficient SOE).46
Detailed Synthesis: Other Psychological Interventions
Characteristics of Studies
Table 15 summarizes the characteristics of 24 studies meeting our inclusion criteria. Further details describing the included studies are provided in Appendix D. For the characteristics and results sections included in this section, we group studies primarily by intervention type (rather than comparator) because of the large heterogeneity across studies.
Two studies assessed TAR (full intervention name: Trauma Affect Regulation: Guide for Education and Therapy). One study compared individually delivered TAR with two comparison groups (PCT and wait-list) in a population of mothers with victimization-related PTSD.59 The second study compared TAR delivered in a group setting with supportive group therapy in a population of incarcerated women victims of interpersonal violence.60 Enrolled populations had a mean age of 31 to 36 years and had about half nonwhite participants (range 43% to 59%).
Four studies assessed BEP. Three of the four BEP studies had wait-list50, 51 or minimal attention comparators;49 one compared BEP with EMDR.154 Three studies conducted by the same research group in the Netherlands had varying sample characteristics; one sampled police officers,51 and the other two had heterogeneous subjects with a variety of index trauma types.50, 154 Treatment lasted for 16 weeks in all four BEP studies, with similar mean age of participants across studies (35 to 40 years of age). Twelve subjects (40%) of the Swiss sample were taking psychotropic medications, “mostly antidepressants.”
Three studies evaluated IRT.52, 142, 156 One IRT study that tested efficacy versus wait-list involved women with a history of sexual trauma (N=168).52 Another study compared IRT with psychoeducation in male Vietnam-era combat veterans with no medical disorders known to affect sleep (e.g., narcolepsy, untreated sleep apnea).156 A third study tested the comparative effectiveness of IRT with PE among men and women with mixed trauma types. All studies included a 3-month followup posttreatment.
Four studies assessed the effectiveness of NET for PTSD among asylum seekers and refugees. Sample sizes ranged from 32 to 277. Duration of treatment ranged from 3 to 12 weeks. Three studies used the PDS to assess PTSD symptom severity, and one used the CAPS.55 All samples contained males (25% to 69%) and females (31% to 75%) with mean ages ranging from 28 to 35 years. One study compared NET (n=17), SC (n=14), and psychoeducation (n=12) in a Ugandan refugee settlement with Sudanese refugees.161 The second study, also conducted in a Ugandan refugee settlement, compared NET (n=111), trauma counseling (n=111), and a nontreatment symptom monitoring group (n=55) among Rwandan and Somalian refugees.54 The third study compared NET (n=16) with treatment as usual (n=16) in a sample of asylum seekers living in Germany who were originally from Turkey, the Balkans, or Africa.53 The fourth study compared NET (n=17) with a wait-list control (n=17) in a sample of refugees and asylum seekers from Africa and the Middle East who were living in Switzerland.55
Two studies tested MEST. One enrolled Iranian combat male veterans and compared outcomes against those who received a control treatment160 while the other tested the comparative effectiveness of MEST and CPT among men and women with mixed trauma types.124 Both included followup assessments 3 months postintervention.
Two studies tested MBSR in samples of male and female war veterans. One tested MBSR plus treatment as usual (TAU) versus TAU,159 and the other tested MBSR versus an active control, group PCT.136 MBSR is a treatment that uses meditation to increase awareness of present mental and physical processes. In MBSR, the instructor leads participants through meditative exercises that focus on noticing sensations, thoughts, and emotions without judgment, and the participants practice short guided meditation exercises outside of group sessions. Three studies assessed other (unique) psychological interventions including the following: IPT,132 EFT,155 and neurofeedback training.162 Appendix A details characteristics of each of these treatments. The study assessing IPT had two active comparator groups: PE and relaxation therapy.132 The other two studies compared an intervention with an inactive control. The enrolled population in each study had different trauma types (Table 15).
Four studies assessed the efficacy of SS; three different active control approaches contained components to treat SUDs alone or to provide psychoeducation about women’s health issues.56, 57, 157 One of these three studies compared the addition (to TAU) of a voluntary SS intervention with a treatment-as-usual control group, which comprised incarcerated women enrolled in a residential substance use treatment program in a minimum security wing.56 Another active control involved TAU in a SUD clinic at a Veteran’s Administration outpatient mental health clinic.58 Three of the studies enrolled women generally in their 30s; one enrolled male veterans with a mean age of 54.58 Sample sizes ranged from 49 to 353;56, 57, 157 one of these was a pilot study (N=49) that may have been underpowered.56 One study enrolled a sample of incarcerated women;56 two enrolled community-based samples of women seeking substance abuse treatment.57, 157 Followup assessments were conducted at 3 and 6 months in all studies; one study each conducted additional assessments at 9 months57 or 12 months.157
Trauma Affect Regulation
PTSD Symptoms
Two studies assessing TAR in populations of women with interpersonal victimization reported between-group changes in CAPS scores (low SOE). The study that compared TAR, PCT, and wait-list reported greater improvement in CAPS-assessed PTSD symptoms for those treated with TAR than those in the wait-list group (between-group mean difference, −17.4; p<0.001).59 The study that compared TAR with usual group care for incarcerated women found a similar reduction in CAPS-assessed PTSD symptoms between groups (between-group mean difference, −2.7; p=NS).60
Remission
Both studies reported on the percentage of participants with full remission from baseline to followup with inconsistent findings. One study found a higher rate of remission among the TAR group than among the wait-list group (RD, 0.21; p<0.001);59 the other found a lower rate of remission among the TAR group than among the usual-care group (RD, −0.11; insufficient SOE).60
Loss of Diagnosis
Both studies reported on loss of PTSD diagnosis from baseline to followup; one found a higher rate of remission in the TAR group than in the wait-list group (RD, 0.26), and the other found a similar rate of remission in both groups (RD, 0.01; p=NR; insufficient SOE).60
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
The study that compared TAR with a wait-list control reported greater decreases in depression symptoms and anxiety symptoms for the TAR than for the wait-list group (BDI between-group mean difference, −4.1; p<0.01; STAI between-group mean difference, −6.3; p=0.19; insufficient SOE).
Brief Eclectic Psychotherapy
PTSD Symptoms
Two studies reported measures of PTSD symptoms for BEP compared with an inactive comparator.49, 50 One only reported subscale scores, however, for the Structured Interview for PTSD (SI-PTSD) measure between groups.50 The study that used CAPS reported significantly greater decreases in PTSD symptoms in the BEP versus the wait-list group (between-group mean difference=−10.8; 1 study; N=30; insufficient SOE).49, 51
The study that compared PTSD symptoms between BEP and EMDR groups reported that both treatments were equally effective in reducing PTSD symptom severity, but EMDR resulted in faster recovery.154 The study reported significant decreases in PTSD symptoms within both treatment groups using the IES-R and the SI-PTSD but greater decreases from baseline to the first assessment for those treated with EMDR than for those treated with BEP (between-group mean difference on SI-PTSD −10.80; 95% CI, −15.23 to −6.37).154 The between-group difference did not remain significant at the second assessment, conducted after both groups had completed treatment (insufficient SOE).
Remission
One study (N=30) reported data on symptom remission. At the end of treatment, a greater proportion of BEP than inactive comparator group subjects had remitted (RD 0.13) as defined by having a CAPS score of less than 20.49 Difference persisted at the 6-month followup (RD 0.19). None of the subjects in the wait-list group achieved complete remission at either assessment (insufficient SOE).
Loss of PTSD Diagnosis
All three BEP studies reported efficacy for loss of PTSD diagnosis at the end of treatment and followup assessments using different assessment measures. The RDs ranged from 0.13 to 0.58 in individual studies.49–51 We concluded that evidence supports the efficacy of BEP for loss of PTSD diagnosis (low SOE).
The study that compared BEP with EMDR reported similar rates at the end of treatment (RD=0.08 slightly favoring EMDR), but EMDR subjects had quicker time to loss of PTSD diagnosis than BEP subjects (RD at mid-treatment 0.40 favoring EMDR; insufficient SOE).154
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
All three studies comparing BEP with wait-list reported on reduction of depression and anxiety symptoms. Two used the Hospital Anxiety and Depression Scale (HADS) as an outcome measure; both studies reported that BEP subjects had significantly greater decreases in depression symptoms at the end of treatment and followup than wait-list subjects and at later followup (low SOE).49, 50 One study used the Symptom Checklist-90 (SCL-90) as a multidimensional indicator of psychopathology and reported that BEP had greater decreases in depression symptoms than wait-list at the end of treatment (data not reported, p<0.01) that persisted at the 3-month followup.51
Two studies reported that BEP had significantly greater decreases in anxiety symptoms (low SOE) as assessed by the HADS (Cohen’s d=0.8, p<0.05 and d=0.9, p<0.05 for one study at the end of treatment and at followup;49 for the other study d=0.5450). The study using the SCL-90 reported that BEP had greater decreases in anxiety symptoms at the end of treatment and at the 3-month followup (data not reported, p-values of <0.05 and <0.01).51
The study comparing BEP with EMDR reported measures of depression and anxiety symptoms (using the HADS depression and the HADS anxiety).154 Similar to findings for other outcomes (e.g., PTSD symptoms), the study reported greater improvement from baseline to the first assessment for those treated with EMDR than for those treated with BEP but no significant difference between groups at the second assessment (insufficient SOE, see Appendix F for detailed data).
Return to Work or Active Duty
Two studies reported outcomes related to work (insufficient SOE)—one reported the percentage of subjects on sick leave;50 the other reported the percentage who had returned to work.51 The former study found fewer subjects on sick leave for the BEP group compared with those on the wait-list, but the difference was not statistically significant (d=0.33, p=0.06).50 The second study reported significantly greater rates of returning to work among BEP subjects than those in the inactive comparator group (RD 0.26; p<0.05) for return to work.51
Imagery Rehearsal Therapy
PTSD Symptoms
One IRT study found efficacy for decreases in CAPS-assessed PTSD symptoms among IRT-treated versus wait-list control groups (between-group mean difference, −21.0; p=0.001; low SOE).52
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
One study that assessed the efficacy of IRT on depressive symptoms using the HAM-D found greater, but not significantly different, decreases in depression among IRT participants compared with wait-list participants (insufficient SOE).52 This study also found significant (p<0.04) differences in changes in anxiety symptoms between groups at the end of treatment, but differences resulted from the IRT group having decreases in symptoms and the wait-list group having increases in symptoms.
Narrative Exposure Therapy
PTSD Symptoms
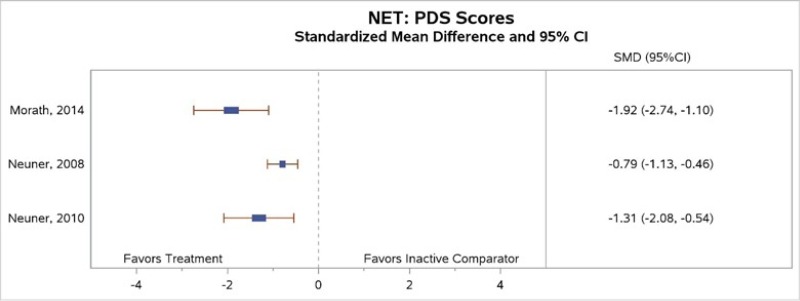
All three studies that compared NET with an inactive comparator found that NET subjects had greater decreases in PTSD symptoms at the end of treatment (moderate SOE; Figure 16).53–55 One study reported a reduction (but no data) in PTSD symptoms for subjects in the intervention group at the followup assessment 6 months after the end of treatment;53 another reported that the intervention led to significantly greater decreases in PTSD symptoms than no treatment (i.e., monitoring group) from baseline to the 6-month followup (d=1.4 and 0.08, respectively, p<0.001).54
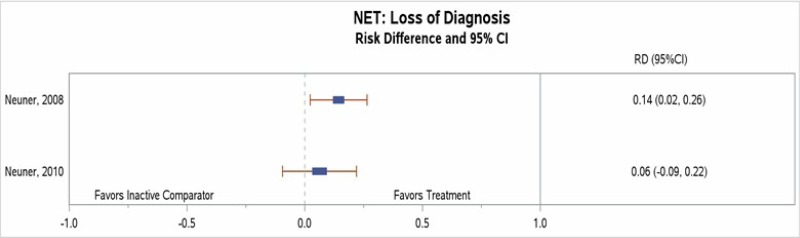
Loss of PTSD Diagnosis
Two studies of NET and an inactive control reported data on loss of PTSD diagnosis (low SOE; Figure 17).53, 54 One of these studies also had an active comparator group (trauma counseling), for which we did not intend to assess comparative effectiveness.54 Both studies with inactive comparators favored NET for loss of PTSD diagnosis at the end of treatment (RD of 0.06 and 0.14 in the two studies).54, 161
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
Two studies evaluated the efficacy of NET on coexisting psychiatric conditions.53, 55 Both studies that compared NET with an inactive control reported greater decreases in depressive symptoms for NET subjects compared with inactive comparator subjects at the end of treatment; however, only one comparison likely attained statistical significance (Cohens d=0.54 but p=NR; insufficient SOE).53, 55, 161
One small study (N=32) found greater decreases on Composite International Diagnostic Interview-Pain scores for NET versus inactive comparator subjects at the end of treatment (insufficient SOE).53
Interpersonal Therapy
One study compared IPT with two active comparators, PE and relaxation, in a population with PTSD primarily related to interpersonal trauma.132 The prior CBT-Exposure section, above, details the comparative effectiveness of PE versus relaxation and PE versus IPT. In this section, we focus on the comparative effectiveness of IPT versus relaxation only.
PTSD Symptoms
Participants randomized to IPT therapy had a similar decrease in PTSD symptom scores as assessed by the CAPS compared with the relaxation therapy group (between-group mean difference, −6.3 favoring IPT; p=0.097).132 On the PSS, however, participants in the IPT group had greater decreases in scores than the relaxation group (between-group mean difference, −18.2 favoring IPT; p=0.008; insufficient SOE).132
Remission
The study that compared IPT versus relaxation found similar rates of PTSD remission (defined as CAPS score <20) across groups (RD 0.04 favoring IPT; insufficient SOE).132
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
The comparative effectiveness of IPT and relaxation for change in depression symptoms as assessed by the HAM-D did not differ at the end of treatment (between-group mean difference, 0.3; insufficient SOE).132
Quality of Life
IPT subjects had greater increases in quality-of-life ratings as assessed by the Quality of Life Enjoyment and Satisfaction Questionnaire163 than the relaxation therapy group (between-group mean difference, 10.1 favoring IPT; p<0.001; insufficient SOE).132
Functional Impairment
IPT subjects had greater increases in interpersonal functioning as measured by the Inventory of Interpersonal Problems than those in the relaxation group (between-group mean difference, −0.46 favoring IPT; p=0.001; insufficient SOE).132
Memory Specificity Training
PTSD Symptoms
One study enrolling Iranian combat veterans compared MEST with a no treatment control group; participants in the MEST group had significantly fewer PTSD symptoms on the IES-R than the control group at followup (insufficient SOE).160
The MEST comparative effectiveness study found that reductions in PTSD symptoms were not similar and not statistically different among subjects randomized to the MEST group and the CPT group. The MEST group participants experienced similar decreases in symptoms after attending only about half of the sessions attended by the CPT group participants (insufficient SOE).124
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
The study reported no significant difference between participants in the MEST group and controls160 or MEST versus CPT124 on depression symptoms measured by the BDI-II (scores not reported; insufficient SOE for both efficacy and comparative effectiveness).
Mindfulness-Based Stress Reduction
Of the two MBSR studies that met review inclusion criteria, this section describes the findings for the study that tested the efficacy of MBSR plus TAU versus TAU.159 The other study tested MBSR versus an active comparison group for which we were not interested in comparative effectiveness (group PCT).136 One study compared IPT with two active comparators, PE and relaxation, in a population with PTSD primarily related to interpersonal trauma.132 The prior CBT-Exposure section above details the comparative effectiveness of PE versus relaxation and PE versus IPT. In this section, we focus on the comparative effectiveness of IPT versus relaxation only.
PTSD Symptoms
No significant differences were found between reduction in PTSD symptoms at posttreatment between those in the MBSR+TAU group versus the TAU group (insufficient SOE).159
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
Differences in posttreatment decreases in depressive symptoms as measured by the Patient Health Questionnaire (PHQ-9) were not significantly significant across treatment groups (MBSR+TAU versus TAU; insufficient SOE).132
Neurofeedback
The single neurofeedback (NF) study that met review inclusion criteria tested NF training versus a wait-list comparison group.162
PTSD Symptoms
The NF group had significantly greater decreases in PTSD as measured by the CAPS (between-group mean difference, −26.8, p<0.05) and by the Davidson Trauma Scale (DTS) (mean=−20.7, p<0.05) than the wait-list group at the posttreatment assessment (insufficient SOE).162 Between-group differences were sustained at the 1-month followup assessment.
Loss of PTSD Diagnosis
In the single small study that met review inclusion criteria, loss of diagnosis comparisons favored the NF group over the wait-list group (RD, 0.40, p=0.0002; insufficient SOE).162
Emotional Freedom Techniques
PTSD Symptoms
One study enrolling U.S. veterans compared EFT with wait-list control; participants in the EFT group had a greater decrease in PTSD symptoms measured by the PCL-M than controls at the end of treatment assessment (between-group mean difference, −22.1; p<0.0001; insufficient SOE).155
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
A single study found significantly greater decreases in psychiatric symptoms at the end of treatment for EFT than inactive comparator subjects for both domains tested from the Symptom Assessment-45 Questionnaire (insufficient SOE).155
Seeking Safety
PTSD Symptoms
Four studies tested the efficacy or effectiveness of SS.56–58, 157 Three compared SS with usual care,56–58 and two compared SS with an active comparator, but not ones for which we sought to determine comparative effectiveness (i.e., psychoeducation,157 relapse prevention57).
The three studies comparing SS with usual care each found that the intervention participants had greater decreases in PTSD symptoms than usual-care participants; however, between-group differences did not reach statistical significance (meta-analysis not performed because of heterogeneity in sample and study characteristics, low SOE for no difference).56–58 Figure 18 shows the SMD and CIs for between-group differences in PTSD symptoms.
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
Analyses of data from two57 studies compared substance use outcomes between SS and usual-care subjects. At the end of treatment, one study found no between-group differences for abstinence and substance use severity at the end of treatment,56 and the other study found significantly greater decreases in drug use (but not alcohol use) among SS subjects compared with usual-care subjects at the end of treatment assessment (insufficient SOE).58
KQ 1a. Variability in Efficacy or Comparative Effectiveness of Psychological Interventions by Patient Characteristics or Type of Trauma
This subquestion of KQ 1 evaluated whether the efficacy or comparative effectiveness of any of the psychological interventions differed by patient characteristics or type of trauma experienced. To answer this question, we present findings from included studies that reported outcomes for subgroups of interest (defined by patient or trauma factors) and compare the efficacy or comparative effectiveness across subgroups. Four studies provided information about efficacy or comparative effectiveness across different subgroups of interest.
Key Points
- Three studies compared the efficacy and one study examined the comparative effectiveness of interventions across subgroups of participants defined by patient characteristics or type of trauma experienced.
- These studies compared subjects with child- versus adult-onset sexual abuse (CPT vs. wait-list and PE vs. wait-list), child- versus adult-onset traumatic event exposure (EMDR vs. placebo), borderline personality disorder versus without borderline personality disorder (DBT vs. usual-care wait-list), and comparative effectiveness between subjects with versus without major depressive disorder (PE vs. IPT vs. relaxation).
- Insufficient SOE exists to determine the efficacy or comparative effectiveness between subgroups.
Detailed Synthesis: Patient Characteristics or Trauma Type
Characteristics of Included Studies
Table 16 summarizes the characteristics of the five included studies (2 of which examined different moderators of the same trial). Each study included a subgroup analyses of trials that have been described in previous parts of this report. Each examined a different treatment comparison, although three studies (2 of which examined different moderators of the same trial) included PE as one of the treatment arms.3, 132, 135 Only one of the four studies (2 moderator studies from the single trial) did not include a followup after posttreatment assessment.132, 135 All used the CAPS as the primary outcome measure. Additional details describing the included studies can be found in Appendix F.
One study examined the efficacy and comparative effectiveness of CPT and PE (versus a wait-list comparator) within a sample of female rape survivors enrolled in a clinical trial (n=171)3 who also had childhood sexual abuse (n=121).125 The second study compared EMDR, fluoxetine, and placebo in subjects with a variety of trauma types including child sexual abuse, child physical abuse, child sexual and physical abuse, adult sexual assault, adult physical assault, domestic violence, other adult victimization, traumatic loss, war/terror/violence, and injury/accident.47 The authors reported subgroup analyses for those with child-onset trauma and those with adult-onset trauma. The third study that included subgroup comparisons tested the efficacy of DBT against a wait-list, treatment-as-usual group of child abuse survivors.23 The efficacy of DBT was compared between those with and without borderline personality disorder. The fourth and fifth studies, which tested moderators from the same trial, examined the comparative effectiveness of PE, IPT, and relaxation training among a group of adults with chronic PTSD of mixed trauma types.132, 135 Subgroup analyses compared the effectiveness of different treatments among adults with and without major depressive disorder, trauma type, gender, and age of primary trauma exposure.
Efficacy or Comparative Effectiveness by Patient Characteristic or Trauma Type
Each of the five studies examined a different patient characteristic or trauma type and treatment comparison (insufficient SOE for each analysis). The study that compared the efficacy and comparative effectiveness of CPT, PE, and wait-list) between women rape victims with versus without childhood sexual abuse found similar efficacy of CPT and PE across both subgroups (those with versus without childhood sexual abuse).125
The second study that compared the efficacy and comparative effectiveness of EMDR, fluoxetine, and placebo between those with childhood-onset (prior to age 18) versus adult-onset trauma47 found no significant differences in the efficacy of EMDR as compared with placebo by trauma onset (child vs. adult) as tested by interaction analysis. Interestingly, however, in the main effects analysis, adults with childhood-onset PTSD had worse outcomes than those with adult-onset PTSD, regardless of what intervention they received.
The third study that reported on whether the efficacy of DBT (as compared with a wait-list usual-care comparison group) varied between women with childhood sexual abuse-related PTSD with versus without borderline personality disorder23 found no differences in efficacy across subgroups. DBT appeared to have similar efficacy, regardless of the presence of comorbid borderline personality disorder.
The fourth and fifth studies examined whether the comparative effectiveness of PE, IPT, and relaxation therapy differed among those with versus without comorbid major depressive disorder,132 and trauma type (sexual, physical, or interpersonal), gender, and age of primary trauma exposure (18 years old or younger versus 19 years old or older).135 In the first study, the authors reported no significant subgroup differences in PTSD symptom changes at posttreatment in the comparative effectiveness of any of the treatments tested; the effect of the treatment response (CAPS score at posttreatment <20) between groups did not significantly differ at posttreatment. The authors reported that among subjects in the PE treatment group, those with comorbid major depressive disorder had significantly higher attrition rates than those without major depressive disorder (50% vs. 5.6%, respectively, p<0.05).132 In the second moderator study, whereas those without sexual trauma exposure had no differences in efficacy across treatment groups, those with sexual trauma exposure had greater efficacy with IPT as compared with PE or relaxation training (p<0.05). Gender and age of primary trauma exposure, however, did not moderate the efficacy findings.
KQ 2. Efficacy and Comparative Effectiveness of Different Pharmacological Treatments
To answer this question, we present findings from placebo-controlled efficacy trials (indirect evidence) followed by evidence from head-to-head trials (direct evidence) to assess the comparative effectiveness of pharmacotherapies rated as having low or medium risk of bias. We used meta-analyses to pool data when five or more studies (or 3 or more studies with low heterogeneity across studies) tested similar interventions and reported similar outcome data; when three or four studies that tested a single intervention were determined to have substantial heterogeneity in sample, intervention, or study characteristics or two or fewer studies testing the same intervention presented data for an outcome, we qualitatively synthesized the findings and present findings from the individual studies. We also conducted network meta-analysis to use both the indirect and direct evidence to determine the comparative effectiveness of pharmacotherapies. We detail the comparative effectiveness of interventions that demonstrated efficacy of at least moderate SOE. In the bulleted text below, we summarize the main overall key points and then the key points for each medication class and report the SOE where appropriate.
We used the same set of outcomes of interest described previously in the chapter focused on psychological interventions (KQ 1). In brief, the primary outcomes of interest that investigators used to determine the effectiveness of treatments for adults with PTSD include PTSD symptoms, loss of PTSD diagnosis, and symptom remission, as defined by study authors based on loss of symptoms below a predefined threshold level. We also comment on other outcomes of interest, such as prevention or reduction of coexisting medical or psychiatric conditions (especially depression, anxiety, and substance use problems). As in the KQ 1 section above, for continuous outcomes such as PTSD, depression, and anxiety symptoms or ratings of quality of life or functioning, we present the between-group mean difference for single studies or the SMD when describing more than one study to indicate the between-group difference in pre- to posttreatment or pre- to followup assessments. For dichotomous outcomes like remission and loss of PTSD diagnosis, we report the RD between groups.
For outcomes with evidence from three or more studies with low heterogeneity across trials or five or more studies testing the same intervention types, we present the pooled estimate from meta-analysis and the 95 percent CI. When three or four studies determined to have substantial heterogeneity in sample, intervention, or study characteristics or two or fewer studies testing the same intervention present data for an outcome, we qualitatively synthesized the findings and present findings from the individual studies.
All included studies are cited in the detailed synthesis section and related tables and figures presented in this section for each treatment. Section headings within each detailed synthesis section include each outcome reported by at least one included study of that treatment type. If an outcome does not appear in the section, no included study testing the intervention of interest reported data on it.
Appendices contain additional information about the risk of bias assessments (Appendix E), individual study characteristics and findings for each outcome presented in evidence tables (Appendix F), characteristics and consistency of findings of high risk of bias studies not synthesized in the text (Appendix G), forest plots depicting individual and pooled study findings (Appendix H), and detailed information about each component contributing to the SOE grade (Appendix I).
Key Points: Overall—Efficacy of Pharmacological Treatments
- For PTSD symptom reduction, fluoxetine (selective serotonin reuptake inhibitor [SSRI]), paroxetine (SSRI), and venlafaxine (serotonin and norepinephrine reuptake inhibitor [SNRI]) have evidence of efficacy (moderate SOE). Low SOE supports the efficacy of prazosin (alpha blocker), topiramate (anticonvulsant), olanzapine and risperidone (atypical antipsychotics), and sertraline (SSRI).
- For PTSD symptom remission, paroxetine (SSRI) and venlafaxine (SNRI) have evidence of efficacy (moderate SOE).
Table 17 summarizes the efficacy and SOE for all included medications for primary outcomes of interest, PTSD symptoms, remission, and loss of PTSD diagnosis, although no drug efficacy studies included loss of PTSD diagnosis as an outcome measure.
Key Points: Overall—Comparative Effectiveness of Pharmacological Treatments
- Very few head-to-head trials were identified.
- –
Two studies provided moderate SOE for no differences between venlafaxine and sertraline for depression symptom reduction and low SOE provided no difference for PTSD symptoms reduction, quality of life, and disability.
- –
Our network meta-analysis of 33 trials (4,817 subjects) that included CAPS-measured PTSD symptom outcomes found no significant differences between the three pharmacological treatments that had at least moderate SOE of efficacy: fluoxetine, paroxetine, and venlafaxine.
Key Points: Alpha Blockers
- Low SOE supports the efficacy of prazosin on PTSD symptoms reduction.
Key Points: Anticonvulsants
- Low SOE supports the efficacy of topiramate on PTSD symptoms reduction.
Key Points: Atypical Antipsychotics
- Low SOE supports the efficacy of olanzapine and risperidone on PTSD symptoms reduction.
- Low SOE supports the efficacy of risperidone on anxiety symptoms reduction.
Key Points: Selective Serotonin Reuptake Inhibitors
- Moderate SOE supports the efficacy of fluoxetine for PTSD symptoms and low SOE for the anxiety symptoms. Low SOE does not support the efficacy of fluoxetine for depressive symptoms.
- Moderate SOE supports the efficacy of paroxetine for PTSD symptoms, remission, depression symptoms, and disability.
- Low SOE supports the efficacy of sertraline for PTSD symptoms reduction and quality of life (low SOE) and supports no differences in efficacy between sertraline and placebo for depression symptoms.
- Moderate SOE supports no differences in effectiveness between venlafaxine and sertraline for depression and low SOE supports no difference in effectiveness for PTSD symptom reduction, quality of life, and disability.
Key Points: Serotonin and Norepinephrine Reuptake Inhibitors
- Moderate SOE supports the efficacy of venlafaxine for PTSD symptoms, remission, depression symptoms, quality of life, and disability/functional impairment
- Moderate SOE supports no differences in effectiveness between venlafaxine and sertraline for depression and low SOE supports no difference in effectiveness for PTSD symptoms, quality of life, and disability (low SOE for no differences).
Detailed Synthesis: Placebo-Controlled Trials of Alpha-Blockers
Characteristics of Studies
We found three studies meeting our inclusion criteria that studied the efficacy of alpha-blockers (Table 18), each testing the efficacy of prazosin. All enrolled subjects had moderate to severe PTSD. All studies enrolled all or a large majority of male subjects; average age ranged from 30 to 56 years. Trial durations ranged from 8 weeks75 to 20 weeks.74, 75 Further details describing the included studies are provided in Appendix F.
Results for Placebo-Controlled Trials of Alpha-Blockers
PTSD Symptoms
All three studies reported greater pre- to posttreatment decreases in CAPS-assessed PTSD symptoms for subjects treated with prazosin than for those receiving placebo (SMD, −0.52, 95% CI, −0.90 to −0.14. I squared=0.0%, 3 studies, N=117; low SOE; Appendix H).74–76
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
One of the included studies assessed depression using the HAM-D.75 The study found that patients treated with prazosin had greater decreases in depression symptoms than those administered placebo, but differences between groups failed to reach statistical significance (between-group mean difference, −5.0; p=0.08; insufficient SOE).
Detailed Synthesis: Placebo-Controlled Trials of Anticonvulsants/Mood Stabilizers
Characteristics of Studies
Table 19 summarizes the six studies that met inclusion criteria. Appendix F contains further details about each study. The studies enrolled subjects with moderate to severe PTSD. Sample sizes ranged from 28 to 232. Treatment duration ranged from 8 to 12 weeks. Three of the included studies focus on combat-related PTSD;77, 164, 165 three enrolled a heterogeneous group of subjects with a variety of index trauma types (e.g., physical and sexual assault/violence, witnessing harm or death, combat, natural disaster, childhood sexual abuse, childhood physical abuse, MVA).78, 79, 166 The studies generally recruited middle-aged adults, with mean ages ranging from ~40 to ~55 years. Three studies enrolled at least two-thirds female subjects;78, 79, 166 three enrolled all or nearly all males. Five studies used some version of the CAPS as the primary outcome; one assessed PTSD symptoms using the PCL.165
Results of Placebo-Controlled Trials of Anticonvulsants/Mood Stabilizers
PTSD Symptoms
Five of the included studies reported CAPS-assessed PTSD symptom changes between groups (Appendix H). Among the three topiramate studies, only one found significant differences across groups,77 although all effect sizes consistently favored topiramate (Figure 19; low SOE).78, 79
One study testing divalproex and another testing tiagabine166 provided insufficient evidence of efficacy for PTSD symptoms due to unknown consistency and imprecise findings.
Remission
Two anticonvulsant study reported between-group differences in remission: one trial of tiagabine166 and one of topiramate.78 Both study defined remission as having a CAPS score of less than 20 at the end of treatment. Neither study found a statistically significant difference between anticonvulsants and placebo (insufficient SOE for tiagabine and for topiramate due to unknown consistency and imprecise findings).
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
Three studies, one that assessed divalproex164 and two that tested topiramate,78, 79 reported between-group changes in depression symptoms (Appendix F). None of the studies reported statistically significant between-group decreases in depression symptoms from pre- to posttreatment, although all point estimates favored anticonvulsants (insufficient SOE).78, 79, 164
Two studies (one divalproex and the other topiramate) reported between-group differences in anxiety symptoms assessed by the Hamilton Anxiety Scale (HAM-A). Neither study found statistically significant reductions in anxiety between groups (see Appendix F; insufficient SOE).78, 164
Disability or Functional Impairment
Two studies, one of tiagabine and one of topiramate, reported between-group differences in disability assessed by the Sheehan Disability Scale (SDS).78, 166 Both studies reported similar changes between subjects treated with medication and those treated with placebo (see Appendix F for details; insufficient SOE).
Detailed Synthesis: Placebo-Controlled Trials of Atypical Antipsychotics
Characteristics of Studies
Figure 20 summarizes the characteristics of the eight studies meeting our inclusion criteria. Appendix F provides details to further describe the studies included in this section.
Evidence of the efficacy of atypical antipsychotics comes from five studies that compared risperidone with placebo and three studies that compared olanzapine with placebo. Relatively small samples (ranging from 15 to 65) tested drug interventions that lasted from 5 weeks to 6 months. Subjects had mean ages generally ranging from 41 to 54. Although two studies enrolled a majority82 or only84 females, three enrolled exclusively males.80, 83, 86, 167
Most studies enrolled subjects with trauma types ranging from combat-related trauma,80, 83, 85, 86, 167 childhood abuse-related trauma,84 mixed types of trauma,82 to other types of trauma not related to combat.81, 168 One study exclusively enrolled subjects with PTSD and concurrent psychotic features,83 although studies frequently excluded subjects with a history of comorbid schizophrenia, bipolar disorder, or recent substance abuse/dependence.81–84, 167
The majority of studies permitted cointerventions. Most studies assessed PTSD symptoms using some version of the CAPS (CAPS total, CAPS-1, CAPS-2);80–86 one used the PCL-M to compare between-group outcomes.167
Results of Placebo-Controlled Trials of Atypical Antipsychotics
PTSD Symptoms
Three studies compared PTSD symptoms between olanzapine and placebo (low SOE). Two studies demonstrated efficacy for CAPS-measured PTSD symptoms (Figure 20).80, 81 Another study82 that did not use CAPS to assess PTSD symptoms but instead used four other measures of PTSD symptoms, including Single Item PTSD Screeners (SIPS), also favored olanzapine, but differences in this very small study did not reach statistical significance (see Figure 21).
For risperidone, four studies compared CAPS-assessed PTSD symptoms between treatment and placebo subjects and found some evidence of efficacy (SMD, −0.26, 95% CI, −0.52 to −0.01; I squared=21.1%; 4 studies; N=422; Figure 20; low SOE). One study found no real differences between groups,83 two suggested benefit but found no significant differences in PTSD symptoms between risperidone and placebo,84, 85 and one study found modest but very imprecise evidence of risperidone benefit.86
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
Two very small studies found slight benefits of varying levels of statistical significance favoring olanzapine for depression symptoms as measured by the Center for Epidemiologic Studies Depression Scale (between-group mean difference, −0.37; N=19; p<0.03)80 and the Montgomery Åsberg Depression rating scale (between-group mean difference, −0.54; N=28; p=0.14; insufficient SOE).81
For risperidone compared with placebo, one study indicated nonsignificant benefit of risperidone over placebo for HAM-D-measured depression symptoms (between-group mean difference, −2.3; N=65; p>0.05) and significant efficacy of risperidone over placebo for HAM-D-measured anxiety symptoms (between-group mean difference, −5.4; N=65; p<0.001).86 Another small study that enrolled subjects experiencing symptoms of psychosis at enrollment reported a greater reduction in Positive and Negative Syndrome Scale–measured psychotic symptoms at the end of treatment among risperidone than placebo subjects (between-group mean difference, −7.7, N=40; p<0.05; low SOE).83
Disability or Functional Impairment
Two very small studies found conflicting evidence of the efficacy of olanzapine on SDS-measured disability compared with placebo (between-group mean difference, 0.3 in 1 study and −4.2 in another, with a combined N of 43; insufficient SOE).
Detailed Synthesis: Placebo-Controlled Trials of Benzodiazepines
We found no studies with low or medium risk of bias meeting our inclusion criteria.169
Detailed Synthesis: Selective Serotonin Reuptake Inhibitors
Characteristics of Studies Table 21 summarizes the characteristics of the 17 studies meeting our inclusion criteria. Further details describing the studies are provided in Appendix F.
Sample sizes ranging from 12 to 563 tested SSRI efficacy over a duration of 5 to 12 weeks of treatment. The mean age of subjects in the samples spanned from 36 to 46 years; females comprised the majority of samples in 9 of 16 studies.47, 62, 64–66, 68, 170, 175, 177 The primary outcome for the majority of studies included some version of the CAPS (CAPS, CAPS-2, or CAPS-Sx); 5 studies identified other primary outcomes, including Treatment Outcome PTSD Scale,61, 62 DTS,178 Duke Global Rating for PTSD,170 IES,71, 172 or Short PTSD Rating Interview (SPRINT).174
Results of Placebo-Controlled Trials of Selective Serotonin Reuptake Inhibitors
PTSD Symptoms
Our meta-analyses found that subjects who received paroxetine, fluoxetine, and sertraline (but not citalopram) had significantly greater decreases in CAPS-assessed PTSD symptoms than subjects who received placebo (Figure 22). The single citalopram study indicated greater decreases in PTSD symptoms among placebo subjects than citalopram subjects, although differences did not reach statistical significance (insufficient SOE). Each of the four fluoxetine studies (five comparisons shown because one study included two fluoxetine arms that compared different doses of the drug) favored fluoxetine (SMD, −0.28; 95% CI, −0.42 to −0.14; I squared=0.0%; moderate SOE). For paroxetine, two studies (one that compared two doses of paroxetine with placebo) each found significant decreases in PTSD symptoms among paroxetine versus placebo subjects (CAPS SMD of −0.56, −0.46, or −0.44 in each study; moderate SOE). Although only three of the seven sertraline studies indicated significant benefit of sertraline for PTSD symptoms, the meta-analysis of pooled data indicated a significant but modest difference of about 5 points on the CAPS between groups (SMD, −0.20; 95% CI, −0.20; 95% CI, −0.36 to −0.04; low SOE). Studies that used other PTSD symptom assessments had consistent findings.61, 71, 170, 174
Remission
Two studies each favored paroxetine, one significantly so (RD, 0.13; p=0.008 in one large study65 and RD, 0.19; p=0.34 in one small study;174 moderate SOE). The other studies were single trials of fluoxetine47 and sertraline69 that each found slight (but insignificant) between-group differences in remission (insufficient SOE).
Loss of PTSD Diagnosis
A single fluoxetine study favored fluoxetine over placebo for loss of PTSD diagnosis (RD, 0.14, p=0.23) but not significantly so (insufficient SOE).47
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
Eleven of the SSRI studies reported between-group changes in depression symptoms (Figure 23). One small study provided insufficient evidence to determine efficacy of citalopram for reducing comorbid depression in adults with PTSD.175 The three fluoxetine studies had mixed results with limited evidence of no between-group differences (low SOE for no difference); one study evidenced significant benefit of fluoxetine,61 another study that tested two different doses of fluoxetine favored both drug arms but not significantly so,62 and the third study found the placebo group to have nonsignificantly greater decreases in depression than fluoxetine participants (p=ns).47 Both paroxetine studies found significantly greater decreases among intervention group versus placebo group subjects in depression symptoms (moderate SOE).64, 65 Decreases in depression symptoms at end-of-treatment did not differ between sertraline and placebo groups (SMD, −0.14; 95% CI, −0.33 to 0.06, 7 studies, N=1,085; low for no difference).
Four studies assessed the efficacy of SSRIs for anxiety symptoms (Figure 24). Both fluoxetine studies favored the treatment group, but only one significantly so.61, 62 The two sertraline studies found effect sizes in the opposite direction, with one study favoring sertraline and the other favoring placebo (insufficient SOE).68, 70
Quality of Life
Two studies of sertraline66, 69 each demonstrated efficacy for quality of life, but only one significantly so (between-group mean difference, 2.4; p=ns in one study and between-group mean difference, 8.4; p<0.05 in another; low SOE).66, 69
Disability or Functional Impairment
Four studies assessed disability differences across SSRI and placebo groups. One study each of fluoxetine170 and sertraline69 provided limited evidence of group differences in disability assessment (insufficient SOE). Two studies64, 65 provided evidence for the efficacy of paroxetine on pre- to posttreatment changes in disability (moderate SOE).
Detailed Synthesis: Serotonin and Norepinephrine Reuptake Inhibitors
Characteristics of Studies
Table 22 summarizes the characteristics of the two studies meeting our inclusion criteria. Further details describing the included studies are provided in Appendix F. Both studies evaluated venlafaxine extended release among a heterogeneous group of subjects with a variety of index trauma types. Both studies used CAPS to assess the primary outcome.
Results of Serotonin and Norepinephrine Reuptake Inhibitors
PTSD Symptoms
Both studies reported similar significant between-group decreases in CAPS-assessed PTSD symptoms from pre- to posttreatment (SMD of −0.35 and −0.26 across two individual studies; Appendix H; moderate SOE).69, 73
Remission
Both venlafaxine studies reported significant between-group differences in remission at the end of treatment assessment (RD of 0.12 and 0.15 in the 2 studies; moderate SOE). One also reported continued benefit at the 3-month followup assessment (RD, 0.13; moderate SOE).73
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
Both studies found significant benefit of venlafaxine for depression symptoms (HAM-D between-group mean difference of −2.6 and −1.6 in the 2 studies; moderate SOE).69, 73
Quality of Life
Both studies found significant benefit of venlafaxine for quality of life (Quality of Life Enjoyment and Life Satisfaction Short Form between-group mean difference of 2.8 and 4.1 in the 2 studies; moderate SOE).69, 73
Disability or Functional Impairment
Both studies found significant benefit of venlafaxine for disability (SDS between-group difference of −2.1 and −2.0 in the 2 studies; moderate SOE) and functioning (Global Assessment of Functioning between-group difference of 2.8 and 4.0 in the 2 studies; moderate SOE).69, 73
Detailed Synthesis: Placebo-Controlled Trials of Tricyclic Antidepressants
We did not find any trials comparing tricyclic antidepressants with placebo or other medications that had low or medium risk of bias.179–182
Detailed Synthesis: Placebo-Controlled Trials of Other Second-Generation Antidepressants
Table 23 summarizes the characteristics of the two studies that met our inclusion criteria. Further details describing the included studies are provided in Appendix F.
Of the two included small, placebo-controlled trials, one assessed bupropion183 and one assessed mirtazapine.184 Both studies enrolled a heterogeneous group of middle-aged subjects with a variety of index trauma types (e.g., military combat or war trauma, childhood sexual abuse, physical abuse, rape, MVA, witnessing a trauma, death or suicide of a loved one).
Results of Other Second-Generation Antidepressants
PTSD Symptoms
Both included studies reported various measures of PTSD symptoms.183, 184 All analyses favored the treatment group, but most comparisons did not indicate significant differences across groups. Overall, we found insufficient evidence to determine the efficacy of either bupropion or mirtazapine for PTSD symptoms (insufficient SOE).
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
The single bupropion and single mirtazapine studies each favored the treatment group for depression symptoms, but differences did not reach statistical significance across groups (insufficient SOE).183, 184 The mirtazapine study reported greater decreases in anxiety symptoms among the treatment versus placebo groups (between-group difference, −1.6, p<0.05; insufficient SOE); the bupropion study did not examine anxiety symptom outcomes.184
Detailed Synthesis: Head-to-Head (Comparative Effectiveness) Pharmacotherapy Trials
Characteristics of Studies
Table 24 summarizes the four studies that met inclusion criteria. Further details are provided in Appendix F. One study enrolled veterans randomized to paroxetine plus naltrexone (arm not eligible), paroxetine plus placebo, desipramine plus naltrexone (arm not eligible), or desipramine plus placebo.185
Results of Head-to-Head Pharmacotherapy Trials
PTSD Symptoms
All four studies assessed PTSD symptoms.69, 175, 185, 186 The study that compared paroxetine+placebo versus desipramine+placebo found similar decreases in PTSD symptoms across groups (CAPS between-group difference of −3.2 favoring desipramine+placebo, p=ns, insufficient SOE).185 The studies that tested venlafaxine extended release (ER) versus sertraline also found similar decreases in PTSD symptoms across groups (CAPS between-group difference of −2.1 favoring sertraline, p=ns and Harvard Trauma Questionnaire between-group difference of −0.09 favoring sertraline, p=ns, low SOE for no difference).69, 186 The fourth head-to-head trial favored sertraline over citalopram for PTSD symptom outcome comparisons; however, differences did not reach statistical significance (CAPS between-group difference of −11.1; p=ns; insufficient SOE).175
Remission
The one head-to-head trial that reported remission favored venlafaxine over sertraline for PTSD symptoms, but differences did not reach statistical significance (CAPS between-group mean difference of −5.9; p=ns, insufficient SOE).69
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
All four studies assessed depression symptoms.69, 175, 185, 186 The study that compared paroxetine+placebo versus desipramine+placebo found similar decreases in depression symptoms across groups (HAM-D between-group difference of −1.3 favoring paroxetine+placebo, p=ns, insufficient SOE).185 The studies that tested venlafaxine ER versus sertraline also found similar decreases in depression symptoms across groups (HAM-D between-group mean difference of −0.1 favoring venlafaxine, p=ns in one study and −0.7 in the other; moderate SOE for no difference). The fourth head-to-head trial favored citalopram over sertraline for depression symptoms; however, differences did not reach statistical significance (BDI between-group difference of −2.9; p=ns; insufficient SOE).175
One study that compared anxiety symptoms across groups found no differences between citalopram and sertraline groups (insufficient SOE).186
The study that tested paroxetine+placebo versus desipramine+placebo among veterans with comorbid alcohol dependence found greater decreases in the percentage of heavy drinking days (p=0.009) and drinks per drinking days (p=0.027) among subjects in the desipramine+placebo group than among those in the paroxetine+placebo group (low SOE).185
Quality of Life
Two studies compared the efficacy of venlafaxine ER and sertraline for quality-of-life outcomes. One study favored venlafaxine69 and the other favored sertraline,186 but the differences across treatments did not reach statistical significance in either study (low SOE for no difference).
Disability or Functional Impairment
The findings for disability differences across groups mirrored those for quality-of-life differences. That is, two studies compared the efficacy of venlafaxine ER and sertraline for disability outcomes. One study favored venlafaxine69 and the other favored sertraline,186 but the differences across treatments did not reach statistical significance in either study (low SOE for no difference).
Network Meta-Analysis of Pharmacotherapy Trials
We conducted network meta-analyses on pre- to posttreatment decreases in PTSD symptoms as measured by the CAPS to determine the comparative effectiveness of drug treatments using all efficacy and comparative effectiveness evidence compiled to answer KQ 2. We did this after checking for any obvious transitivity which would preclude the use of network meta-analysis due to inconsistencies in the sample or treatment characteristics. We did not find any such inconsistencies.
Our network meta-analysis included 33 trials and 13 active treatments (4,491 subjects) that included CAPS-measured PTSD symptoms. A network diagram illustrates the number of subjects contributing to each comparison; thickness of lines connecting each drug-drug or drug-placebo comparison indicates the number of trials with available data for that comparison (Figure 25). We conducted two tests to assess the consistency for the network meta-analysis. First, we compared consistency and inconsistency models that did not differ significantly (χ2(3)=0.06, p=0.997). Next, when possible given the network structure, we tested for differences between direct and indirect estimates using network sidesplits. Direct and indirect estimates did not differ significantly for any of the sidesplit comparisons (Table 25).
Figure 26 presents the network meta-analysis findings for each between-group treatment comparison of pre- to posttreatment change in CAPS-assessed PTSD symptoms (SMD and 95% CI displayed for each comparison). We report the findings on the pharmacological interventions for which analyses in the prior section of this report determined at least moderate SOE of efficacy for PTSD symptoms (fluoxetine, paroxetine, and venlafaxine).
Our network meta-analysis evidenced no significant differences between effectiveness of paroxetine, fluoxetine, and venlafaxine.
Indirect evidence from placebo-controlled trials contributed the majority of evidence to the network meta-analysis. Only four head-to-head comparisons contributed data to the network, none of which compared effectiveness between interventions determined to have at least moderate SOE of efficacy for PTSD symptoms (fluoxetine, paroxetine, and venlafaxine); because indirect comparisons provided the basis for most of the network meta-analysis, we believe the findings only support a low SOE of benefit.
KQ 2a. Variability in Efficacy or Comparative Effectiveness of Pharmacological Interventions by Patient Characteristics or Type of Trauma
This KQ evaluated whether the efficacy or comparative effectiveness of any of the pharmacological interventions differed by patient characteristics or type of trauma experienced. To answer this question, we present findings from included studies that reported outcomes for subgroups of interest (defined by patient or trauma factors) and compare the efficacy or comparative effectiveness across subgroups. One study provided information about the efficacy of a pharmacological intervention across different subgroups of interest.
Key Point
- One study compared the efficacy of fluoxetine versus placebo between subjects with child- versus adult-onset trauma and found no differences in efficacy by trauma onset (insufficient SOE for a single study of unknown consistency and imprecise findings).
Detailed Synthesis: Patient Characteristics or Trauma Types
Characteristics of Included Studies
Table 26 summarizes the characteristics of the included study previously described in this report. The study had an end of treatment assessment at 8 weeks and a 6-month followup that included the CAPS as the primary outcome measure of PTSD symptoms. Additional details describing the included study can be found in Appendix F.
The analysis conducted to answer this KQ had a small sample size, and therefore did not have the statistical power to detect anything but a very large difference. Many factors other than patient characteristics and trauma type varied across studies, which prevented us from conducting further subgroup analyses using studies that did not, a priori, seek to examine differences in efficacy or comparative effectiveness of interventions across subgroups. Thus, findings should be considered hypothesis generating.
The single study compared EMDR, fluoxetine, and placebo in subjects with a variety of trauma types including child sexual abuse, child physical abuse, child sexual and physical abuse, adult sexual assault, adult physical assault, domestic violence, other adult victimization, traumatic loss, war/terror/violence, and injury/accident.47 The authors reported subgroup analyses for those with child-onset trauma and those with adult-onset trauma.
Efficacy or Comparative Effectiveness by Patient Characteristic or Trauma Type
The study compared the efficacy of fluoxetine versus placebo between those with childhood-onset (prior to age 18) versus adult-onset trauma.47 No significant index trauma onset by treatment differences was found in the efficacy of fluoxetine compared with placebo by trauma onset (child versus adult) as tested by interaction analysis.
KQ 3. Psychotherapy Versus Pharmacotherapy for Adults With PTSD
This KQ focused on studies that directly compared a psychological treatment with a pharmacological treatment.
Key Point
- One study that tested the comparative effectiveness of EMDR versus fluoxetine for reduction in PTSD symptoms, rates of symptom remission, and loss of PTSD diagnosis and found insufficient evidence to draw conclusions.
Detailed Synthesis
Characteristics of Studies
We found one medium risk of bias study that met our inclusion criteria. Table 27 summarizes the characteristics of the study. Further details are provided in Appendix F.
One study compared subjects with varying trauma types randomized to 8 weeks of fluoxetine, EMDR, or placebo.47 Prior KQ 1 and KQ 2 results sections include findings from the placebo comparisons.
Results for Psychotherapy Versus Pharmacotherapy
PTSD Symptoms
EMDR and fluoxetine groups did not have significant differences in pre- to posttreatment decreases in CAPS-assessed PTSD symptoms (between-group mean difference, −10.1 favoring fluoxetine; p=0.13; insufficient SOE). EMDR group subjects, however, had significantly greater decreases in PTSD symptoms than fluoxetine group subjects measured at the 6-month followup assessment (between-group mean difference, −16.3; p<0.005; insufficient SOE).
Remission
Comparisons favored EMDR-treated subjects compared with fluoxetine-treated subjects for remission at both the end of treatment and 6-month followup assessments, but only the followup comparisons reached statistical significance (end of treatment RD, 0.15; p=0.17; 6-month followup RD, 0.58, p<0.001; insufficient SOE).
Loss of PTSD Diagnosis
The percentages of subjects no longer meeting diagnostic criteria for PTSD were similar for EMDR compared with fluoxetine at the end of treatment (RD, 0.03 favoring EMDR; p=0.82; insufficient SOE) and 6-month followup assessment (RD, 0.15, p=0.20; insufficient SOE).
Prevention or Reduction of Comorbid Medical or Psychiatric Conditions
Comparisons favored EMDR-treated subjects compared with fluoxetine-treated subject for depression symptoms at both the end of treatment and 6-month followup assessments, but only the followup comparisons reached statistical significance (BDI end of treatment between-group mean difference, −1.9; p=ns; 6-month followup between-group mean difference, −6.8, p<0.001; insufficient SOE).
KQ 3a. Variability in Comparative Effectiveness of Psychological Versus Pharmacological Interventions by Patient Characteristics or Type of Trauma
This KQ evaluated whether the efficacy or comparative effectiveness of psychological and pharmacological interventions differed by patient characteristics or types of trauma experienced. To answer this question, we present findings from included studies that reported outcomes for subgroups of interest (defined by patient or trauma factors) and compare the efficacy or comparative effectiveness across subgroups. One study provided information about the comparative effectiveness of a psychological and a pharmacological intervention across different subgroups of interest.
Key Point
- One study evaluated the comparative effectiveness of EMDR versus fluoxetine between those with child- and adult-onset trauma (insufficient SOE).
Detailed Synthesis: Patient Characteristics or Trauma Types
Characteristics of Included Studies
Table 28 summarizes the characteristics of the one included study previously described in this report. The study had an end of treatment assessment at 8 weeks and a 6-month followup that included the CAPS as the primary outcome measure of PTSD symptoms. Additional details describing the included study can be found in Appendix F.
One study compared EMDR, fluoxetine, and placebo in subjects with a variety of trauma types.47 The authors reported comparisons between the comparative effectiveness of EMDR versus fluoxetine between those with child- and adult-onset trauma.
Comparative Effectiveness by Patient Characteristics or Trauma Type
The study examined the comparative effectiveness of EMDR versus fluoxetine between those with childhood-onset (prior to age 18) versus adult-onset trauma.47 Analyses indicated no significant differences in the comparative effectiveness of EMDR and fluoxetine between those with child- and adult-onset trauma (insufficient SOE).
KQ 4. Adverse Effects of Treatments for PTSD
For this question, we evaluated the studies included in KQs 1 through 3. In addition, we searched for non-RCTs and observational studies (specifically, prospective cohort studies with an eligible comparison group, and case-control studies). We did not find any nonrandomized trials or observational studies that met our inclusion criteria (e.g., prospective cohort studies or case-control studies with a sample size of at least 500; see the Methods section), nor did we exclude any observational studies solely for having a smaller sample size. Therefore, the results for this KQ included AE data from studies included in KQs 1 through 3. Throughout this section, we describe risks of various AEs reported as absolute RDs between intervention and control. Appendix F includes detailed information about specific AE findings for each study. Appendix G contains detailed information about the inputs to determine the SOEs of each study.
Key Points: Adverse Effects of Psychological and Pharmacological Treatments for Adults With PTSD
- Studies typically did not report the use of methods to systematically capture AE information collected by standardized measures.
- The few head-to-head trials provided insufficient evidence to compare AEs across different interventions.
- Insufficient SOE provides information regarding AEs associated with psychological treatments.
- Insufficient SOE provides information regarding most specific AEs such as mortality, suicidality, self-harmful behaviors, and withdrawals due to AEs for pharmacological treatments.
- Placebo comparisons of specific AEs between pharmacological treatments with at least moderate SOE of efficacy indicated small increased risk of the following AEs:
- –
nausea, somnolence, and diarrhea for fluoxetine versus placebo (low SOE)
- –
nausea, dry mouth, and somnolence for paroxetine versus placebo (low SOE)
- –
nausea (moderate SOE), dry mouth (low SOE) and constipation (low SOE) for venlafaxine versus placebo
Detailed Synthesis: Psychological Treatments
Characteristics of Studies
The KQ 1 section of the report described brief characteristics of psychological studies rated low or medium risk of bias that included AE assessments. Appendix F contains full information about AE reporting and outcomes for each study.
Withdrawals Due to Adverse Effects
Just 3 of the 31 CBT-M studies,31, 32, 36 1 of the 25 CBT-exposure studies;18 and 2 of the 10 EMDR studies43, 48 reported information about withdrawals due to AEs (insufficient SOE).
Mortality
Just four CBT-exposure studies reported mortality.12, 20, 138, 139 The CBT-exposure groups totaled two deaths across studies; six deaths occurred in the comparator groups (PCT, wait-list, or treatment as usual; insufficient SOE).
Suicide, Suicidal Ideation, or Self-Harmful Behaviors
Eleven of the included studies from KQ 1 reported information about suicide or self-harm—1 of 12 CBT-cognitive intervention studies,127 1 of 10 CBT-coping skills studies,136 5 CBT-exposure studies,12, 20, 53, 138, 139 and 2 of 31 CBT-M studies.23, 144 Studies that did report suicidality reported few occurrences of several different suicidality measures and self-harm behaviors (insufficient SOE).12
Other Specific Adverse Effects
A few studies included other measures of AEs such as symptom deterioration, hospitalizations, and serious AEs (Appendix F), but most tested different interventions, comparators, and outcomes that limit the ability to synthesize the data to make definitive conclusions.
Detailed Synthesis: Pharmacological Treatments
Characteristics of Studies
The KQ 2 section of the report described brief characteristics of pharmacological studies rated low or medium risk of bias that provided the evidence base for the assessment of AEs of pharmacological interventions.
Withdrawals Due to Adverse Effects
All but six47, 63, 76, 82, 165, 175 of the pharmacotherapy studies reported data on withdrawals due to AEs (data shown in Appendix F). The wide variation in interventions and comparators, however, precluded the ability to pool evidence to determine differences across most pharmacotherapy groups (insufficient SOE). Appendix H displays the meta-analysis pooled findings of anticonvulsants, antipsychotics, and SSRIs. The analysis of pooled data likely does not have adequate power to detect significant differences, although RDs of individual and pooled studies do not appear to indicate a clinically meaningful difference in withdrawals due to AEs across treatment groups (insufficient SOE). Appendix G provides additional details for SOE grades.
Mortality
Only three of the pharmacotherapy studies reported mortality.62, 69, 165 Across these studies, one death occurred in the pharmacotherapy group (which authors did not deem related to use of the drug) and one in the comparator group (insufficient SOE).62, 69, 165 Appendix F contains additional details about the specific number of AEs reported in each study.
Suicide, Suicidal Ideation, or Self-Harmful Behaviors
Four of the included medication studies reported data on suicidality using various measures or self-harmful behaviors.62, 76, 165, 174 Few reported events in each study, and the inability to determine the relationship with the drug itself precludes conclusions about these associations (insufficient SOE). Appendix F contains additional details about the specific number of AEs reported in each study.
Other Specific Adverse Effects, by Medication
Limited information about specific AEs reported for most of the medications precluded synthesis of these findings. We therefore focus here on the medications with moderate SOE supporting efficacy (see KQ 2)—fluoxetine, paroxetine, and venlafaxine—to conduct additional meta-analyses for specific AEs. Appendix F contains additional details about the specific number of AEs reported in each study.
SSRIs Compared With Placebo
Fluoxetine Compared With Placebo
Of the five studies that compared fluoxetine with placebo, three reported data on some specific AEs (additional details available in Appendix F).62, 63, 173
Overall, insufficient findings for fluoxetine prevented determination of between-group differences in specific AEs. Three studies (total N=756) contributed data about specific AEs, with most specific AEs only reported by one study each. Evidence from two studies62, 173 suggests increases, although not statistically significant in either study, in the risk of nausea (RD range 0.03 to 0.07; p=ns; N=712; low SOE) and somnolence (RD range 0.04 to 0.06 [variation by dose of fluoxetine]; p=ns; 1 study; N=411). Another small fluoxetine study found large, statistically significant differences in the risk of diarrhea among fluoxetine versus placebo subjects (RD, 0.24, p<0.05; 1 study; N=44; low SOE).
Paroxetine Compared With Placebo
Of the three studies that compared paroxetine with placebo, two reported data for a few specific AEs.65, 174 The third provided more ambiguous summary data of specific AEs.64
Overall, evidence from paroxetine studies was insufficient to determine whether the RD of most specific AEs between drug and placebo. Single studies reported events for just a few outcomes; few events occurred. Evidence from a single study (N=323) did suggest a significant increase in nausea (RD, 0.11; p<0.05), dry mouth (RD, 0,10; p<0.05), and somnolence (RD, 0.13; p<0.05). Studies provided limited data for other specific AEs (insufficient SOE).
Venlafaxine Compared With Placebo
Of the two studies that compared venlafaxine with placebo (total N=687), both reported data on several specific AEs.69, 73 Overall, findings suggest statistically significant increases in the venlafaxine compared with placebo group subjects in the risk of nausea (RD, 0.10 in both individual studies, p<0.05 in both studies; moderate SOE), dry mouth (RD, 0.04; p=ns in one study; RD, 0.08; p<0.05 in the other study; low SOE), and constipation (RD, 0.02; p=ns in one study; RD, 0.09; p<0.05 in the other study, low SOE). No studies found significant differences in insomnia, fatigue, somnolence, or decreased appetite between venlafaxine- versus placebo-treated adults with PTSD (insufficient SOE).
Detailed Synthesis: Head-to-Head Studies of Psychological and Pharmacological Interventions
No KQ 3 studies reported AEs.
Contextual Question (CQ) 1a. Components of Effective Psychological Treatments
For this CQ, we searched for articles that examined the components of effective psychological treatments (e.g., frequency or intensity of therapy and/or aspects of the therapeutic modality). We found only one recently published article that addressed this CQ.187 The article, authored by “pioneer” creators of an empirically based psychotherapy to treat PTSD, focuses on components of their treatment believed to be most critical in its effectiveness. The review article synthesizes the collected information to conclude that most frequently identified components include psychoeducation, coping skills and emotion regulation, cognitive processing and restructuring (i.e., “meaning making”), IE, emotions, and memory processing.
CQ 1b. Degree of Fidelity of Psychological Interventions Effective in Trial Settings When Implemented in Clinical Practice Settings
For this CQ, we searched for articles that aimed to determine the degree of fidelity of psychological interventions effective in trial settings when implemented in clinical practice settings. We found no direct evidence to help answer this CQ. We comment on the fidelity assessment of each psychological intervention in our risk of bias assessment included in Appendix E, but none of these studies or others we identified in our searches specifically tested the application of implementing efficacious interventions in clinical settings.
Figures
Figure 3Standardized mean change in PTSD symptoms (measured by the Clinician-Administered PTSD Scale) for CPT compared with inactive comparators
CAPS = Clinician-Administered PTSD Scale; CI = confidence interval; CPT = cognitive processing therapy; PTSD = posttraumatic stress disorder; SMD = standardized mean difference.
Figure 7Standardized mean change from baseline to end of treatment in PTSD symptoms (any measure) for exposure therapy compared with inactive comparator
CI = confidence interval; PTSD = posttraumatic stress disorder; PE = prolonged exposure; SMD = standardized mean difference; VRE = virtual reality exposure.
Figure 8Standardized mean change from baseline to end of treatment in PTSD symptoms (CAPS) for exposure therapy compared with inactive comparator
CAPS = Clinician-Administered PTSD Scale; CI = confidence interval; PE = prolonged exposure; SMD = standardized mean difference; VRE = virtual reality exposure.
Figure 10Standardized mean change from baseline to end of treatment in depression symptoms (BDI) for exposure therapy compared with inactive comparator
BDI: Beck Depression Inventory; CI = confidence interval; PE = prolonged exposure; SMD = standardized mean difference; VRE = virtual reality exposure.
Figure 22Standardized mean change from baseline in CAPS for selective serotonin reuptake inhibitors compared with placebo
CAPS = Clinician-Administered PTSD Scale; CI = confidence interval; PTSD = posttraumatic stress disorder; SMD = standardized mean difference; SSRI = selective serotonin reuptake inhibitor.
Tables
Table 4Outcome measure tools commonly used in the included trials
| Abbreviated Name | Complete Name | Range of Scores |
|---|---|---|
| BDIa | Beck Depression Inventory | 0 to 63 |
| CAPSb | Clinician-Administered PTSD Scale | 0 to 136 |
| DGRP | Duke Global Rating for PTSD scale | 1 = very much improved; 2 = much improved; >2 = nonresponders |
| DTS | Davidson Trauma Scale | 0 to 136 |
| GAF | Global Assessment of Functioning | 0 to 100 |
| HADS | Hospital Anxiety and Depression Scale | 0 to 21 |
| HAM-A or HAS | Hamilton Anxiety Rating Scale | 0 to 56 |
| HAM-Da | Hamilton Depression Rating Scale | 0 to 54 |
| IES | Impact of Event Scale | 0 to 75 |
| IES-R | Impact of Event Scale-Revised | 0 to 88 |
| MADRS | Montgomery-Åsberg Depression Rating Scale | 0 to 60 |
| MISS or M-PTSD | Mississippi Scale for Combat-related PTSD | 35 to 175 |
| PCLc | PTSD Checklist | 17 to 85 |
| PSS-I | PTSD Symptom Scale Interview | 0 to 51 |
| PSS-SR | PTSD Symptom Scale Self-report Version | 0 to 51 |
| PTDS or PDS | Posttraumatic Diagnostic Scale | 0 to 51 |
| Q-LES-Q-SF | Quality of Life Enjoyment and Life Satisfaction Short Form | 0 to 70 (raw score) |
| SCL-90-R | Symptom Checklist- 90-Revised | 0 to 360 |
| SDS | Sheehan Disability Scale | 0 to 30 |
| SF-12 | Medical Outcome Study Self-Report Form (12 item) | 0 to 100 |
| SF-36 | 36-Item Short Form Health Survey | 0 to 100 |
| SI-PTSD or SIP | Structured Interview for PTSD | 0 to 68 |
| SPRINT | Short PTSD Rating Interview | 0 to 32 |
| STAI | State–Trait Anxiety Inventory | 20 to 80 |
| WAS | Work and Social Adjustment Scale | 0 to 40 |
- a
A 3‑point improvement has been considered clinically meaningful.119
- b
Some experts suggest that a reduction of 15 points on the Clinician-Administered PTSD Scale (CAPS) constitutes a clinically significant reduction.120
- c
Some researchers have considered a reduction of 5 or more points to indicate a clinically significant response.121
Table 5Classes and categories of psychological treatments for posttraumatic stress disorder
| Class of Treatment | Categories of Treatment | Subcategories of Treatment |
|---|---|---|
| Cognitive behavioral therapy (CBT) | CBT-cognitive interventions | Cognitive processing therapy (CPT) Cognitive restructuring (CR) Cognitive therapy (CT) Meta Cognitive Therapy (MCT) |
| CBT-coping skills | Relaxation training Stress inoculation training (SIT) Structured approach therapy (SAT) | |
| CBT-exposure | Imaginal exposure (IE), In vivo exposure Prolonged exposure (PE) or modified PE (mPE) Concurrent Treatment of PTSD and Substance Use Disorders using Prolonged Exposure (COPE) Virtual reality exposure (VRE) Written exposure therapy (WET) | |
| CBT-Ma | See footnotea | |
| Eye movement desensitization and reprocessing (EMDR) | None | NA |
| Other psychotherapies | Brief eclectic psychotherapy (BEP) | NA |
| Emotional freedom techniques (EFT) | NA | |
| Hypnosis | NA | |
| Interpersonal therapy (IPT) | NA | |
| Imagery rehearsal therapy (IRT) | NA | |
| Memory specificity training (MEST) | NA | |
| Mindfulness-based stress reduction (MBSR) | NA | |
| Narrative exposure therapy (NET) | NA | |
| Neurofeedback training (NF) | NA | |
| Seeking Safety (SS) | NA | |
| Trauma affect regulation (TAR) | NA |
- a
Mixed CBT trials had elements of the following types: breathing retraining, cognitive restructuring, crisis/safety planning, exposure (imaginal, in vivo, or both), guided imagery, mindfulness training, psychoeducation, relapse prevention, relaxation training, self-monitoring, skills training, and stress management.
BEP = brief eclectic psychotherapy; CBT = cognitive behavioral therapy; CBT-M = CBT mixed; COPE = Concurrent Treatment of PTSD and Substance Use Disorders using Prolonged Exposure; CPT = cognitive processing therapy; CR = cognitive restructuring; CT = cognitive therapy; EFT = emotional freedom techniques; EMDR = eye movement desensitization and reprocessing; IE = imaginal exposure; IPT = interpersonal therapy; IRT = imagery rehearsal therapy; MBSR = mindfulness-based stress reduction; MEST = memory specificity training; mPE = modified PE; NA = not applicable; NET = narrative exposure therapy; NF = neurofeedback training; PCT = present-centered therapy; PDT = psychodynamic therapy; PE = prolonged exposure; SAT = structured approach therapy; SIT = stress inoculation training; SS = Seeking Safety; TAR = trauma affect regulation; VRE = virtual reality exposure; WRE = written exposure therapy.
Table 6Summary of efficacy and strength of evidence of PTSD psychological treatments
| Treatment | Outcome | N Trials (Subjects) | Findings | SOE |
|---|---|---|---|---|
| Cognitive processing therapy | PTSD symptomsa | 5 (399)1–4, 6 | Reduced PTSD symptoms SMD −1.35 (95% CI, −1.77 to −0.94) | Moderate |
| Loss of PTSD diagnosis | 4 (299)1–4 | Greater loss of PTSD diagnosis RD 0.44 (95% CI, 0.26 to 0.62) | Moderate | |
| Depression symptomsb | 5 (399)1–6 | Reduced depression symptoms SMD −1.09 (95% CI, −1.52 to −0.65) | Moderate | |
| Cognitive therapy | PTSD symptomsa | 4 (283)5, 7–9 | Reduced PTSD symptoms SMD of individual studies range from −2.0 to −0.3 | Moderate |
| Loss of PTSD diagnosis | 4 (283)5, 7–9 | Greater loss of PTSD diagnosis RD 0.55 (95% CI, 0.28 to 0.82) | Moderate | |
| Depression symptomsb | 4 (283)5, 7–9 | Reduced depression symptoms Between-group mean differences of individual trials ranged from −11.1 to −8.3 | Moderate | |
| Cognitive behavioral therapy-exposure | PTSD symptomsa | 13 (885)3, 10–21 8 (689)3, 10, 11, 13, 16, 18, 20, 21 | Reduced PTSD symptoms SMD −1.23 (95% CI, −1.50 to −0.97) SMD CAPS −1.12 (95% CI, −1.42 to −0.82) | High |
| Loss of PTSD diagnosis | 6 (409)3, 13, 14, 16, 17, 21 | Greater loss of PTSD diagnosis RD 0.56 (95% CI, 0.35 to 0.78) | Highc | |
| Cognitive behavioral therapy-exposure (continued) | Depression symptomsb | 10 (715)3, 11–15, 18–21 | Reduced depression symptoms SMD −0.76 (95% CI, −0.91 to ‑0.60) | High |
| Cognitive behavioral therapy-mixed | PTSD symptomsa | 21 (1,349)12, 14, 22–40 11 (709)22, 23, 27–29, 34–39 | Reduced PTSD symptoms SMD −1.01 (95% CI, −1.28 to −0.74) SMD −1.24 (95% CI, −1.67 to −0.81) | Highc |
| Loss of PTSD diagnosis | 9 (474)22–24, 31–34, 39, 41 | Greater loss of PTSD diagnosis RD 0.29 (95% CI, 0.11 to 0.41) | Highc | |
| Depression symptomsb | 15 (929)12, 14, 22–24, 28, 29, 33, 35–40, 42 | Reduced depression symptoms SMD −0.87 (−1.14 to −0.61) | Highc | |
| Eye movement desensitization and reprocessing | PTSD symptomsa | 8 (449)13, 16, 43–48 | Reduced PTSD symptoms SMD −1.08 (95% CI, −1.82 to −0.35) | Moderated |
| Loss of PTSD diagnosis | 7 (427)13, 16, 43–45, 47, 48 | Greater loss of PTSD diagnosis RD 0.43 (95% CI, 0.25 to 0.61) | Moderate | |
| Depression symptomsb | 7 (347)13, 43–48 | Reduced depression symptoms SMD −0.91 (95% CI, −1.58 to ‑0.24) | Moderate | |
| Brief eclectic psychotherapy | Loss of PTSD diagnosis | 3 (96)49–51 | Greater loss of PTSD diagnosis RD of individual studies ranged from 0.13 to 0.58 | Low |
| Depression symptomsb | 3 (96)49–51 | Reduced depression symptoms | Low | |
| Imagery rehearsal therapy | PTSD symptomsa | 1 (168)52 | Reduced PTSD symptoms Between-group mean difference −21.0; p<0.05 | Low |
| Narrative exposure therapy | PTSD symptomsa | 3 (232)53–55 | Reduced PTSD symptoms SMD range from −1.95 to −0.79 across 3 individual studies | Moderate |
| Loss of PTSD diagnosis | 2 (198)53, 54 | Greater loss of PTSD diagnosis RD of 0.06 and 0.43 in individual studies | Low | |
| Seeking Safety | PTSD symptomsa | 3 (232)56–58 | Reduced PTSD symptoms SMD of individual trials ranged from −0.22 to 0.04 | Low for no difference |
| Trauma affect regulation | PTSD symptomsa | 2 (173)59, 60 | Reduced PTSD symptoms Between-group mean difference of −17.4 and −2.7 in individual studies | Low |
NOTE: Outcomes graded as insufficient are not included in this table.
- a
SMD from the Clinician-Administered PTSD Scale; SMD from various PTSD symptom scales.
- b
SMD from the Beck Depression Inventory; SMD from various depression symptom scales.
- c
SOE increased from moderate to high because of additional evidence of efficacy published since prior PTSD review.
- d
SOE increased from low to moderate because of additional evidence of efficacy published since prior PTSD review.
CAPS = Clinician-Administered PTSD Scale; CI = confidence interval; N = number of subjects; PTSD = posttraumatic stress disorder; RD = risk difference; SMD = standardized mean difference; SOE = strength of evidence.
Table 7Characteristics of included cognitive intervention trials
| Study | Arm (N) | Duration of Treatment (Followup) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | Percent Female | Percent Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Chard et al., 20052 | CPT (36) MA (35) | 17 weeks (3 and 12 months) | Female Childhood sexual abuse | 65.5 to 68.3 | 33 | 100 | 19 | Medium |
| Ehlers et al., 20035 | CT (28) SHB (28) RA (29) | Mean of 9 weeks, 0 to 3 booster sessions (3, 6, and 9 months) | Male and female MVA | PDS (frequency) 30.0 PDS (distress) 30.8 | 39 | 72 | 97 | Medium |
| Ehlers et al., 20058 | CT (14) WL (14) | 4 to 12 weeks plus up to 3 monthly boosters (3 and 6 months) | Male and female Mixed | CAPS (frequency) 31.6 to 42.0 CAPS (intensity) 29.0 to 36.5 | 37 | 54 | 4 | Medium |
| Ehlers et al., 20149 | Intensive CT (30) Standard CT (31) Supportive Therapy (30) WL (30) | 14 weeks (27 weeks, 40 weeks for all but WL) | Chronic PTSD Mixed | 73 | 39 | 59 | 30 | Low |
| Forbes et al., 20124 | CPT (30) TAU (29) | 12 weeks (3 months) | Male and female Military related | 65.8 to 75.5 | 53 | 3 | 0 | Medium |
| Galovski et al., 20126 | Modified CPT (53) Symptom-monitoring delayed treatment (47) | 4–18 sessions for Modified CPT arm; 10 weeks for WL arm (followup data gathered after cross-over not reported). | Physical/sexual assault (as a child or an adult) | 74 to 77 | 40 | 69 | 58 | Medium |
| Marks et al., 1998122 Lovell, et al., 2001123 | PE (23) CR (13) CR+PE (24) Relax (21) | 10 sessionsb (mean of 16 weeks), (1, 3, and 6 months) | Male and female Mixed | NR | 38 | 36 | NR | Medium |
| Maxwell et al., 2016124 | MEST (8) CPT (8) | 6 weeks (post, 3 months) | Male and female Mixed | MPSS-SR 54.13 to 63.50 | NR | 81 | 44 | Medium |
| Monson et al., 20061 | CPT (30) WL (30) | 10 weeks (1 month) | Male and female Combat | 76.7 to 79.1 | 54 | 10 | 4 | Medium |
| Mueser et al., 20087 | CT (54) UC (54) | 12 to 16 sessions (post)b | Male and female Mixed | 74.5 to 76.2 | 44 | 79 | 16 | Medium |
| Resick et al., 20023 Resick, et al., 2003125 Resick, et al., 2012126 | CPT (62) PE (62) MA (47) | 6 weeks (3 and 9 months, 5 to 10 years) | Female Sexual assault | 69.9 to 76.6 | 32 | 100 | 29 | Medium |
| Resick et al., 2015127, 128 | Group CPT-C (56) Group PCT (52) | 6 weeks (2 weeks post-tx, 6 months after start of treatment, 12 months after start of treatment) | Military trauma (although could also have had PTSD attributed to other previous trauma) | PCL-S 58 to 59 | 32 | 7 | 43 | Medium |
| Tarrier et al., 1999129, 130 | IE (35) CT (37) | 16 sessions (112 days) (6 and 12 months) | Male and female Mixed | 71.1 to 77.8 | 39 | 42 | NR | Medium |
| Wells et al., 201419 | MCT (11) PE (11) WL (10) | 8 weekly sessions (post) | Mixed | PDS 33 to 38 | 41 | 38 | NR | Medium |
- a
Data reported are mean CAPS total or range of mean CAPS total scores across groups unless otherwise specified.
- b
Number of treatment sessions is reported when duration of treatment was not specified.
CAPS = Clinician-Administered PTSD Scale; CPT = cognitive processing therapy; CT = cognitive therapy; CR = cognitive restructuring; IE = imaginal exposure; MA = minimal attention (a type of wait-list group); MCT = meta cognitive therapy; MEST = memory specificity training; MPSS-SR = Modified PTSD Symptom Scale-Self-Report; MVA = motor vehicle accident; N = total number randomized/assigned to intervention and control groups; NR = not reported; PCT = present-centered therapy; PDS = Posttraumatic Diagnostic Scale; PE = prolonged exposure; PTSD = posttraumatic stress disorder; RA = repeated assessments (a type of wait-list group); SHB = self-help booklet based on principles of CBT; TAU = treatment as usual; UC = usual care; WL = wait-list; y = year.
Table 8Characteristics of included coping skills trials
| Study | Arm (N) | Duration (Followup) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % F | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Carlson et al., 199846 | Relax (13) EMDR (10) TAU (12) | 6 weeks (3 and 9 months) | Male Vietnam combat veterans | M-PTSD 117.5 to 119.4 | 48.5 | 0 | 45.7 | Medium |
| Foa et al., 199914 Zoellner et al., 1999134 | SIT (26) PE (25) PE+SIT (30) WL (15) | 9 weeks (3, 6, and 9 months) | Female Assault | PSS-I 29.4 to 32.9 | 35 | 100 | 36 | Medium |
| Markowitz et al., 2015132 Markowitz et al., 2016135 | PE (38) IPT (40) Relax (32) | 14 weeks (post) | Chronic PTSD Mixed | 68.9 to 72.1 | 40 | 77 | 35 | Medium |
| Marks et al., 1998122 Lovell, et al., 2001123 | Relax (21) PE (23) CR (13) CR+PE (24) | 10 sessionsb (mean of 16 weeks) (1, 3, and 6 months) | Male and female Mixed | NR | 38 | 36 | NR | Medium |
| Sautter et al., 2015131 | SAT (29) PFE (28) | 12 sessions (post, 12 weeks) | U.S. combat veterans | 83 to 86 | 33 | 2 | 6,334 | Medium |
| Taylor et al., 2003133 | Relax (19) PE (22) EMDR (19) | 8 weeks (1 and 3 months) | Male and female Mixed | NR | 37 | 75 | 23 | Medium |
- a
Data reported are mean CAPS or range of mean CAPS scores across groups unless otherwise specified.
- b
Number of treatment sessions is reported when duration of treatment was not specified.
CPT = cognitive processing therapy; CR = cognitive restructuring; EMDR = eye movement desensitization and reprocessing; F = female; IPT = interpersonal therapy; M-PTSD = Mississippi Scale for Combat-related PTSD; N = total number randomized/assigned to intervention and control groups; NR = not reported; PE = prolonged exposure; PFE = PTSD Family Education; PSS-I = PTSD Symptom Scale—Interview; PTSD = posttraumatic stress disorder; relax = relaxation; SAT=structured approach therapy; SIT = stress inoculation training; TAU = treatment as usual; WL = wait-list; y = year.
Table 9Results of the coping skills interventions compared with inactive controls for PTSD
| Study | Arm (N) | Outcome Measure(s) | Between Group Pre-Post Treatment Mean Difference | P-Value |
|---|---|---|---|---|
| Carlson et al., 199846 | Relax (13) TAU (12) | M-PTSD PTSD symptomsa IES-Total | −0.2 −0.8 5.7 | NS NR NS |
| Foa, 1999 et al.,14 Zoellner, 1999134 | SIT (26) WL (15) | PSS-I | −10.5 | <0.05 |
- a
This was a global self-rating on a 0 to 10 scale with 10 = “worst.”
Note: Results are only presented for the relevant arms for this section (coping skills and inactive comparators).
IES = Impact of Event Scale; M-PTSD = Mississippi Scale for Combat-related PTSD; N = total number randomized to intervention and control groups; NR = not reported; NS = not significant; PSS-I = Posttraumatic Stress Disorder Symptom Scale-Interview; PTSD = posttraumatic stress disorder; relax = relaxation; SIT = stress inoculation training; TAU = treatment as usual; WL = wait-list.
Table 10Results at the end of treatment for depression and anxiety symptoms for coping skills interventions compared with inactive controls
| Study | Arm (N) | Outcome Measure(s) | Between Group Pre-Post Treatment Mean Difference | P-Value |
|---|---|---|---|---|
| Carlson et al., 199846 | Relax (13) TAU (12) | BDI STAI-State subscale STAI-Trait subscale | −7.3 −5.3 −1.3 | NR NS NS |
| Foa et al., 199914 Zoellner et al., 1999134 | SIT (26) WL (15) | BDI STAI-State subscale | −8.5 −11.4 | <0.05 NS |
Note: Results are only presented for the relevant arms for this section (coping skills and inactive comparators).
BDI = Beck Depression Inventory; N = total number randomized to treatment and control groups; NR = not reported; NS = not statistically significant at p<0.05; relax = relaxation; SIT = stress inoculation training; STAI = State-Trait Anxiety Inventory; TAU = treatment as usual; WL = wait-list.
Table 11Characteristics of included CBT-exposure trials
| Study | Arm (N) | Duration (Followup) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % F | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Asukai et al., 201010 | PE (12) UC (12) | 8 to 15 weekly sessions (3 and 12 months) | Male and female Mixed | 84.3 to 84.6 | 29 | 88 | 100 | Medium |
| Basoglu et al., 200711 | In vivo (16) WL (15) | 1 sessionb (4, 8, 12, 24 weeks and 12 months) | Male and female Natural disaster | 62.3 to 63.1 | 34 | 87 | NR | Medium |
| Bryant et al., 200341 | IE (20) IE+CR (20) SC (18) | 8 weeks | Male and female Mixed | CAPS-I intensity 32.5 to 32.9 | 35 | 52 | NR | Medium |
| Bryant et al., 200842 | PE (31) PE+CR (28) IE (31) In vivo (28) | 8 weeks | Male and female Mixed | 71.4 to 76.8 | 37 | NR | 8 | Medium |
| Coffey et al., 2016140 | mPE+MET-PTSD (40) mPE (45) HLS (41) | 9 to 12 sessions (post, 3 months, 6 months) | Mixed, combat related trauma excluded | 76 to 82 | 34 | 46 | 21 | Medium |
| Foa et al., 199914 Zoellner et al., 1999134 | PE (25) SIT (26) PE+SIT (30) WL (15) | 9 weeks (3, 6, and 9 months) | Female Assault | PSS-I 29.4 to 32.9 | 35 | 100 | 36 | Medium |
| Foa et al., 200512 | Total 190 PE (NR) PE+CR (NR) WL (NR) | 12 weeks; 9 to 12 weekly sessions (3, 6, and 12 months) | Female Assault | PSS-I 31.1 to 34.0 | 31 | 100 | 51 | Medium |
| Fonzo et al., 2017137 Fonzo et al., 201721 | PE (36) WL (30) | 9 to 12 weekly or biweekly sessions | Male and female Mixed | 66 to 71 | 36 | 65 | NR | Medium |
| Gamito et al., 2010141 | VRE (5) IE (2) WL (3) | 12 sessionsb | Male Combat | NR | 64 | 0 | NR | Medium |
| Langkaas et al., 2017142 | PE (31) IRT (34) | 10 weeks (post, 12 months) | Male and female Mixed | PSS-I 33.2 to 34.9 | 45 | 58 | NR | Medium |
| Markowitz et al., 2015132 Markowitz et al., 2016135 | PE (38) IPT (40) Relax (32) | 14 weeks | Chronic PTSD | 68.9 to 72.1 | 40 | 77 | 35 | Medium |
| Marks et al., 1998122 Lovell et al., 2001123 | PE (23) CR (19) PE+CR (24) Relax (21) | 10 sessions,b mean of 16 weeks (1, 3, and 6 months) | Male and female Mixed | CAPS Severity 2.6 to 3.2 | 38 | 36 | NR | Medium |
| Mills et al., 201220 | COPE+TAU (55) TAU (48) | 13 sessions (post) | Mixed | 89 to 91 | 34 | 62 | NR | Medium |
| Nacasch et al., 201115 | PE (15) TAU (15) | 9 to 15 weeks (12 months) | Male and female Combat | PSS-I 36.8 to 37.1 | 34 | NR | 100 | Medium |
| Reger et al., 201618 | VRE (54) PE (54) WL (54) | 10 sessions (post, 3 months, 6 months) | Activity duty military | 78 to 80 | 30 | 4 | 40 | Medium |
| Resick et al., 20023 Resick, et al., 2003125 Resick, et al., 2012126 | PE (62) CPT (62) WL (47) | 6 weeks (3 and 9 months, 5 to 10 years) | Female Sexual assault | 69.9 to 76.6 | 32 | 100 | 29 | Medium |
| Rothbaum et al., 200513 | PE (24) EMDR (26) WL (24) | 4.5 weeks (6 months) | Female Sexual assault | Data reported in graphs only | 34 | 100 | 32 | Medium |
| Ruglass et al., 2017 143 | COPE (39) Relapse Prevention (43) AMCG (28) | 12 weeks (post, 1, 2, and 3 months) | Male and female Mixed 65% with clinical PTSD | 46.39 to 57.70 | 44 | 36 | 82 | Medium |
| Schnurr et al., 2003139 | Group exposure (180) PCT (180) | 30 weeks, 5 subsequent monthly boosters (12 months total) | Male Combat | 80.4 to 82.1 | 51 | 0 | 34 | Low |
| Schnurr et al., 2007138 | PE (141) PCT (143) | 10 weeks (3 and 6 months) | Female Mixed | 77.6 to 77.9 | 45 | 100 | 46 | Medium |
| Sloan et al., 201217 | WET (22) WL (24) | 10 sessions for WET group/5 weeks for WL group (post, 18 weeks) | MVA | NR | 41 | 65 | 63 | Low |
| Tarrier et al., 1999129, 130 | IE (35) CT (37) | 16 sessions (16 weeks) (6 and 12 months) | Male and female Mixed | 71.1 to 77.8 | 39 | 42 | NR | Medium |
| Taylor et al., 2003133 | PE (22) EMDR (19) Relax (19) | 6 months | Male and female Mixed | NR | 37 | 75 | 23 | Medium |
| van den Berg et al., 201516 | PE (53) EMDR (55) WL (47) | 8 weeks (post, 6 months) | Psychotic disorders and PTSD Mixed | 70 | 41 | 54 | NR | Low |
| Wells et al., 201419 | MCT (11) PE (11) WL (10) | 8 sessions (post) | Mixed | PDS 33 to 38 | 41 | 38 | NR | Medium |
- a
Data reported are mean CAPS or range of mean CAPS scores across groups unless another instrument is specified.
- b
Number of treatment sessions is reported when duration of treatment was not specified
AMCG = active monitoring control group; CAPS = Clinician-Administered PTSD Scale; CAPS-I = Clinician-Administered PTSD Scale Interview; CBT = cognitive behavioral therapy; COPE = Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure; CPT = cognitive processing therapy; CR = cognitive restructuring; EMDR = eye movement desensitization and reprocessing; F = female; G = group; HLS = health information control condition; IE = imaginal exposure; in vivo = in vivo exposure; MCT = metacognitive therapy; IRT = imagery rehearsal therapy; MET-PTSD = trauma-focused motivational enhancement therapy; mPE = modified prolonged exposure; MVA = motor vehicle accident; N = number; NR = not reported; PCT = present-centered therapy; PDS = Posttraumatic Diagnostic Scale; PE = prolonged exposure; PSS-I = PTSD Symptom Scale—Interview; PTSD = posttraumatic stress disorder; relax = relaxation; SC = supportive counseling; SIT = stress inoculation training; TAU = treatment as usual; UC = usual care; VRE = virtual reality exposure; WET = written exposure therapy; WL = wait-list; y = year.
Table 12Characteristics of included CBT-mixed intervention trials
| Study | Arm (N) | Duration tx (Followup) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % F | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Acosta et al., 2017149 | Web CBT plus UC (81) UC (81) | 12 weeks (post, 1 month, 3 months) | Combat veterans with PTSD and substance abuse 79% of sample had clinical PTSD | 78.6 | 32 | 7 | 13 | Medium |
| Blanchard et al., 200336 | CBT-M (27) SC (27) WL (24) | 8 to 12 weeks (3 months) | Male and female MVA 83% of sample had clinical PTSD | 65.0 to 68.2 | 41 | 73 | 10.2 | Medium |
| Bohus et al., 201323 | DBT (43) UC-WL (39) | 24 weeks (post, 6 weeks, 12 weeks) | Child abuse survivors with and without borderline personality disorder | 83 to 88 | 36 | 100 | NR | Medium |
| Bryant et al., 200341 | IE (20) CBT-Mb (IE+CR) (20) SC (18) | 8 weeks (6 months) | Male and female Mixed | CAPS-I 32.5 to 32.9 CAPS-F 36.0 to 38.3 | 35 | 52 | NR | Medium |
| Bryant et al., 200842 | PE (31) CBT-Mb (exp+CR) (28) IE (31) In vivo (28) | 8 weeks (6 months) | Male and female Mixed | 71.4 to 76.8 | 37 | NR | 8 | Medium |
| Cloitre et al., 200237 | CBT-M (31) WL (27) | 12 months | Female Childhood abuse | 69 | 34 | 100 | 54 | Medium |
| Cloitre et al., 2010148 Cloitre et al., 2016150 | CBT-M (33) CBT-M (38) CBT-M (33) | 16 weeks (3 and 6 months) | Female Mixed childhood abuse | 63.1 to 64.5 | 36 | 100 | 64 | Medium |
| Cottraux et al., 200831 | CBT-M (31) SC (29) | 16 weeks (1 and 24 months) | Male and female Mixed | PCLS 60.8 | 39 | 70 | NR | Medium |
| Engel et al., 201526, c* | Online CBT and stress inoculation training, nurse guided (43) Optimized UC (37) | 6 weeks (post [6 weeks], 12 weeks, 18 weeks) | Veterans of recent military conflicts PTSD | PCL 58.56 to 55.16 | 36 | 19 | 45 | Medium |
| Fecteau et al., 199938 | CBT-M (22) WL (21) | 4 weeks (6 months) | Male and female MVA | 70.9 to 77.3 | 41 | 70 | NR | Medium |
| Foa et al., 199914 Zoellner et al., 1999134 | PE (25) SIT (26) CBT-Mb (PE+SIT) (30) WL (15) | 9 weeks (3, 6, and 12 months) | Female Assault | PSS-I 29.4 to 32.9 | 35 | 100 | 36 | Medium |
| Foa et al., 200512 | Total 190 PE (NR) CBT-Mb (PE+CR) (NR) WL (NR) | 12 weeks, 9 to 12 weekly sessions (3, 6, and 12 months) | Female Assault | PSS-I 31.1 to 34.0 | 31 | 100 | 51 | Medium |
| Haller et al., 2016145, c | Group ICBT for depression and SUD plus CPT-M (trauma-focused CPT modified to include substance use prevention) (individual) (61) Group ICBT for depression and SUD plus individual ICBT for depression and SUD (62) | 12 sessions (up to 16 weeks) (post, 3 months, 6 months, 9 months, 12 months) | Veterans with MDD or dysthymia, past 90-day alcohol, cannabinoid, or stimulant dependence, and trauma exposure 82.1% of sample had clinical PTSD | PCL 56.99 | 47 | 11 | 36 | Medium |
| Harned et al., 2014144 | DBT plus DBT PE (17) DBT (9) | 1 year (3 months) | PTSD with borderline personality disorder and intentional self-injury | PSS-I 30 to 33 | 33 | 100 | 19 | Medium |
| Hinton et al., 200534 | CBT-M (20) WL (20) | 12 weeks | Male and female Cambodian refugees | 74.9 to 75.9 | 52 | 60 | 100 | Medium |
| Hinton et al., 2009151 | CBT-M (12) CBT-M (12) | 12 weeks | Cambodian refugees Witnessed genocide | 75.4 to 77.3 | 50 | 60 | 100 | Medium |
| Hinton et al., 2011152 | CBT-M (12) Relax (12) | 14 weeks (12 weeks) | Female Trauma NR | PCL 69.8 to 71.1 | 50 | 100 | 100 | Medium |
| Hollifield et al., 200732 | Acupuncture (29) (arm not eligible) CBT-M (28) WL (27) | 12 weeks (3 months) | Male and female Mixed | PSS-SR 30.8 to 32.5 | 42 | 48 | 24 | Medium |
| Ivarsson et al., 201424 | Internet-based CBT (31) Delayed treatment (WL) attention control (31) | 8 weeks (post) | Chronic PTSD Mixed | IES-R 55 | 46 | 82 | NR | Medium |
| Johnson et al., 201129 | CBT-M (35) UC (35) | 8 months (1 week, 3 and 6 months) | Female Interpersonal violence 87% of sample had clinical PTSD | 53.3 to 62.7 | 33 | 100 | 57 | Medium |
| Kubany et al., 200335 | CBT-M (19) WL (18) | 8 to 11 sessionsd (3 months) | Female Interpersonal violence | 80.1 to 80.2 | 35 | 100 | 51 | Medium |
| Kubany et al., 200428 | CBT-M (63) WL (62) | 4 to 5.5 weeks (3 and 6 months) | Female Interpersonal violence | 74.1 to 74.4 | 42 | 100 | 47 | Medium |
| Litz et al., 200733 | CBT-M (24) SC (21) | 8 weeks (3 and 6 months) | Male and female Combat | PSS-I 26.7 to 29.2 | 39 | 22 | 30 | Medium |
| Maguen et al., 201725 | CBT (17) WL (16) | 6 weeks (post) | Endorsed killing or responsible for the death of another in a war zone, PTSD | PCL 48.6 to 52.9 | 61 | 0 | 39 | Medium |
| Marks et al., 1998122 Lovell et al., 2001123 | PE (23) CR (13) CBT-Mb (CR+PE) (24) Relax (21) | 10 sessionsd (mean of 16 weeks), (1, 3, and 6 months) | Male and female Mixed | CAPS Severity 2.6 to 3.2 | 38 | 36 | NR | Medium |
| McDonagh et al., 200539 | CBT-M (29) PCT (22) WL (23) | 14 weeks (3 and 6 months) | Female Childhood sexual abuse | 67.7 to 72.0 | 41 | 100 | 7 | Medium |
| McGovern et al., 201527, c | ICBT for PTSD and SUD plus SC (73) IAC plus SC (75) (arm not eligible) Standard care (73) | 8–12 sessions typically completed in 8–12 weeks (post at 6 months post-baseline) | PTSD and substance abuse | 77.35 | 35 | 59 | 4 | Medium |
| Monson et al., 201222 | CBCT (20 individuals) WL (20 individuals) | 15 sessions (3 months after treatment) | Veterans and their partners | 68.87 to 73.03 | 37 | 75 | 28 | Medium |
| Sannibale et al. 2013146 | IT (integrated CBT for PTSD and AUD) (33) CBT for AUD plus SC (29) | 12 weeks (post, 5 months, 9 months) | Comorbid PTSD and AUD | 68 | 41 | 53 | NR | Low |
| Spence et al., 201130 | CBT-M (23) WL (21) | 8 weeks (3 months) | Male and female Mixed | PCL-C 57.0 to 60.8 | 43 | 81 | NR | Medium |
| van Emmerik et al., 200840 | CBT-M (41) Writing (44) WL (40) | 5 sessionsd (mean of 17 weeks), 13 to 139 weeks | Male and female Mixed 97% of sample had clinical PTSD | IES 46.4 to 49.1 | 40 | 67 | NR | Medium |
- a
Data reported are mean CAPS or range of mean CAPS scores across groups unless another instrument is specified.
- b
The information provided after CBT-M indicates the content of the mixed intervention (see abbreviations below).
- c
Less than 100 percent of sample had clinical PTSD.
- d
Number of treatment sessions is reported when duration of treatment was not specified.
AUD = Alcohol Use Disorder; CAPS = Clinician-Administered PTSD Scale; CBCT = cognitive behavioral couples therapy; CBT-M = cognitive behavioral therapy-mixed; CR = cognitive restructuring; DBT = dialectical behavior therapy; F = female; IAC = individual addiction counseling; ICBT = integrated cognitive behavioral therapy; IE = imaginal exposure; IES = Impact of Event Scale; in vivo = in vivo exposure; IT = integrated treatment; MDD = major depressive disorder; MVA = motor vehicle accident; NR = not reported; PCL = Posttraumatic Stress Disorder Checklist; PCL-C = Posttraumatic Stress Disorder Checklist-Civilian Version; PCLS = Post-Traumatic Stress Disorder Checklist Scale; PDS = Posttraumatic Stress Diagnostic Scale; PE = prolonged exposure; PSS-I = PTSD Symptom Scale—Interview; PSS-SR = Posttraumatic Symptom Scale-Self Report; PTSD = posttraumatic stress disorder; relax = relaxation; SC = supportive counseling; SIT = stress inoculation training; SUD = substance use disorder; UC = usual care; WL = wait-list; writing = structured writing therapy; y = year.
Table 13Results at the end of treatment for disability or functional impairment outcomes for CBT-mixed interventions compared with inactive controls
| Study | Arm (N) | Outcome Measure(s) | Baseline Value | End of Treatment Value | Change From Baseline | P-Value | Effect Size (Cohen’s d) |
|---|---|---|---|---|---|---|---|
| Blanchard et al., 200336 | CBT-M (27) WL (24) | GAF | CBT-M: 53.9 WL: 56.0 | 75.8 60.4 | NR | <0.05 | NR |
| Bohus et al., 201323 | CBT-M (43) UC-WL (39) | GAF | CBT-M: 41.50 WL: 42.79 | CBT-M: 49.44 WL: 43.79 | NR | <0.01 | NR |
| Cloitre, 200237 | CBT-M (31) WL (27) | IIP SAS-SR ISEL | CBT-M: 1.88 WL: 1.70 CBT-M: 2.44 WL: 2.57 CBT-M: 24 WL: 23 | CBT-M: 1.06 WL: 1.60 CBT-M: 2.06 WL: 2.47 CBT-M: 30 WL: 23 | NR | 0.01 0.02 0.01 | NR |
| Cottraux et al., 200831 | CBT-M (31) SC (29) | Global Phobic Disability Subscale of FQ | NR | 4.4 4.0 | −2.14 −2.0 | 0.86 | NR |
| Hollifield et al., 200732 | Acupuncture (29) (intervention not eligible) CBT-M (28) WL (27) | SDI | CBT-M: 4.09 WL: 4.0 | 3.3 3.96 | NR | <0.05 | 0.76 0.04 |
| Spence et al., 201130 | CBT-M (23) WL (21) | SDS | CBT-M: 18.17 WL: 19.42 | 13.22 18.11 | NR | 0.07 | 0.62 |
Note: Results are only presented for the relevant arms for this section (CBT-M and inactive comparators); values entered are means unless otherwise specified; p-values are for the comparison between CBT-M and inactive comparators.
CBT-M = cognitive behavioral therapy mixed; FQ = Fear Questionnaire (a self-rating inventory for evaluation of agoraphobia, social phobia, blood-injury phobia, anxiety-depression, and global phobic disability); GAF = Global Assessment of Functioning; IIP = Inventory of Interpersonal Problems; ISEL = Interpersonal Support Evaluation List; N = number; NR = not reported; SAS-SR = Social Adjustment Scale-Self Report; SC = supportive counseling; SDI = Sheehan Disability Inventory; SDS = Sheehan Disability Scale; UC = usual care; WL = wait-list.
Table 14Characteristics of included EMDR trials
| Study | Arm (N) | Duration (Followup) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % Female | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Acarturk et al., 201644 | EMDR (49) WL (49) | 2 to 7 sessions (time to complete not specified) (1 week post-tx, 5 weeks) | Refugees | IES-R 50 to 62 | 34 | 74 | 100 | Medium |
| Carlson et al.,199846 | EMDR (10) Relaxation (13) WL (12) | Twice a week for 6 weeks (3 and 9 months) | Male Vietnam combat veterans | M-PTSD 117.9 to 119.4 | 49 | 0 | 46 | Medium |
| Hogberg et al., 200748 | EMDR (13) WL (11) | 2 months | Swedish public transportation employees | IES 39 | 43 | 21 | NR | Medium |
| Nijdam et al., 2012154 | BEP (70) EMDR (70) | 17 weeks | Male and female Mixed | IES-R 72.8 to 79.9 | 38 | 56 | 100 | Medium |
| Rothbaum et al., 199745 | EMDR (11) WL (10) | 4 weeks (3 months) | Female Sexual assault | PSS-I 33.3 to 39.0 | 35 | 100 | NR | Medium |
| Rothbaum et al., 200513 | PE (24) EMDR (26) WL (24) | 4.5 weeks (6 months) | Female Sexual assault | Data reported in graphs only | 34 | 100 | 32 | Medium |
| Taylor et al., 2003133 | PE (22) EMDR (19) Relaxation (19) | 8 weeks (1 and 3 months) | Male and female Mixed | NR | 37 | 75 | 23 | Medium |
| ter Heide et al., 201643 | EMDR (37) Stabilization as usual (37) | 9 sessions (2 weeks post, 3 months) | Refugees | 75 to 78 | 40 | 27 | NR | Low |
| van den Berg et al., 201516 | PE (53) EMDR (55) WL (47) | 8 weeks (post, 6 months) | Psychotic disorders and PTSD Mixed | 70 | 41 | 54 | NR | Low |
| van der Kolk et al., 200747 | EMDR (29) Fluoxetine (30) Placebo (29) | 8 weeks (6 months) | Male and female Mixed | 71.2 | 36 | 83 | 33 | Medium |
- a
Data reported are mean or range of mean scores across groups for the PTSD measure listed.
BEP = brief eclectic psychotherapy; CAPS = Clinician-Administered Post Traumatic Stress Disorder Scale; CI = confidence interval; EMDR = eye movement desensitization and reprocessing; IES = Impact of Event Scale; IES-R = Impact of Event Scale-Revised; M-PTSD = Mississippi Scale for Combat-Related Post Traumatic Stress Disorder; N = total number randomized/assigned to intervention and control groups; NR = not reported; PE = prolong exposure; PSS-I = Post Traumatic Stress Disorder Symptom Scale-Interview; PTSD = posttraumatic stress disorder; TAU = treatment as usual; WL = wait-list; y = year.
Table 15Characteristics of included studies of other psychological interventions
| Study | Arm (N) | Treatment Duration (Followup) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % Female | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Boden et al., 201258 | SS (59) TAU (58) | 12 weeks | Male Combat 92% of sample had clinical PTSD | IES-R 46.8 to 47.7 | 54 | 0 | 74 | Medium |
| Church et al., 2013155 | EFT (30) WL (29) | 6 sessions (post/30 days, 3 months, 6 months) | U.S. combat Veterans | PCL-M 63.7 | 52 | 10 | NR | Medium |
| Cook et al., 2010156 | IRT (61) PsychEd (63) | 6 weeks (1, 3, and 6 months) | Male Combat | 79.5 to 81.3 | 59 | 0 | 58 | Medium |
| Ford et al., 201159 | TAR (48) PCT (53) WL (45) | 12 sessionsb (3 and 6 months) | Female Victimization or incarceration 80% of sample had clinical PTSD | 61.9 to 68.7 | 31 | 100 | 59 | Medium |
| Ford et al., 201360 | TAR (41) SGT (39) | 12 group sessions (post/after treatment) | Incarcerated women victims of interpersonal violence 78% of sample had clinical PTSD | 63 to 65 | 36 | 100 | 43 | Medium |
| Gersons et al., 200051 | BEP (22) WL (20) | 16 weeks (3 months) | Male and female police officers Trauma type NR | NR | 37 | 12 | NR | Medium |
| Hien et al., 200457 | Total 107c SS (unclear) RPC (unclear) UC (32) | 12 weeks | Female Mixed w/substance abuse disorders 80% of sample had clinical PTSD | 70.4 to 73.9 | 37 | 100 | 63 | Medium |
| Hien et al., 2009157 Hien et al., 2012158 | SS (176) PsychEdd (177) | 6 weeks | Female Mixed 88% of sample had clinical PTSD | 61.6 to 64.2 | 39 | 100 | 54 | Medium |
| Kearney et al., 2013159 | MBSR+TAU (25) TAU (22) | 8 weeks (post, 4 months) | War veterans Mixed | PCL 60 to 63 | 52 | 21 | 32 | Medium |
| Krakow et al., 200152 | IRT (88) WL (80) | 3 sessions—2 sessions 1 week apart and 1 session 3 weeks later (3 and 6 months) | Female Sexual abuse/assault | 79.6 to 81.9 | 38 | 100 | 21 | Medium |
| Langkaas et al., 2017142 | PE (31) IRT (34) | 10 weeks (post, 12 months) | Male and Female Mixed | PSS-I 33.2 to 34.9 | 45 | 58 | NR | Medium |
| Lindauer et al., 200550 | BEP (12) WL (12) | 16 weeks | Male and female Mixed | NR | 39 | 54 | NR | Medium |
| Markowitz et al., 2015132 Markowitz et al., 2016135 | PE (38) IPT (40) Relax (32) | 14 | Chronic PTSD | 68.9 to 72.1 | 40 | 77 | 35 | Medium |
| Maxwell et al., 2016124 | MEST (8) CPT (8) | 6 weeks (post, 3 months) | Male and female Mixed | MPSS-SR 54.13 to 63.50 | NR | 81 | 44 | Medium |
| Moradi et al., 2014160 | MEST (12) Control (12) | 4 (post, 3 months) | Iranian Combat male veterans | NR | 45 | 0 | 100 | Medium |
| Morath et al., 201455 | NET (17) WL (17) | 12 (4 months after treatment, 1-year followup) | Refugees and Asylum seekers | 88 | 28 | 41 | 100 | Medium |
| Neuner et al., 2004161 | NET (17) Trauma couns (14) PsychEd (12) | 3 to 4 weeks (4 and 12 months) | Male and female Sudanese refugees | PDS 19.5 to 25.2 | 33 | 61 | 100 | Medium |
| Neuner et al., 200854 | NET (111) Trauma couns (111) MG (no intervention) (55) | 3 weeks (6 months) | Male and female Rwandan and Somalian refugees | PDS 21.3 to 26.7 | 35 | 51 | 100 | Medium |
| Neuner et al., 201053 | NET (16) TAU (16) | Weekly or biweekly sessions (median 9)f | Male and female Asylum seekers | PDS 36.9 to 38.9 | 31 | 31 | NR | Medium |
| Nijdam et al., 2012154 | BEP (70) EMDR (70) | 16 weeks | Male and female Mixed | IES-R 72.8 to 79.9 | 38 | 56 | 100 | Medium |
| Polusny et al., 2015136 | MBSR (58) Group PCT (58) | 8 weeks (post, 2 months) | War veterans Mixed | 62 | 58 | 16 | 16 | Medium |
| Schnyder et al., 201149 | BEP (16) MA (14) | 16 weeks (6 months)c | Male and female Mixed 96% of sample had clinical PTSD | 73.4 to 78.6 | 40 | 47 | NR | Medium |
| van der Kolk et al., 2016162 | Neurofeed-back training (28) WL (24) | 12 weeks (post, 1 month) | Mixed | 76 to 79 | 44 | 76 | 24 | Medium |
| Zlotnick et al., 200956 | SS (27) TAU (22) | 6 to 8 weeks (3 and 6 months) | Female Mixed 83% of sample had clinical PTSD | 64.4 to 69.4 | 35 | 100 | 53 | Medium |
- a
Data reported are mean CAPS total or range of mean CAPS total scores across groups unless otherwise specified.
- b
Number of treatment sessions is reported when duration of treatment was not specified.
- c
The article did not report the numbers randomized to each group. It reported the numbers analyzed in each group (41, 34, and 32, respectively). It describes baseline data for 107 subjects analyzed. Of the 128 women who met full study eligibility criteria, 115 (90%) agreed to participate, and 96 of these women were randomly assigned to the two active treatment groups (SS and RPC). Thirty-two of the 128 women became the community care comparison group; they were not randomized to that group.
- d
Psycho Ed in this study is “Women’s Health Education.”
- e
Only the BEP group had a followup assessment; the control group did not.
- f
Treatment was terminated at the discretion of the therapist; range of 5 to 17 sessions provided.
Note: When mean data for baseline PTSD severity were not reported for the total sample but were presented for each study arm, we provide the range across arms.
BEP = brief eclectic psychotherapy; CAPS = Clinician-Administered PTSD Scale; EFT = emotional freedom techniques; F = female; IRT = imagery rehearsal therapy; MA = minimal attention (inactive control group); MBSR = mindfulness-based stress reduction; MEST = memory specificity training; MG = no-treatment monitoring group; MPSS-SR = Modified PTSD Symptom Scale-Self-Report; N = total number randomized/assigned to intervention and control groups; NET = narrative exposure therapy; NR = not reported; PCT = present-centered therapy; PDS = Posttraumatic Stress Diagnostic Scale; PE = prolonged exposure; PTSD = posttraumatic stress disorder; PsychEd = psychosocial education; RPC = relapse prevention condition; SGT = Supportive Group Therapy; SS = Seeking Safety; Ssm = sleep symptom monitoring; SUD = substance use disorder; TAR = Trauma Affect Regulation; TAU = treatment as usual; trauma couns = trauma counseling; UC = usual care; WL = wait-list; y = year.
Table 16Characteristics of included studies that compared efficacy or comparative effectiveness between patients having different characteristics or specific trauma types
| Study | Arm (N) | Duration (Followup) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % Female | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Bohus et al., 201323 | DBT (43) TAU-WL (39) | 24 weeks (post, 6 weeks, 12 weeks) | Child abuse survivors Subgroup analysis: BPD | 83 to 88 | 36 | 100 | NR | Medium |
| Markowitz et al., 2015132, 135 | PE (38) IPT (40) Relaxation (32) | 14 | Chronic PTSD Mixed Subgroup analysis: MDD, trauma type, gender, age of primary trauma exposure | 68.9 to 72.1 | 40 | 77 | 35 | Medium |
| Resick et al., 20023 Resick, et al., 2003125 | CPT (62) PE (62) WL (47) | 6 weeks (3 and 9 months) | Female Sexual assault Subgroup analysis: history of child sexual abuse | 69.9 to 76.6 | 32 | 100 | 29 | Medium |
| van der Kolk et al., 200747 | EMDR (29) Fluoxetine (30) Placebo (29) | 8 weeks (6 months) | Male and female Mixed subgroup analysis: child-onset and adult-onset trauma | 71.2 | 36 | 83 | 33 | Medium |
- a
Data reported are mean CAPS totals or range of mean CAPS total scores across groups unless otherwise specified.
BPD = borderline personality disorder; CAPS = Clinician-Administered PTSD Scale; DBT = dialectical behavior therapy; CPT = cognitive processing therapy; EMDR = eye movement desensitization and reprocessing; F = female; IPT = interpersonal therapy; MDD = major depressive disorder; N = total number randomized/assigned to intervention and control groups; PE = prolonged exposure; PTSD = posttraumatic stress disorder; TAU = trauma affect regulation; WL = wait-list; y = year.
Table 17Summary of efficacy and strength of evidence of PTSD pharmacological treatments
| Treatment | N Trials (Subjects) | Findings | SOE | |
|---|---|---|---|---|
| Fluoxetine (SSRI) | PTSD symptomsa | 4 (835)47, 61–63 | Reduced PTSD symptoms SMD −0.28 (95% CI −0.42 to −0.14) | Moderate |
| Depression symptomsb | 3 (771)47, 61, 62 | Similar reduction in depression symptoms SMD −0.20 (95% CI −0.40 to 0.00) | Low SOE for no differencec | |
| Paroxetine (SSRI) | PTSD symptomsa | 2 (348)64, 65 | Reduced PTSD symptoms SMD of −0.56 to −0.44 in individual studies Both studies favored treatment (2 of 2 studies p<0.05) | Moderate |
| PTSD symptom remission | 2 (348)64, 65 | Greater PTSD symptom reduction RD of 0.13 and 0.19 across 2 individual studies (1 of 2 studies p<0.05) | Moderate | |
| Depression symptomsb | 2 (348)64, 65 | Reduced depression symptoms SMD range −0.60 to ‑0.34 across individual studies Both studies favored treatment (2 of 2 studies p<0.05) | Moderate | |
| Sertraline (SSRI) | PTSD symptomsa | 7 (1,085)66–72 | Reduced PTSD symptoms SMD −0.20 (95% CI −0.36 to −0.04) | Lowd |
| Depression symptomsb | 7 (1,085)66–72 | Similar reduction in depression symptoms SMD −0.14 (95% CI −0.33 to 0.06) | Low for no differencee | |
| Venlafaxine (SNRI) | PTSD symptomsa | 2 (687)69, 73 | Reduced PTSD symptoms SMD −0.35 and −0.26 across two individual studies | Moderate |
| PTSD symptom remission | 2 (687)69, 73 | Greater PTSD symptom remission RD of 0.12 and 0.15 across individual studies | Moderatef | |
| Depression symptomsb | 2 (687)69, 73 | Reduced depression symptoms Between-group mean difference of −2.6 and ‑1.6 across individual studies | Moderateg | |
| Prazosin (alpha blocker) | PTSD symptomsa | 3 (117)74–76 | Reduced PTSD symptoms SMD −0.52 (95% CI, −0.90 to −0.14) | Low |
| Topiramate (anticonvulsant) | PTSD symptomsa | 3 (142)77–79 | Reduced PTSD symptoms SMD range of −1.85 to −0.38 across individual studies | Lowh |
| Olanzapine (antipsychotic) | PTSD symptomsa | 2 (47)80, 81 3 (62)80–82 | Reduced PTSD symptoms SMD of −1.15 and −0.96 across individual studies,80, 81 both significantly favored treatment SMD range −1.15 to 0.89 across individual studies All studies favored treatment (2 of 3 studies p<0.05) | Low |
| Risperidone (antipsychotic) | PTSD symptomsa | 4 (422)83–86 | Reduced PTSD symptoms SMD −0.26 (95% CI, −0.52 to −0.01) | Low |
NOTE: Outcomes graded as insufficient are not included in this table. Insufficient evidence was provided for divalproex (anticonvulsant), tiagabine (anticonvulsant), citalopram (SSRI), all TCAs, buproprion (other second-generation antidepressant [SGA]), and mirtazapine (other SGA). No studies that met inclusion criteria rated as having low or medium risk of bias evaluated lamotrigine (anticonvulsant), any benzodiazepine, desvenlafaxine (SNRI), duloxetine (SNRI), nefazodone (other SGA), or trazodone (other SGA).
- a
SMD from Clinician-Administered PTSD Scale and other various PTSD symptom scales.
- b
SMD from the Beck Depression Inventory and other various depression symptom scales.
- c
SOE changed from moderate in the prior review to low for no difference in the updated review. Only 2 of 3 studies favored treatment; one favored placebo. Imprecision, inconsistency, and effect sizes near the null prompted the change in grade.
- d
SOE changed from moderate in the prior review to low in the updated review. The studies were inconsistent in whether findings favored treatment or the inactive comparator group, and findings were imprecise.
- e
SOE changed from low to low for no difference in the updated review. The studies were inconsistent in whether findings favored treatment or the inactive comparator group, findings were imprecise, and most individual study estimates were close to the null.
- f
SOE changed from insufficient to moderate in the updated review because of consistent evidence across two studies of adequate sample sizes.
- g
SOE changed from low to moderate in the updated review because of consistent evidence across two studies of adequate sample sizes.
- h
SOE changed from moderate in the prior review to low in the updated review. The findings were imprecise; only 1 of 3 individual studies found significant differences between study groups, and the sample sizes were small.
CI = confidence interval; N = number; PTSD = posttraumatic stress disorder; RD = risk difference; SGA = second-generation antidepressant; SMD = standardized mean difference; SNRI = serotonin and norepinephrine reuptake inhibitor; SOE = strength of evidence; SSRI = selective serotonin reuptake inhibitor; TCA = tricyclic antidepressant.
Table 18Characteristics of included placebo-controlled trials of alpha-blockers
| Study | Arm Dose mg/Day (N) | Duration (Weeks) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % Female | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Raskind et al., 200374 | Prazosin 2 to 10 mg (5) Placebo (5) | 20 | Male Combat veterans | 79.1 to 83.6 | 53 | 0 | NR | Medium |
| Raskind et al., 200775 | Prazosin 2 to 15 mg (20) Placebo (20) | 8 | Male and female Combat veterans | 70.0 | 56 | 5 | 35 | Medium |
| Raskind et al., 201376 | Prazosin 1 mg titrated to a max of 20 mg for men or 10 mg for women (32) Placebo (35) | 15 | Veterans Active-duty soldiers, Combat trauma | 77.3 to 85.7 | 30 | 15 | 37 | Medium |
- a
Data reported are mean CAPS or range of mean CAPS scores across groups unless otherwise specified.
Note: When mean data for baseline PTSD severity were not reported for the total sample but were presented for each study arm, we provide the range across arms.
CAPS = Clinician-Administered PTSD Scale; F = female; mg = milligram; N = total number randomized/assigned to intervention and control groups; NR = not reported; PTSD = posttraumatic stress disorder; y = year.
Table 19Characteristics of included placebo-controlled trials of anticonvulsants, by drug
| Study | Arm (Dose mg/Day) (N) | Duration (Weeks) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % Female | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Akuchekian et al., 200477 | Topiramate (12.5 to 500) (34) Placebo (33) | 12 | Male Combat veterans | 49.8 | 40 | 0 | 100 | Medium |
| Batki et al., 2014165 | Topiramate (25 to 300) (14) Placebo (16) | 12 | Veterans w/AUD, warzone and/or civilian related trauma | 72.8 to 83.1 | 50 | 7 | 7 | Low |
| Davidson et al., 2007166 | Tiagabine (4 to 16) (116) Placebo (116) | 12 | Male and female Mixed | 82.6 | 423 | 66 | NR | Medium |
| Davis et al., 2008164 | Divalproex (1,000 to 3,000) (44) Placebo (41) | 8 | Male and female Combat veterans | 75.2 to 77.3 | 55 | 2 | NR | Low |
| Tucker et al., 200778 | Topiramate (25 to 400) (20) Placebo (20) | 12 | Male and female Mixed | 88.3 to 91.1 | 41 | 79 | 11 | Medium |
| Yeh et al., 201179 | Topiramate (25 to 200) (17) Placebo (18) | 12 | Male and female Mixed | 66.1 to 78.8 | 40 | 68 | NR | Medium |
- a
Data reported are mean CAPS or range of mean CAPS scores across groups unless otherwise specified.
AUD = alcohol use disorder; CAPS = Clinician-Administered PTSD Scale; F = female; mg = milligram; N = total number randomized/assigned to intervention and control groups; NR = not reported; PTSD = posttraumatic stress disorder; y = year.
Table 20Characteristics of included placebo-controlled trials of atypical antipsychotics, by drug
| Study | Arm Dose mg/Day (N) | Duration (Weeks) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % F | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Bartzokis et al., 200586 | Risperidone 1 to 3 mg (33) Placebo (32) | 16 | Male Combat veterans | 98.6 to 102.2 | 52 | 0 | 32 | Medium |
| Butterfield et al., 200182 | Olanzapine 5 to 20 mg (10) Placebo (5) | 10 | Male and female Mixed | SIP 39.7 to 45.9 | 43 | 93 | 46 | Medium |
| Carey et al., 201281 | Olanzapine 5 to 10 mg (14) Placebo (14) | 8 | Adults w/non-combat related chronic PTSD, Noncombat | 79.4 to 81.6 | 41 | 61 | NR | Medium |
| Hamner et al., 200383 | Risperidone 1 to 6 mg (20) Placebo (20) | 5 | Male Combat veterans | 89.1 to 90.3 | 52 | 0 | 54 | Medium |
| Krystal et al., 201185 | Risperidone 1 to 4 mg (147) Placebo (149) | 24 | Male and female Combat | 78.2 | 54 | 3 | 34 | Low |
| Monnelly et al., 2003167 | Risperidone 0.5 to 2 mg (8) Placebo (8) | 6 | Male Combat veterans | PCL-M 72 to 73 | 51 | 0 | 20 | Medium |
| Reich et al., 200484 | Risperidone 0.5 to 8 mg (12) Placebo (9) | 8 | Female Childhood abuse | 65.5 to 73.9 | 28 | 100 | 14 | Medium |
| Stein et al., 200280 | Olanzapine 10 to 20 mg (10) Placebo (9) | 8 | Male Combat veterans | 84.0 to 86.1 | 52 | 0 | NR | Medium |
- a
Data reported are mean CAPS or range of mean CAPS scores across groups unless otherwise specified.
Note: When mean data for baseline PTSD severity, sex, or race were not reported for the total sample but were presented for each study arm, we provide the range across arms.
CAPS = Clinician-Administered PTSD Scale; F = female; mg = milligram; N = total number randomized/assigned to intervention and control groups; NR = not reported; PCL-M = PTSD Checklist – Military Version; PTSD = posttraumatic stress disorder; SIP = Structured Interview for PTSD; y = year.
Table 21Characteristics of included placebo-controlled trials of selective serotonin reuptake inhibitors, by drug
| Study | Arm Dose mg/Day (N) | Duration (Weeks) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % Female | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Brady et al., 200066 | Sertraline 25 to 200 mg (94) Placebo (93) | 12 | Male and female Mixed | 75.1 to 76.6 | 40 | 73 | 16 | Medium |
| Brady et al., 200567 | Sertraline 150 mg (49) Placebo (45) | 12 | Male and female Mixed, alcohol dependence | 57.6 to 60.1 | 37 | 46 | NR | Medium |
| Connor et al., 1999170 Meltzer-Brody et al., 2000171 | Fluoxetine 10 to 60 mg (27) Placebo (27) | 12 | Male and female Mixed | DTS 73.7 to 79.4 | 37 | 91 | 7 | Medium |
| Davidson et al., 200669 | Total 538b Venlafaxine 37.5 to 375 mg (179) Sertraline 25 to 200mg (173) Placebo (179) | 12 | Male and female Mixed | ~82 | NR | NR | NR | Medium |
| Davidson et al.,200168 | Sertraline 25 to 200 mg (100) Placebo (108) | 12 | Male and female Mixed | 73.5 to 73.9 | 37 | 78 | 17 | Medium |
| Friedman et al., 2007 70 | Sertraline 25 to 200 mg (86) Placebo (83) | 12 | Male and female Mixed (71% combat) | 72.1 to 73.8 | 46 | 20 | 71 | Medium |
| Li et al., 2017172 | Sertraline 135 mg (36) Placebo (36) | 12 | Male and female Mixed | IES-R 63.9 to 64.8 | 46 | 12 | 100 | Low |
| Marshall et al., 200164 | Paroxetine 20 mg (188) Paroxetine 40 mg (187) Placebo (188) | 12 | Male and female Mixed | 74.3 to 75.3 | 42 | NR (~2:1 F:M) | <10% | Medium |
| Martenyi et al., 200261; Martenyi et al., 2006173 | Fluoxetine 20 to 80 mg (226) Placebo (75) | 12 | Male and female Combat and victim/witness of war | 80.5 to 81.3 | 38 | 19 | 9 | Medium |
| Martenyi et al., 200762 | Fluoxetine 20 mg (163) Fluoxetine 40 mg (160) Placebo (88) | 12 | Male and female Mixed | 75 to 79 | 41 | 72 | 23 | Medium |
| Panahi et al., 201171 | Sertraline 50 to 200 mg (35) Placebo (35) | 10 | Male Combat | IES-R 65.1 to 65.4 | 46 | 0 | 100 | Low |
| Simon et al., 2008174 | Paroxetine 12.5 to 62.5 mg (11) Placebo (14) | 10 | Male and female Mixed (60% exposure to war; combat % NR), refractory to exposure | SPRINT 16.1 to 17 | 46 | 56 | 26 | Medium |
| Tucker et al., 200165 | Paroxetine 20 to 50 mg (163) Placebo (160) | 12 | Male and female Mixed | 73.2 to 74.3 | 41 | 66 | 28 | Medium |
| Tucker et al., 2003175 Tucker et al., 2004176 | Citalopram 20 to 50 mg (25) Sertraline 50 to 200 mg (23) Placebo (10) | 10 | Male and female Mixed | 83.9 to 94.2 | 39 | 74 | 14 | Medium |
| van der Kolk et al., 199463 | Fluoxetine 20 to 60 mg (33) Placebo (31) | 5 | Male and female Mixed (48% combat) | NR | 40 | 34 | NR | Medium |
| van der Kolk et al., 200747 | Fluoxetine (30) EMDR (29) Placebo (29) | 8c | Male and female Mixed | 71.2 | 36 | 83 | 33 | Medium |
| Zohar et al., 200272 | Sertraline 50 to 200 mg (23) Placebo (19) | 10 | Male and female Israeli military veterans | 91.2 to 93.3 | 40 | 12 | NR | Medium |
- a
Data reported are mean CAPS or range of mean CAPS scores across groups unless otherwise specified.
- b
The Ns for each are the number analyzed; the number randomized to each group was not reported (overall N was 538; 531 were included in the analysis).
- c
Study was 8 weeks of treatment but also included a 6-month posttreatment followup.
CAPS = Clinician-Administered PTSD Scale; DTS = Davidson Trauma Scale; EMDR = eye movement desensitization and reprocessing; F = female; IES-R = Impact of Event Scale-Revised; mg = milligram; N = total number randomized/assigned to intervention and control groups; NR = not reported; PTSD = posttraumatic stress disorder; SPRINT = Short PTSD Rating Interview; y = year.
Table 22Characteristics of included placebo-controlled trials of serotonin and norepinephrine reuptake inhibitors
| Study | Arm Dose mg/Day (N) | Duration (Weeks) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % Female | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Davidson et al., 200673 | Venlafaxine ER 37.5 to 300 mg (161) Placebo (168) | 24 | Male and female Mixed | 81 to 82.9 | 41 | 54 | NR | Medium |
| Davidson et al., 200669 | Total 538b Venlafaxine 37.5 to 375 mg (179) Placebo (179) | 12 | Male and female Mixed | ~82 | NR | NR | NR | Medium |
- a
Data reported are mean CAPS or range of mean CAPS scores across groups unless another instrument is specified.
- b
The Ns for each are the number analyzed and include a third study arm (sertraline 25 to 200 mg); the number randomized to each group was not reported (overall N was 538; 531 were included in the analysis).
CAPS = Clinician-Administered PTSD Scale; ER = extended release; F = female; mg = milligram; N = total number randomized/assigned to intervention and control groups; NR = not reported; PTSD = posttraumatic stress disorder; y = year.
Table 23Characteristics of included placebo-controlled trials of other second-generation antidepressants
| Study | Arm Dose mg/Day (N) | Duration (Weeks) | Population Trauma Type | Baseline PTSD Severity | Mean Age (Y) | % Female | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Becker et al., 2007183 | Total 30a Bupropion 100 to 300 mg (18) Placebo (10) | 8 | Male and female Mixed | NR | 50 | 21 | 71 | Medium |
| Davidson et al., 2003184 | Total 29b Mirtazapine 15 to 45 mg (17) Placebo (9) | 8 | Male and female Mixed | SPRINT 21.7 to 25.0 | 46 | NR | NR | Medium |
- a
Thirty subjects were randomized; exact numbers randomized to each group NR; authors reported that 18 received bupropion and 10 received placebo; 2 dropped out prior to treatment.
- b
A total of 29 subjects were randomized: 3 subjects dropped out early, 17 received mirtazapine, and 9 received placebo.
Note: When mean data for baseline PTSD severity were not reported for the total sample but were presented for each study arm, we provide the range across arms.
F = female; mg = milligram; N = total number randomized/assigned to intervention and control groups; NR = not reported; PTSD = posttraumatic stress disorder; SPRINT = Short PTSD Rating Interview; y = year.
Table 24Characteristics of included head-to-head pharmacotherapy trials
| Study | Arm Dose mg/Day (N) | Duration (Weeks) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % Female | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Davidson et al., 200669 | Total 538b Venlafaxine 37.5 to 375 mg (179) Sertraline 25 to 200 mg (173) Placebo (179) | 12 | Male and female Mixed | ~82 | NR | NR | NR | Medium |
| Petrakis et al., 2012185 | Paroxetine 40 mg + Naltrexone 50 mg (22) (arm not eligible) Paroxetine 40 mg + Placebo (20) Desipramine 200 mg + Naltrexone 5 Placebo 0 mg (22) (arm not eligible) Desipramine 200 mg + (24) | 12 | Male and female Veterans w/alcohol dependence | 62.5 to 77.8 | 47 | 9 | 25 | Medium |
| Sonne et al., 2016186 | Sertraline 25 to 200 mg (109) Venlafaxine 37.5 to 375 mg (98) | 24-28 | Trauma, affected refugees, Catastrophic experience Unknown what portion of the sample had clinical PTSD | HTQ 3.18 to 3.24 | 44 | 40 | NR | Medium |
| Tucker et al., 2003175 Tucker et al., 2004176 | Citalopram 20 to 50 mg (25) Sertraline 50 to 200 mg (23) Placebo (10) | 10 | Male and female Mixed | 83.9 to 94.2 | 39 | 74 | 14 | Medium |
- a
Data reported are mean CAPS or range of mean CAPS scores across groups unless otherwise specified.
- b
The Ns for each are the number analyzed; the number randomized to each group was not reported (overall N was 538; 531 were included in the analysis).
CAPS = Clinician-Administered PTSD Scale; F = female; mg = milligram; HTQ = Harvard Trauma Questionnaire; N = total number randomized/assigned to intervention and control groups; NR = not reported; PTSD = posttraumatic stress disorder; y = years.
Table 25Consistency of network meta-analysis finding
| Drug A | Drug B | Direct | Indirect | Difference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p | Coefficient | 95% CI | p | Coefficient | 95% CI | p | ||
| Citalopram | Sertraline | −0.45 | −1.02, 0.12 | 0.122 | −0.60 | −1.98, 0.79 | 0.398 | 0.15 | −1.36, 1.65 | 0.849 |
| Despiramine | Paroxetine | 0.14 | −0.28, 0.56 | 0.507 | −0.41 | −124.40, 123.59 | 0.995 | 0.55 | −123.45, 124.54 | 0.993 |
| Sertraline | Venlafaxine | −0.07 | −0.32, 0.17 | 0.556 | −0.08 | −0.37, 0.20 | 0.566 | 0.01 | −0.36, 0.39 | 0.955 |
Table 26Characteristics of included pharmacological trials that compared efficacy or comparative effectiveness between subgroups defined by patient characteristics or trauma types
| Study | Arm (N) | Duration (Followup) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % Female | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| van der Kolk et al., 200747 | EMDR (29) Fluoxetine (30) Placebo (29) | 8 weeks (6 months) | Male and female Mixed subgroup analysis: child-onset and adult-onset trauma | 71.2 | 36 | 83 | 33 | Medium |
- a
Data reported are mean CAPS total or range of mean CAPS total scores across groups unless otherwise specified.
CAPS = Clinician-Administered PTSD Scale; EMDR = eye movement desensitization and reprocessing; N = total number randomized/assigned to intervention and control groups; PTSD = posttraumatic stress disorder; y = year.
Table 27Characteristics of included studies directly comparing psychotherapy with pharmacotherapy
| Study | Arm (N) | Duration (Followup) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % Female | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| van der Kolk, 200747 | Fluoxetine (30)b EMDR (29) Placebo (29) | 8 weeks (6 months) | Male and female Mixed | 71 | 36 | 83 | 33 | Medium |
- a
Data reported are mean CAPS total score (1 week). The mean CAPS total score (1 month) was 74.0.
- b
Titrated from 10 mg/day to max 60 mg/day (mean = 30 mg/day, mode = 40 mg/day).
CAPS = Clinician-Administered Post Traumatic Stress Disorder Scale; EMDR = eye movement desensitization and reprocessing; N = total number randomized/assigned to intervention and control groups; PTSD = posttraumatic stress disorder; y = year.
Table 28Characteristics of included psychological versus pharmacological trials that examined comparative effectiveness between subgroups defined by patient characteristics or trauma types
| Study | Arm (N) | Duration (Followup) | Population Trauma Type | Baseline PTSD Severitya | Mean Age (Y) | % Female | % Non-white | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| van der Kolk et al., 200747 | EMDR (29) Fluoxetine (30) Placebo (29) | 8 weeks (6 months) | Male and female Mixed subgroup analysis: child-onset and adult-onset trauma | 71.2 | 36 | 83 | 33 | Medium |
- a
Data reported are mean CAPS total or range of mean CAPS total scores across groups unless otherwise specified.
CAPS = Clinician-Administered PTSD Scale; EMDR = eye movement desensitization and reprocessing; N = total number randomized/assigned to intervention and control groups; PTSD = posttraumatic stress disorder; y = year.
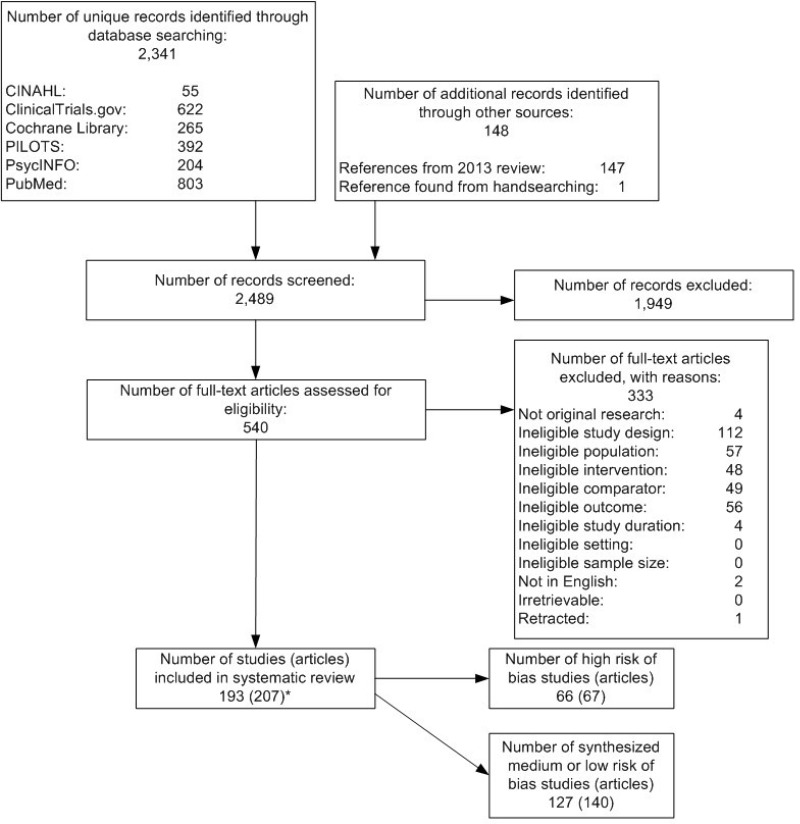
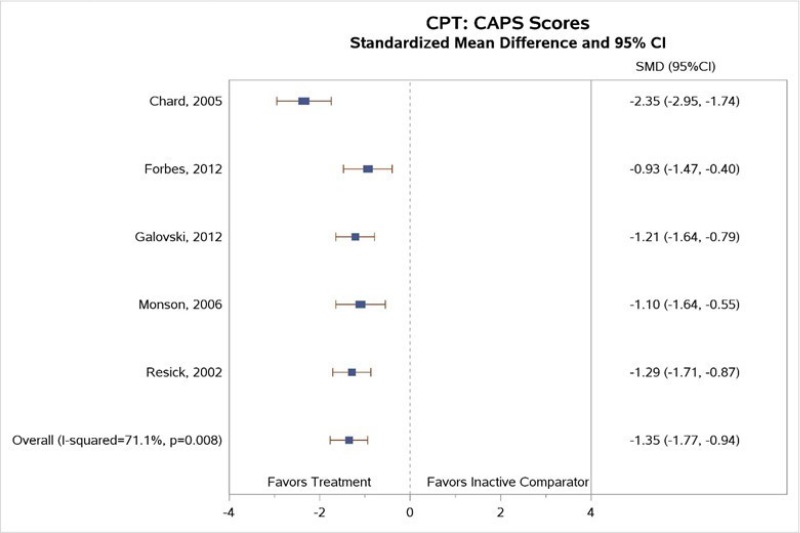
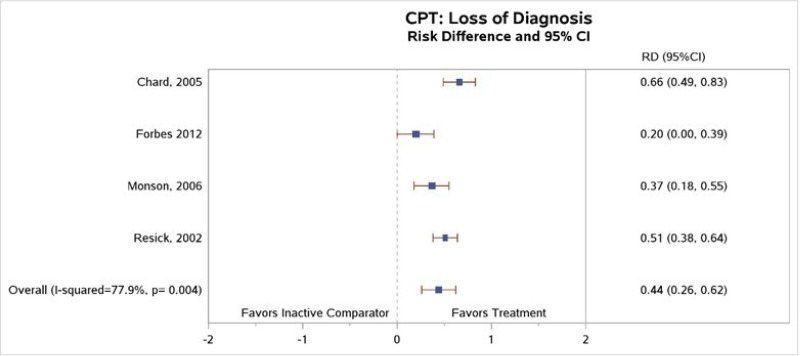
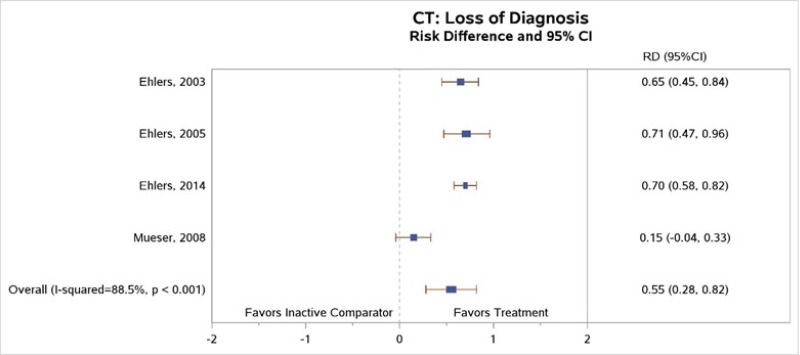
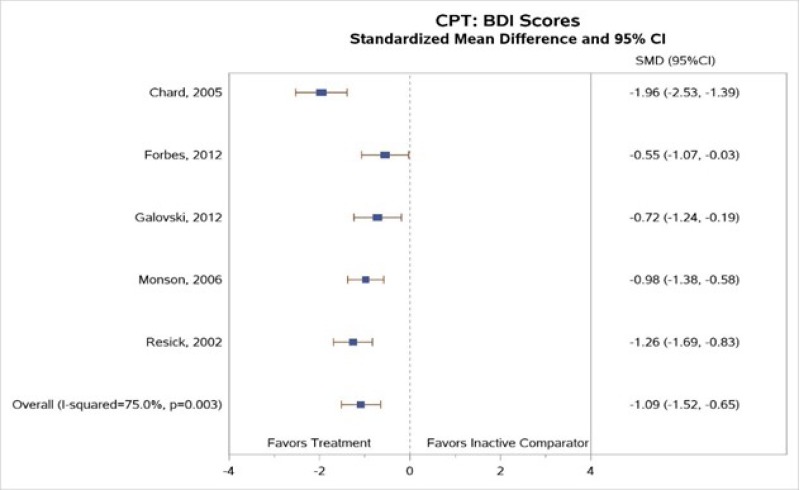
![Click on image to zoom Figure 7 is titled “Standardized mean change from baseline to end of treatment in PTSD symptoms (any measure) for exposure therapy compared with inactive comparator.” The figure displays a forest plot reporting the standardized mean difference in PTSD symptoms, exposure therapy versus inactive comparators. This figure is described further in the “PTSD symptoms” as follows: “Our meta-analysis of pooled data from these studies (Figure 7) found a greater decrease in PTSD symptoms for subjects treated with exposure than for those in control groups; the effect size was very large (SMD −1.23; 95% CI, −1.50 to −0.97, 13 trials [14 comparisons], N = 885); I-squared, 67.5%; high SOE).”](/books/NBK525134/bin/ch4f6.jpg)
![Click on image to zoom Figure 8 is titled “Standardized mean change from baseline to end of treatment in PTSD symptoms (CAPS) for exposure therapy compared with inactive comparator.” The figure displays a forest plot reporting the standardized mean difference in CAPS scores, exposure therapy versus inactive comparators. This figure is described further in the “PTSD symptoms” section as follows: “Our meta-analysis of the trials reporting CAPS scores found a greater decrease in PTSD symptoms among subjects treated with exposure than those in an inactive comparator group (SMD, −1.12; 95% CI, −1.42 to −0.82; 8 trials [9 comparisons], N=689); I-squared 68.66%; SOE) (Figure 8).”](/books/NBK525134/bin/ch4f7.jpg)
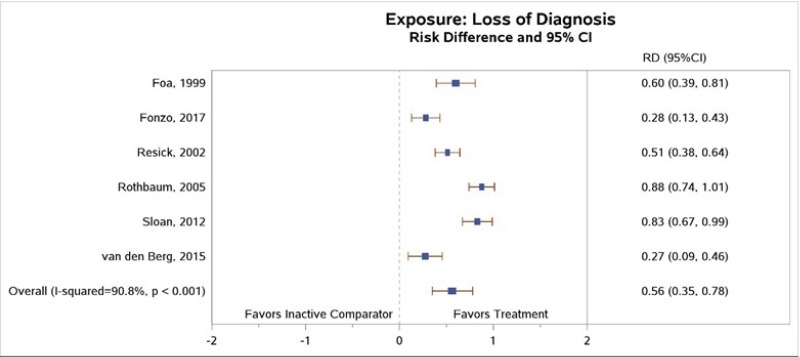
![Click on image to zoom Figure 10 is titled “Standardized mean change from baseline to end of treatment in depression symptoms (BDI) for exposure therapy compared with inactive comparator.” The figure displays a forest plot reporting standardized mean difference in depression (as measured by the Beck Depression Inventory), exposure therapy versus inactive comparators. This figure is described further in the “Prevention or Reduction of Comorbid Conditions” section as follows: “Results of our meta-analysis indicated a greater reduction in BDI depressive symptom scores for subjects treated with exposure than for those in wait-list or usual-care inactive comparator groups (SMD, −0.76; 95% CI, −0.91 to −0.60; I2=19.4%, 10 trials [11 comparisons], N=7,152, Figure 10; high SOE.”](/books/NBK525134/bin/ch4f9.jpg)